Schwann-like adipose-derived stem cells as a promising therapeutic tool for peripheral nerve regeneration:effects of cholinergic stimulation
2021-11-26RobertaPiovesanaAlessandroFaroniAdaMariaTataAdamReid
Roberta Piovesana, Alessandro Faroni, Ada Maria Tata, Adam J. Reid
Schwann-like adipose-derived stem cells and nerve injury:Peripheral nerve injuries(PNIs) are a common clinical problem usually as a consequence of trauma. Despite optimal surgical management, PNI has a lifelong impact on function and wellbeing of the patient.
The peripheral nervous system (PNS)has regenerative capability, in contrast to the central nervous system (CNS),and is dependent on the plasticity of the peripheral glia, Schwann cells (SCs). Despite this regenerative capability, PNI recovery of sensorial and motor function is always incomplete causing pain, cold intolerance,paralysis and impairment of activities of daily living. Therefore, development of innovative approaches enhancing PNS regeneration after injury is of great clinical relevance(Faroni et al., 2015).
It is widely accepted that the current microsurgical approach in isolation to PNI is insufficient to address the complex cellular and molecular mechanisms occurring in the nerve injury environment; however, there are no available adjunctive therapies or pharmacological treatments that improve outcomes for patients. In addition, greater understanding of PNI and regeneration has demonstrated that timing of any intervention is fundamental for optimal recovery (De Stefano et al., 2013; Faroni et al., 2015).
Cell-based therapies for PNI have demonstrated experimental potential and appear to be a promising paradigm for clinical intervention towards improved functional outcomes, especially in the presence of a nerve gap. The use of SCs has been investigated with some experimental success; however, their clinical application is limited, due to the necessity of sacrifice of a functional healthy nerve and considering that SC harvest and amplification timescales would delay treatments beyond the clinical window of opportunity.
As a consequence, attention has turned to stem cells with the first description of a mesenchymal to Schwann cell‘differentiation’ in rat bone-marrow-derived mesenchymal stem cells (BM-MSCs) under appropriate conditionin vitro(Dezawa et al.,2001). BM-MSCs present problems linked with the painful withdrawal and the low number of isolated MSCs. Subsequently, the same protocol was applied to differentiate adipose-derived stem cells (ASCs) towards Schwann-like phenotype (dASCs; Figure 1A)(Kingham et al., 2007). Although ASCs share a lot of features with BM-MSCs, the higher number of stem cells successfully isolated and the faster proliferation, as well as the easier isolation through surgical liposuction procedure, make this cell population more appealing for regenerative medicine.
After differentiation, ASCs transform their undifferentiated-flattened fibroblast-like morphology to assume a spindle, polarized structure similar to SCs and express glial cell markers [e.g. S100 calcium binding protein-β(S100-β), low-affinity nerve growth factor receptor (p75NTR) and glial fibrillary acidic protein (GFAP)] (Kingham et al., 2007). The increase of their neurotrophic potential promotes regeneration in bothin vitroandin vivoexperimental models highlighting the great potential of these cells as an alternative to SC therapies.
Acetylcholine (ACh) and Schwann-like dASCs:Neurotransmitters are known pharmacological modulators of SC physiology and myelination (Loreti et al., 2006, 2007;Uggenti et al., 2014; Faroni et al., 2016,2019). Exploration of neurotransmitter receptor manipulation to regulate dASC physiology has been demonstrated with GABA-B receptor ability to modulate BDNF and NGF secretion (Faroni, et al., 2013),whilst P2X7 receptor controls cell death(Faroni, et al., 2013). Recently, we have demonstrated that rat ASCs (Piovesana et al., 2018), dASCs (Piovesana et al., 2019) and native SCs (Loreti et al., 2006) all express muscarinic receptor subtypes, with a greater expression of M2 subtype. The presence of ACh in non-neuronal tissue has been largely established and via M2 subtype it is able to suspend ASC activity, reducing their cell growth and migration. After the removal of cholinergic stimulation, ASCs are able to rescue their proliferation, a necessary condition for the maintenance of stem cell self-renewal (Piovesana et al., 2018).
Following differentiation, M2 receptor activation in dASCs by orthosteric selective agonist arecaidine propargyl ester (APE),produces similar effects observed in SCs(Loreti et al., 2007; Uggenti et al., 2014).In particular, M2 receptors inhibit cell proliferation in a reversible manner and cell migration; moreover they assume a more pronounced spindle-shaped morphology with an upregulated expression of SC differentiation markers (i.e. Egr2/Krox20, myelin protein zero) (Piovesana et al., 2019). These data suggest that M2 receptor selective stimulation supports dASC phenotype promoting their differentiation(Additional Table 1) (Piovesana et al., 2019).
Muscarinic receptors and neurotrophic factor production:The first relevant aspect supporting neuronal survival and axon elongation after nerve injury is the local production of neurotrophic factors.Exogenous administration of neurotrophic factors or other small molecules and peptides have not proven successful experimentally, perhaps due to the very particular temporospatial control of native SCs in the neurotrophic production, but also due to interactions and potential sideeffects which could impair axonal growth or significantly increase neuropathic pain(Faroni et al., 2015). Pharmacological stimulation of the resident cells to produce neurotrophic factors or transplantation of neurotrophic factor producing cells are a potential adjunct to address nerve regeneration.
Both ASCs and dASCs represent a rich source of neurotrophic growth factors. We have recently demonstrated that rat dASCs produce and release higher basal levels of proNGF and release higher concentration of mature NGF than SCs (Piovesana et al.,2020). Furthermore, we have determined for the first time that cholinergic stimulation regulates the production and secretion of NGF in both rat dASCs and SCs (Piovesana et al., 2020). The pharmacological modulation of all muscarinic receptors, using the nonselective agonist muscarine, as well as the selective activation of M2 receptor subtype,using APE treatment, regulate the production and secretion of NGF both in rat dASCs and SCs, and significantly downregulate the proNGF-B isoform (25 kDa) expression,mainly involved in the apoptosis (Piovesana et al., 2020). The negative modulation of proNGF-B suggests an improvement of dASC and SC capability in reducing neuronal death(Additional Table 1).
Native SCs are highly productive of neurotrophic factors, although cholinergic stimulation increases NGF production even within 24 hours of M2 receptor stimulation (Piovesana et al., 2020). These findings represent the first evidence for the positive role of cholinergic receptors in the modulation of NGF expression and release in the PNS.
Muscarinic stimulation upregulates tissue plasminogen activator (tPA) activity both in dASCs and SCs, indicating that cholinergic stimulation may efficiently promote the proNGF cleavage and therefore the production of the active form of NGF(mature NGF; mNGF) (Piovesana et al.,2020). Interestingly dASCs produce higher extracellular levels of MMP9 than SCs,an enzyme required to rapidly degrade mNGF and restore the microenvironment homeostasis.
These results indicate that muscarinic challenge can promote an NGF-mediated neuro-reparative response by dASCs, with promise to improve neuronal survival and axon regeneration via negative modulation of proNGF-B in native glial cells but also in the transplanted cells.
We corroborated the idea that non-selective treatment activating all muscarinic receptor subtypes generates a balance of their effects on dASC phenotype and on NGF metabolism;however it is now evident, that muscarine and APE treatments show comparable effects, suggesting that M2 receptor may be the main subtype involved in NGF metabolism both in dASCs and SCs.
Altogether these data demonstrate that dASCs are efficient producers of NGF and that the muscarinic receptors promote NGF production and maturation. Therefore, our findings highlight a new pharmacological target that could be used to enhance dASC phenotype and increase a local release of NGF by dASCs that could reduce the wellknown effects of mechanical allodynia caused by the injection of exogenous NGF.
Human ASCs can differentiate towards SC-like phenotype:Human adipose tissue is also enriched in resident ASCs, useful for regenerative medicine. Human ASCs are positive for the typical markers CD29,CD44, CD90, CD73, CD105, CD271 and negative for CD14, CD20 and CD45 (Tomita et al., 2013; Faroni et al., 2016). After isolation they are able to differentiate in the three specific mesodermal lineagesin vitro, producing fat droplets as adipogenic differentiation, proteoglycans as chondrogenic differentiation and calcium deposits for osteogenic differentiation (Faroni et al., 2016). The protocol used in rat ASCs was reproduced in human ASCs, with a good percentage of SC-like cells (Tomita et al., 2013). After 3-5 days of growth factors exposure in this differentiation protocol,dASCs assume an elongated spindle-shaped morphology that is maintained duringin vitropassage, increased cell proliferation and higher levels of neurotrophic factors(e.g. NGF, BDNF and GDNF) compared to ASCs (Faroni et al., 2016). However,whilst human dASCs have this regenerative potential, they require continuous chemical stimulation and trans-differentiation seems unlikely. Growth factor withdrawal following this differentiation protocol results in a reversion of dASC phenotype and significant reduction of growth factor expression to an undifferentiated state within 72 hours (Faroni et al., 2016). On the other hand, 8 weeks following human dASC cell transplantation in athymic nude rats show a small number of human cells associated with myelinated axons (Tomita et al., 2013), proposing that thein vivoenvironment (e.g., axon contacts,resident SCs or paracrine signals) could induce or change ASC characteristics and perhaps maintain dASC phenotype.
Further research on human cells is required to understand whether environmental factors or other molecules can stabilize the dASC phenotypein vitroand alsoin vivo.Here, we hypothesize that there may be a role for manipulation of muscarinic receptors to modulate the regenerative properties of ASCs and dASCs towards a clinically relevant intervention.
Future perspective and conclusions:In a regenerative scenario, our results propose
dASCs as a remarkable cell population usefulas substitution of SCs, considering their ability
to create pro-regenerative environment byneurotrophic factor production. The dataobtained in the rat model open the possibilitythat human dASCs may play a strategicrole in the nerve regeneration; however,current ASC differentiation protocols require constant chemical stimulation with different growth factors (i.e. GGF-2, Fsk, PDGF,bFGF) and our previous data demonstrate that growth factor withdrawal results in reversion to ASC phenotype. Therefore,we seek to identify new pharmacological treatments to stabilize the phenotype and improve physiological activities, especially in the maintenance of neurotrophic factor production. Starting from the evidence that muscarinic stimulation promotes spindle-like morphology and the upregulated expression of differentiation markers in rat SCs and in dASCs, we hypothesize that M2 receptor stimulation may support human dASC phenotype also in absence of growth factors.In conclusion, our data suggest an exciting clinical paradigm of Schwann-like ASCs with cholinergic stimulation mediated by muscarinic receptors that could be transplanted in appropriate scaffolds to enhance and accelerate the peripheral nerve regeneration (Figure 1B).
The present work was supported by Ateneo Sapienza Funds 2017 (RM11715C7F959CA4)to AMT and “Avvio Giovani” Project 2018(AR11816435A0F81D) from Ateneo Sapienza to RP. RP fellowship was also supported by CIB 2018. AF and AJR are supported by the Hargreaves and Ball Trust, the Academy of Medical Sciences (AMS-SGCL7), and by Seed Corn Funding from the Rosetrees Trust and the Stoneygate Trust (M746).
The authors are very grateful to Paul Mellidi for the illustration in Figure 1B.
Roberta Piovesana, Alessandro Faroni,Ada Maria Tata, Adam J. Reid*
Department of Biology and Biotechnologies“Charles Darwin”, Sapienza, University of Rome,Rome, Italy (Piovesana R, Tata AM)Blond McIndoe Laboratories, Division of Cell Matrix Biology and Regenerative Medicine, School of Biological Sciences, Faculty of Biology, Medicine and Health, The University of Manchester,Manchester Academic Health Science Centre,Manchester, UK (Piovesana R, Faroni A, Reid AJ)Research Centre of Neurobiology “Daniel Bovet”,Sapienza, University of Rome, Rome, Italy(Tata AM)
Department of Plastic Surgery & Burns,Wythenshawe Hospital, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, Manchester, UK (Reid AJ)
*Correspondence to:Adam J. Reid, MBChB, FRCS(Plast), PhD, Adam.Reid@manchester.ac.uk.https://orcid.org/0000-0003-1752-3302(Adam J. Reid)
Date of submission:June 18, 2020
Date of decision:July 28, 2020
Date of acceptance:September 19, 2020
Date of web publication:November 27, 2020
https://doi.org/10.4103/1673-5374.300433
How to cite this article:Piovesana R, Faroni A,Tata AM, Reid AJ (2021) Schwann-like adiposederived stem cells as a promising therapeutic tool for peripheral nerve regeneration: effects of cholinergic stimulation. Neural Regen Res 16(6):1218-1220.
Copyright license agreement:The Copyright License Agreement has been signed by all authors
before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional Table 1:Summary of our results obtained in rat dASCs, compared with untreated cells (Piovesana et al., 2019, 2020).
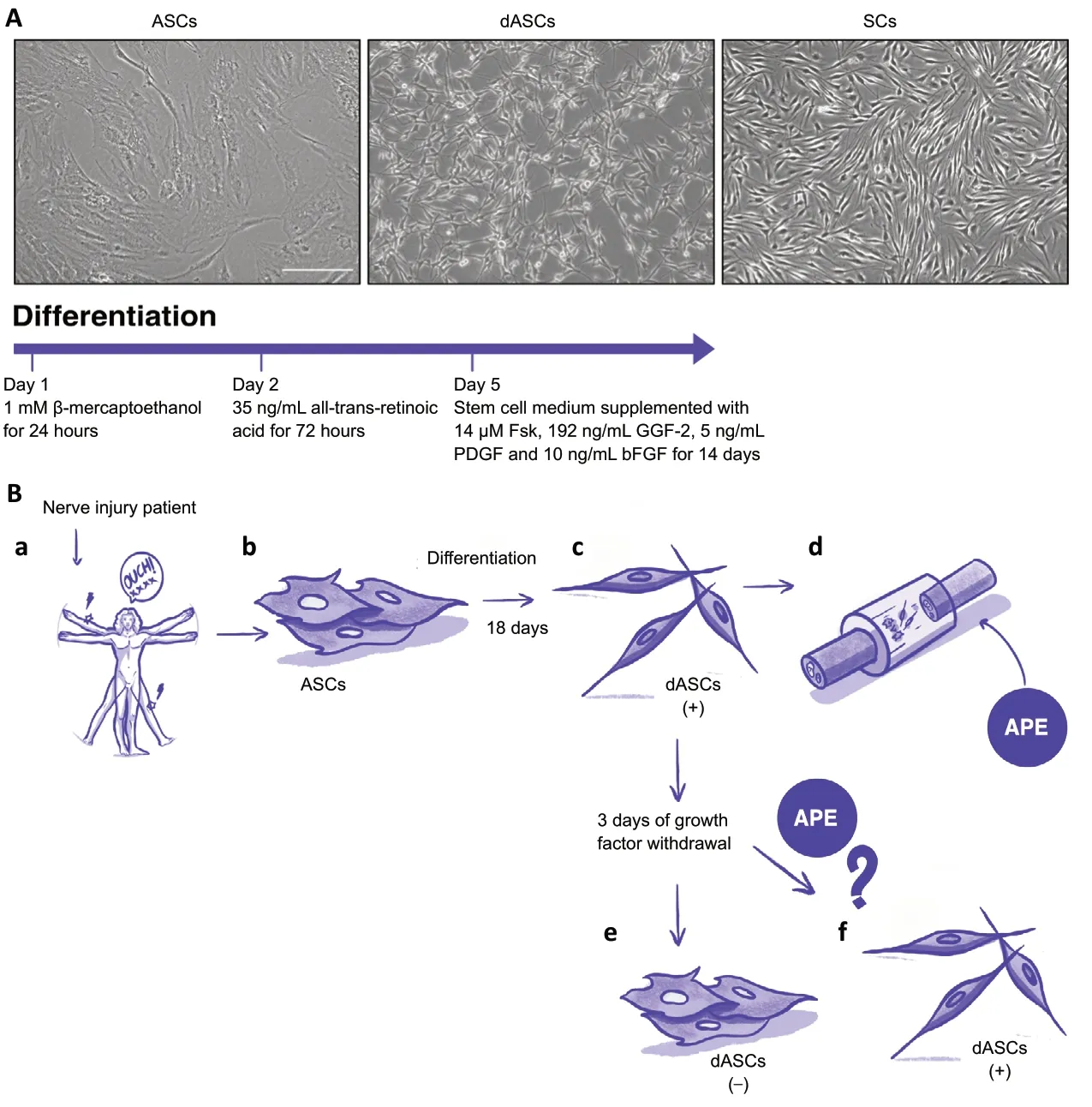
Figure 1 |ASC differentiation and potential clinical application.
杂志排行
中国神经再生研究(英文版)的其它文章
- Entacapone promotes hippocampal neurogenesis in mice
- Electroacupuncture improves learning and memory functions in a rat cerebral ischemia/reperfusion injury model through PI3K/Akt signaling pathway activation
- Normobaric oxygen therapy attenuates hyperglycolysis in ischemic stroke
- MicroRNA-670 aggravates cerebral ischemia/reperfusion injury via the Yap pathway
- Corticospinal excitability during motor imagery is diminished by continuous repetition-induced fatigue
- TP53-induced glycolysis and apoptosis regulator alleviates hypoxia/ischemia-induced microglial pyroptosis and ischemic brain damage
