Microglial depletion and repopulation: a new era of regenerative medicine?
2021-11-26AlexandraBarnettFultonCrewsLeonColeman
Alexandra M. Barnett, Fulton T. Crews, Leon G. Coleman
Microglia have multiple functions and phenotypes that can prevent or worsen neuropathology.Microglial depletion and repopulation methods provide a promising technique for understanding microglial biology. Their utility as therapeutic modalities is now under consideration. As resident immune cells in the central nervous system (CNS),microglia maintain the local environment and promote neuronal vitality. However, persistent proinflammatory signaling due to aberrant microglial activation can be detrimental. This is seen in settings such as traumatic brain injury(TBI) (Henry et al., 2020), stroke (Li et al., 2017),and alcohol use disorder (AUD) (Coleman et al.,2020), when the loss of homeostatic control results in persistent proinflammatory signaling that contributes to ongoing neuropathology(Figure 1). Thus, selective replacement of chronically proinflammatory-activated microglia could improve functional outcomes. Depletion of microglia from the CNS microenvironment has served as a helpful tool to understand the contribution of microglia to brain disease. In this perspective, we discuss our recent findings regarding microglial depletion and repopulation in AUD as well as the benefits and detriments of microglial depletion and repopulation and their potential therapeutic applications in other neuropathological disease models. We recently reported that microglial depletion and repopulation protected against long-term proinflammatory activation in a model of AUD(Coleman et al., 2020). In a primaryex vivobrain slice culture model, binge ethanol caused a persistent induction of proinflammatory cytokines.Microglial depletion and repopulation after ethanol treatment, using methods described below,resulted in a normalization of proinflammatory cytokines to baseline levels, and an increase in protective trophic factors such as brain-derived neurotrophic factor. Thus, repopulation of microglia could potentially reverse neuropathology associated with chronic proinflammatory signaling in AUD and other proinflammatory neurological diseases (Figure 1).
Various preclinical methods have been used to deplete microglia to study their role in neuropathology. This includes pharmacological approaches such as colony-stimulating factor 1 receptor (CSF1R) inhibitors (Rice et al., 2015) and genetic approaches that induce the diphtheria toxin receptor in microglia (Lund et al., 2018).Microglia require trophic support through CSF1,and CSF1R inhibitors made by plexxikon (PLX)block this support, leading to depletion of more than 90% of microgliain vivoover approximately 3 weeks when administered in their diet. These studies generally find microglial depletion can protect against a broad range of insults. Though microglia are important in maintaining steadystate conditions in the brain, neither short- nor long-term depletion of microglia has been found to have robust impact on cognition or behavior(Elmore et al., 2015). Thus, CSF1R pharmacological depletion with agents that can successfully cross the blood-brain barrier and deplete microglia,such as CSF1R antagonists or similar agents,could have little toxicity while protecting from chronic proinflammatory pathology. However,additional studies are needed to determine the consequences of the loss of microglia on neurons and astrocytes. We will discuss the efficacy of CSF1R inhibition in disease settings as this approach has potential translational relevance.
Microglial depletion with CSF1R antagonists has been shown to induce neuroprotective outcomes in certain disease models. This method has been shown to reduce neuroinflammation and improve recovery following induction of extensive neuronal loss in the hippocampus caused by diphtheria toxin injection (Rice et al., 2015). Further, microglial depletion with CSF1R antagonist PLX3397 reduces induction of proinflammatory gene expression in brain caused by acute binge ethanol (Coleman et al., 2020). In models of intracerebral hemorrhage,depletion promotes brain recovery and reduces inflammation, while also preserving the bloodbrain barrier to reduce leukocyte infiltration (Li et al., 2017). These settings are examples wherein microglial depletion is beneficial, reducing proinflammatory gene induction. However, microglial depletion does not always yield beneficial outcomes. In ischemic stroke models, depletion results in increased infarct size, cell death,inflammatory mediator levels, and leukocyte infiltration into the CNS (Jin et al., 2017). The different findings in different pathological states support the view that elimination of microglia exerts context-dependent effects. Nonetheless,depletion seems to be beneficial in several inflammation-related pathological states. One important setting that could potentially benefit from microglial depletion and has yet to be examined is neurogenic shock secondary to trauma. However, as microglia are necessary for CNS homeostasis and response to pathology and current depletion methods also impact peripheral monocytes, long-term sustained depletion is likely not a clinically viable approach.
Upon removal of the depleting agent, microglia quickly repopulate to normal levels. Microglia rapidly repopulate over 1-2 weeks and restore the empty CNS niche after depletion (Elmore et al., 2015), and, depending on the method of depletion, may repopulate from different sources (Lund et al., 2018; Weber et al., 2019).Recently, studies using PLX5622 indicate microglia repopulate from proliferation of the residual pool of resident microglia (Weber et al., 2019),whereas repopulation after genetic ablation with induction of the diphtheria toxin receptor also involves migration of peripheral monocytes that can be relatively more proinflammatory(Lund et al., 2018). In the healthy brain,repopulated microglia are able to perform similar maintenance functions as resident microglia, and their transcriptomic profiles suggest microglial phenotype is a normal resting state (Elmore et al., 2015). However, in cases of neuropathology(e.g., TBI, aging) repopulated microglia can have different transcriptomic profiles and functional activities from the pre-depleted microglia in those disease states (Weber et al., 2019; Henry et al., 2020) and response to systemic immune challenge. Therefore, repopulation may be a promising strategy in the study and treatment of neuropathology if repopulated microglia behave more similarly to naïve ones.
Repopulated microglia can be beneficial in disease settings characterized by prolonged proinflammatory responses. This includes settings such as TBI (Henry et al., 2020), aging (Elmore et al., 2018), and AUD (Coleman et al., 2020).Microglial repopulation during the chronic phase of TBI resolved neuroinflammation and related neurodegeneration and improved motor and cognitive functions (Henry et al., 2020).Repopulated microglia following binge ethanol administrationex vivoreversed proinflammatory gene expression, increased expression of trophic factors, and decreased responses to Toll-like receptor agonists (Coleman et al., 2020). In the aged brain, microglia assume a “primed” or more pro-inflammatory, state that may contribute to long-term neurodegeneration (O’Neil et al.,2018). Repopulation of microglia in the aged brain partially restores their transcriptomic profile,reverting age-associated upregulation of some genes (i.e.,Apoe,Tgfb2) to control levels (O’Neil et al., 2018). Microglial repopulation can also rescue long-term potentiation and reverse ageassociated deficits in spatial learning (Elmore et al., 2018). However, similar to depletion,microglial repopulation can be detrimental in certain settings. For instance, microglial depletion with repopulation in TBI early post-injury period increased neurodegeneration and accelerated cognitive decline (Hanlon et al., 2019). Therefore,the chronic proinflammatory state induced with many neurological diseases may be improved by microglial repopulation in the chronic phase,but depleting microglia during injury can be detrimental.
The specific effects of microglial depletion and repopulation on microglial biology remain unclear and may depend on the method of depletion and the setting whereby microglial function is assessed.Though repopulated microglia differ in their gene expression in disease models, consensus is lacking on the exact functional differences of repopulated microglia, and the method of depletion (i.e.,CSF1R antagonismversusgenetic/diphtheria removal) can result in repopulation from different sources as stated above. Regarding the function of repopulated microglia, our work usingex vivobrain slice culture found that repopulated microglia have lower induction of proinflammatory genes in response to direct stimulation to Toll-like receptor agonists, and have increased expression of trophic factors (Coleman et al., 2020). In studies using stressed mice, microglial repopulation after depletion with CSF1R antagonism attenuated induction of microglial proinflammatory genes in low dose systemic lipopolysaccharide (0.5 mg/kg)(Weber et al., 2019). However, other studies using depletion models in aged brain did not find significant differences in functional responses of repopulated microglia to systemic lipopolysaccharide (Elmore et al., 2018). Some methodological differences may account for this. For example, Weber et al. (2019) measured isolated microglia whereas Elmore et al.(2018) measured genes in whole brain, and we administered Toll-like receptor agonists directly to brain slice culture. Nonetheless, mice with repopulated microglia show improvements in multiple behavioral assays, including measures of spatial learning and memory and cognition (Elmore et al., 2018; Weber et al., 2019). These findings support that repopulation reverses some aspects of proinflammatory responses of microglia that can be beneficial in various settings. Microglial repopulation is not a “one size fits all” approach.Rather, it is a promising intervention that could be protective in specific situations.
For microglial repopulation to be employed as a therapeutic method there are multiple important considerations. First is the method of depletion. Ideally, an approach will be developed that does not cause off-target toxicity and will specifically deplete microglia, without impacting peripheral monocytes and systemic immunity.One possible approach could be using intranasal delivery of CSF1R antagonists loaded into braintargeted nanoparticles, or intranasal nanoparticle delivery of CSF1R siRNAs. Secondly, ideally a method of depletion would be employed that causes microglia to repopulate from the CNS pool rather than peripheral monocytes or bone marrow, as infiltrating monocytes may be more proinflammatory and “disease-like” (Lund et al.,2018). Additionally, the timing of depletion and subsequent repopulation is critical. This timing would need to be optimized in each specific disease state to cause beneficial outcomes.Microglia are known to be protective during acute brain injury but can be detrimental directly after.This is perhaps most clear in the setting of TBI where microglial depletion and repopulation early post-injury accelerates cognitive decline (Hanlon et al., 2019) while in the chronic phase it dampens pro-inflammatory signaling and promotes recovery(Henry et al., 2020). Further, when microglia are absent during neuronal lesion, neuronal loss is increased, while their absence following injury reduced inflammation and promoted recovery(Rice et al., 2015). Lastly, there must be close consideration of the nature of the disease state that is being treated and the duration of depletion that would be required for a benefit, and if this would be clinically appropriate. Thus, current work suggests that microglial depletion and/or repopulation can be neuroprotective during the chronic hyperinflammatory phases following injury or stress when proinflammatory responses increase pathology and are no longer beneficial,with careful consideration given to the method of microglial depletion.
Looking forward, microglial depletion and repopulation are exciting new approaches that not only help unravel the secrets of microglial biology and their role in disease progression but could have therapeutic potential. These studies also highlight the diversity of microglial states that impact neuropathology and the potential therapeutic value of reducing proinflammatory microglia. One can imagine clinics in the notso-distant future, where people check in for their microglial depletion to reverse symptoms associated with aging or other conditions.However, the short- and long-term effects of depletion and repopulation in humans and nonhuman primates on brain function as a whole and its effects on neurons and other cell types need to be investigated further. Depletion and repopulation seem to have limited side effects in rodents, and early investigations in humans suggest safety. CSF1R antagonists were well tolerated in a phase II clinical trial for glioblastoma(Butowski et al., 2016) and crossed the blood-brain barrier, though they did not cause the degree of depletion that is seen in rodents. Studies in nonhuman primates and humans are needed to find the best way to deplete microglia while sparing peripheral monocytes. Future studies might also discover pharmacological approaches that can specifically alter microglial functional state without requiring depletion and repopulation. In conclusion, the specific short-term depletion and subsequent repopulation of microglia in chronic neurological disease states holds great promise,and future work will hopefully lead to targeted and precise strategies for depletion that are clinically relevant.
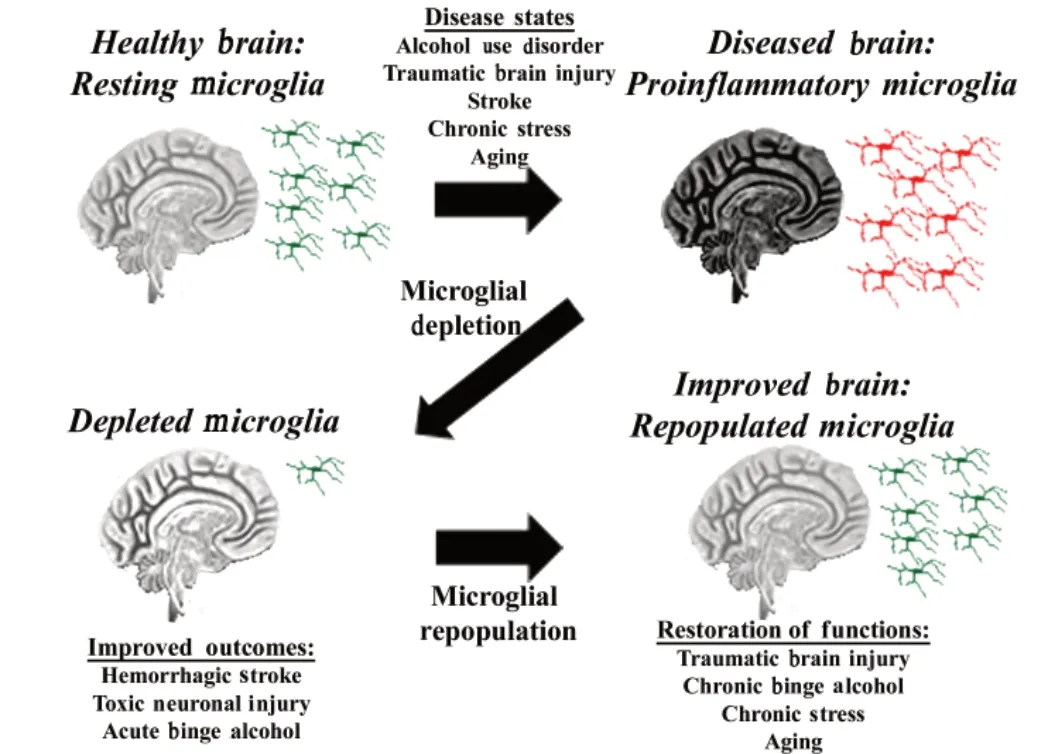
Figure 1 |Microglial Repopulation for Chronic Neuroinflammation.
This work was supported by the National Institutes of Health, National Institute on Alcoholism and Alcohol Abuse (P60AA011605-Fulton Crews,U01AA020023-Fulton Crews, U24AA020024-Fulton Crews, U54AA019767-Fulton Crews,T32AA007573-Fulton Crews; K08AA024829-Leon G Coleman, Jr; K08AA024829S1-Leon G Coleman, Jr,and the Bowles Center for Alcohol Studies).
We thank Jian Zou for his encouragement to pursue the possibility of microglial repopulation.
Alexandra M. Barnett, Fulton T. Crews,Leon G. Coleman*
Bowles Center for Alcohol Studies, The University of North Carolina at Chapel Hill, School of Medicine, Chapel Hill, NC, USA (Barnett AM,Crews FT, Coleman LG)Department of Pharmacology, The University of North Carolina at Chapel Hill, School of Medicine,Chapel Hill, NC, USA (Crews FT, Coleman LG)Department of Psychiatry, The University of North Carolina School of Medicine, Chapel Hill, NC, USA(Crews FT)
*Correspondence to:Leon G. Coleman, MD, PhD,leon_coleman@med.unc.edu.https://orcid.org/0000-0003-1693-3799(Leon G. Coleman)
Date of submission:June 17, 2020
Date of decision:July 29, 2020
Date of acceptance:August 20, 2020
Date of web publication:November 27, 2020
https://doi.org/10.4103/1673-5374.300439
How to cite this article:Barnett AM, Crews FT,Coleman LG (2021) Microglial depletion and repopulation: a new era of regenerative medicine?Neural Regen Res 16(6):1204-1205.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the
terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Entacapone promotes hippocampal neurogenesis in mice
- Electroacupuncture improves learning and memory functions in a rat cerebral ischemia/reperfusion injury model through PI3K/Akt signaling pathway activation
- Normobaric oxygen therapy attenuates hyperglycolysis in ischemic stroke
- MicroRNA-670 aggravates cerebral ischemia/reperfusion injury via the Yap pathway
- Corticospinal excitability during motor imagery is diminished by continuous repetition-induced fatigue
- TP53-induced glycolysis and apoptosis regulator alleviates hypoxia/ischemia-induced microglial pyroptosis and ischemic brain damage
