Astaxanthin alleviates pathological brain aging through the upregulation of hippocampal synaptic proteins
2021-11-26NingLiuLiangZengYiMingZhangWangPanHongLai
Ning Liu , Liang Zeng, Yi-Ming Zhang Wang Pan, Hong Lai
Abstract Oxidative stress is currently considered to be the main cause of brain aging. Astaxanthin can improve oxidative stress under multiple pathological conditions. It is therefore hypothesized that astaxanthin might have therapeutic effects on brain aging. To validate this hypothesis and investigate the underlying mechanisms, a mouse model of brain aging was established by injecting amyloid beta (Aβ)25-35 (5 µM,3 µL/injection, six injections given every other day) into the right lateral ventricle. After 3 days of Aβ25-35 injections, the mouse models were intragastrically administered astaxanthin (0.1 mL/d, 10 mg/kg) for 30 successive days. Astaxanthin greatly reduced the latency to find the platform in the Morris water maze, increased the number of crossings of the target platform, and increased the expression of brain-derived neurotrophic factor, synaptophysin, sirtuin 1, and peroxisome proliferator-activated receptor-γ coactivator 1α. Intraperitoneal injection of the sirtuin 1 inhibitor nicotinamide (500 µM/d) for 7 successive days after astaxanthin intervention inhibited these phenomena. These findings suggest that astaxanthin can regulate the expression of synaptic proteins in mouse hippocampus through the sirtuin 1/peroxisome proliferator-activated receptor-γ coactivator 1α signaling pathway, which leads to improvements in the learning, cognitive, and memory abilities of mice. The study was approved by the Animal Ethics Committee, China Medical University, China (approval No. CMU2019294) on January 15, 2019.
Key Words: brain aging; cognitive; factor; hippocampus; learning; memory; oxidative stress; pathways; synapse Chinese Library Classification No. R453; R741; Q629.4
Introduction
At present, most developed countries and some developing countries in the world are faced with aging populations.With increasing aged populations, the aging of the brain has gradually become clearer. Brain aging refers to the aging phenomenon that occurs gradually in brain tissue morphology, structure, and function, leading to learning,cognitive, and memory dysfunctions (Baghel et al., 2019).How to effectively delay cognitive dysfunction caused by brain aging has therefore become a hot topic in related fields. The mechanisms of brain aging are multifactorial; however, it is generally considered that oxidative stress is the leading cause of brain aging.
Recent studies have demonstrated that silencing information regulator 2-related enzyme 1 (sirtuin 1 or SIRT1) plays a vital role in nerve development, repair, and protection (Toklu et al.,2017; Yerra et al., 2017). SIRT1 substrates include histones,non-histones (involved in apoptosis, neuronal protection,organ metabolism, cell aging, and tumorigenesis, for example),and various transcription factors (Ma et al., 2016; Yan et al., 2016; Lin et al., 2017). SIRT1 can regulate intracellular reactive oxygen species levels and protect cells from oxidative stress injury (Liu et al., 2019). Nicotinamide (NAM) can inhibit the expression of SIRT1 (Hwang and Song, 2017; Pan et al.,2020). Peroxisome proliferator-activated receptor gamma co-activator-1α (PGC-1α), a relatively newly discovered protein in the oxidative stress system, plays an essential role as a transcriptional regulation factor that can induce the expression of cellular antioxidant enzymes. PGC-1α is a core control factor that regulates mitochondrial biosynthesis.Studies have shown that SIRT1 is an upstream protein of PGC-1α that can adjust the initiation and sustained response of PGC-1α in the oxidative stress state (Guo et al., 2014; Tan et al., 2015). Thus, SIRT1 possesses the positive biological effect of stimulating PGC-1α (Thirupathi and de Souza, 2017; Visioli and Artaria, 2017).
Astaxanthin (AST) affects the quenching and scavenging of singlet oxygenin vitro(Jackson et al., 2004). Furthermore, AST can reduce lipid peroxidation and protein damage in diabetic rats, decrease the oxidation of apolipoproteins, and be used as an inhibitor to prevent arteriosclerosis and ischemic brain injury (Marin et al., 2011; Song et al., 2014; Ma et al., 2015).In human lymphocytes, oxidative stress caused by a fatty acid mixture and mitochondrial damage can be reduced by AST,which can maintain and improve DNA function (Wolf et al.,2010; Campoio et al., 2011; Park et al., 2011).
Based on the aforementioned observations, we aimed to determine whether hippocampal synaptic proteins are involved in the potential protective mechanisms by which AST regulates oxidative stress in the hippocampus of aging mice.
Materials and Methods
Animals
Sixty specific-pathogen-free male Institute of Cancer Research mice, aged 6 months, were purchased from Liaoning Changsheng Co. Ltd., Benxi, China (license No. SCXK 2015-0003). These mice had free access to water and food and were kept at 55 ± 5% humidity, 22 ± 2°C, and in a 12-hour light/dark cycle. The experimenters alleviated the suffering of the mice as much as possible during the experiments and complied with the regulations and ethical standards of China Medical University. The study was approved by the Institutional Animal Care and Use Committee, China Medical University (approval No. CMU2019294) on January 15, 2019.This study followed the Animal Research: Reporting ofIn VivoExperiments (ARRIVE) guidelines.
Pseudo-aging model establishment
The mice were randomly divided into control, amyloid beta(Aβ), Aβ + AST, and Aβ + AST + NAM groups (n= 15 per group).The mice in the control group did not receive any treatment.The mice in all three Aβ groups received right lateral ventricle injections of Aβ25-35(5 µM, 3 µL/injection, six injections given every other day; Sigma, Milwaukee, WI, USA). The mice in the Aβ + AST and Aβ + AST + NAM groups were then treated with AST (0.1 mL/day, 10 mg/kg; Sigma) by oral gavage for 30 consecutive days from 3 days after completing the Aβ25-35injections. The mice in the Aβ + AST + NAM group were also intraperitoneally injected with NAM (500 µM/day, Sigma) for 7 consecutive days after the AST treatment (Figure 1).
Morris water maze
Two weeks after the mouse models were prepared, the Morris water maze test (Liu et al., 2011) was used to assess spatial memory and learning. The maze (Zhenghua Biological Instrument Equipment Co. Ltd., Huaibei, China) consisted of a circular stainless-steel tank (120 cm in diameter and 35 cm in height), which was divided into four quadrants by four fixed points on its perimeter. The tank contained an escape platform of 10 cm3that was the same color as the rest of the tank (to eliminate any vision-related false-positive results).The platform was placed in a constant quadrant of the tank throughout the trials and was kept 1.5 cm below the water surface. In brief, groups of mice were familiarized with the maze environment on day 1, when they were allowed to swim freely for 2 minutes in the tank (without the platform).The experiment was then implemented four times per day for 5 consecutive days. Each mouse was placed in the water,facing the wall, and the position of the drop point was changed in every test. Each mouse was given 120 seconds to find the submerged platform, and was permitted to stay on the platform for 20 seconds. If the mouse failed to find the platform, it was carefully driven onto the platform and allowed to remain there for 20 seconds. The escape latency time was recorded for each test, which was taken as the learning capability of the mouse. The probe experiment started on day 6, and was performed with the platform removed. Mice were permitted to swim freely in the tank for 120 seconds. Thetimes that the mouse swam across the platform location were counted by a computer, and this measurement was taken as the spatial memory acquisition ability of the mouse.
Tissue preparation
After the behavioral trials, mice (n= 15 per group) were anesthetized with sodium pentobarbital (50 mg/kg,intraperitoneal injection) and decapitated. The hippocampi were quickly removed and divided into two equal parts. One part of each hippocampus was embedded in paraffin and prepared as 5-µm sections for immunofluorescence. The other part of each hippocampus was immediately put into liquid nitrogen and conserved at -80°C, to be used in western blot assays.
Immunofluorescence staining
Brain-derived neurotrophic factor (BDNF) is a hippocampal synaptic protein (Low et al., 2015; Kandola et al., 2019).Synaptophysin (SYN) is closely related to the synaptic structure and function of vesicle protein adsorption, and is recognized as a significant symbol of synaptic plasticity (Cheng et al.,2011; Ma et al., 2019). The paraffin-embedded sections were dewaxed with xylene and rehydrated through graded alcohol solutions. Sections were then washed in phosphate-buffered saline (PBS; 0.1 M, pH 7.2) and placed in citrate buffer (0.1 M, pH 6.0), and were then heated in a water bath (80-90°C)for 10 minutes for antigen retrieval. The sections were then cooled for 30 minutes and placed into PBS for 3 minutes.After rinsing with PBS, the sections were incubated with 5%bovine serum albumin for 30 minutes at 37°C. They were then incubated at 4°C overnight with rabbit anti-SYN (1:500; Cat#17785; PTG, Houston, TX, USA), rabbit anti-BDNF (1:500; Cat#25699; PTG), rabbit anti-SIRT1 (1:500; Cat#13161; PTG) and rabbit anti-PGC-1α (1:500; Cat# 20658; PTG). After five rinses with PBS, the sections were incubated for 1 hour at 37°C with biotinylated goat anti-rabbit IgG (1:1000; Cat# 00001-2; PTG).They were then rinsed three times with PBS before being incubated with 4′,6-diamino-2-phenylindole and hydrogen peroxide in PBS for 10 minutes. Next, the sections were washed with PBS and coverslipped with glycerin. The optical densities of the immunopositive cells and the average optical densities were recorded and analyzed using ImageJ software(version 1.52a; National Institutes of Health, Bethesda, MD,USA).
Western blot analysis
Hippocampal tissue was first mixed at a ratio of 1:5 with lysis buffer. Next, to homogenize the tissue, the mixture was centrifuged at 12,000 ×gfor 30 minutes at 4°C. The supernatant was then collected and the protein concentration was measured using a bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL, USA). The protein samples were segregated using 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Millipore, Boston,MA, USA). The membranes were blocked for 3 hours with Tris-buffered saline with 0.1% Tween-20 and 5% dried skim milk, and they were then incubated with primary antibodies overnight at 4°C. The primary antibodies were rabbit anti-BDNF (1:500), rabbit anti-SYN (1:500), rabbit anti-SIRT1 (1:500), rabbit anti-PGC-1α (1:500), and mouse anti-glyceraldehyde-3-phosphate dehydrogenase (1:1000;Cat#10494; PTG). Next, the membranes were incubated with biotinylated goat anti-rabbit IgG (1:1000; Cat# 00001-2; PTG) at room temperature for 1 hour, and enhanced chemiluminescence (Pierce Biotechnology) and a ChemDoc XRS with Quantity One software (BioRad, Hercules, CA,USA) were used to analyze the bands. Band intensities were analyzed using ImageJ software.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA, USA). All data are expressed as the mean ± standard deviation. The twotailed independent samplest-test was used for comparisons between two groups. Differences were taken to be statistically significant atP< 0.05.
Results
AST improves cognitive function in aging miceThe Morris water maze results illustrated that the escape latencies and trajectories had different degrees of change in the different groups (Figure 2A). The average escape latency of mice in the Aβ group tended to increase, indicating that spatial memory and learning abilities were decreased. The average escape latency of the Aβ group was significantly longer than that of the control group (P= 0.0027). However,the average escape latency of the Aβ + AST group was considerably shorter than that of the Aβ group (P= 0.0034),and was not significantly different from that of the control group (P= 0.2968). In contrast, the average escape latency of t he Aβ + AST + NAM group was significantly longer than that of the Aβ + AST group (P= 0.0035), and was not significantly different from that of the Aβ group (P= 0.4051). In the probe trial, there were differences between groups in the number oftimes the mice crossed the platform (Figure 2B). The number of times crossing the platform was significantly lower in the Aβ group than in the control group (P= 0.0081). However,the number of times crossing the platform was higher in the Aβ + AST group than in the Aβ group (P= 0.0142), but was not significantly different from that of the control group(P= 0.3041). In contrast, the number of times crossing the platform was lower in the Aβ + AST + NAM group than in the Aβ + AST group (P= 0.0279), and was not significantly different from that of the Aβ group (P= 0.5170).
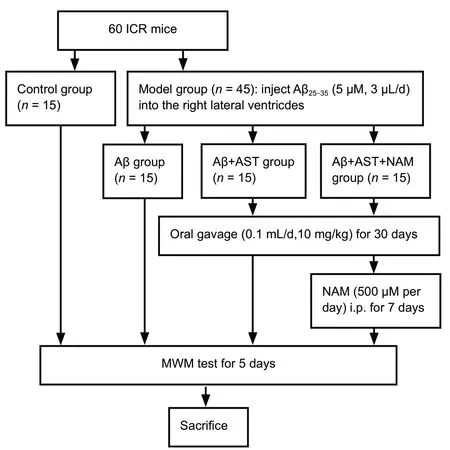
Figure 1 |Study flowchart.
AST restores the immunoreactivities of hippocampal synaptic proteins in aging mice
Immunofluorescent staining results revealed that, compared with the control group, BDNF (P= 0.0003) and SYN (P= 0.0018)immunoreactivities in the pyramidal cells of the hippocampal CA3 region were significantly lower in the Aβ group. Compared with the Aβ group, the Aβ + AST group had stronger BDNF (P= 0.0334) and SYN (P= 0.0021) immunoreactivities. However,compared with the control group, the Aβ + AST group had no significant differences in BDNF (P= 0.0622) or SYN (P=0.0813) immunopositivity. Compared with the Aβ + AST group,the fluorescence intensity of SIRT1 (P= 0.0031) was weaker in the Aβ + AST + NAM group, and this was accompanied by a lower fluorescence intensity of PGC-1α (P= 0.0020).However, the SIRT1 (P= 0.0979) and PGC-1α (P= 0.1413)immunoreactivities were similar between the Aβ + AST +NAM and Aβ groups. These findings indicate that NAM may reduce SIRT1 and PGC-1α expression, while AST may enhance SIRT1 and PGC-1α expression. This may ultimately decrease the degree of oxidative stress, thus improving learning and memory abilities in mice (Figure 3).
AST restores the expression of synaptic proteins in the hippocampus of aging mice
Western blot results revealed that the expression levels of SYN(P= 0.0001) and BDNF (P= 0.0015) were considerably lower in the Aβ group than those in the control group. However, AST treatment increased the expression levels of SYN (P= 0.0005)and BDNF (P= 0.0044) in Aβ mice, to levels that were not significantly different from those in the control group (SYN,P= 0.0932; BDNF,P= 0.0844). The expression levels of SYN (P=0.0049), BDNF (P= 0.0029), SIRT1 (P= 0.0011), and PGC-1α (P= 0.0214) were markedly lower in the Aβ + AST + NAM group than in the Aβ + AST group (Figure 4).

Figure 2 |Effects of AST on cognitive functions in an aging mouse model,as measured by the Morris water maze.
Discussion

Figure 4 |AST restores the expression of hippocampal synaptic proteins and pathway proteins in aging mice.
Oxidative stress is currently considered to be the leading cause of brain aging (Popa-Wagner et al., 2020); however, the precise mechanisms of oxidative stress-induced impairment of memory, learning, and cognition are not well understood.Using the Morris water maze test, previous studies illustrated that brain aging leads to considerably decreased memory,learning, and cognitive functions (Macklin et al., 2017; Haider and Tabassum, 2018; Ishola et al., 2019). In the present study,the Morris water maze results demonstrated that injections of Aβ25-35into the lateral ventricles of mice resulted in decreased spatial memory and learning abilities. The mice in the Aβ group had shorter travel distances and longer escape latencies, and their swimming trajectories were dispersed.AST treatment improved and enhanced the spatial memory and learning abilities of these mice.
BDNF is an important protein for central nervous system development and neuronal survival, differentiation, growth,and development. It can prevent nerve damage and death,improve the pathological state of neurons, and promote the regeneration of damaged neurons and the differentiation of biological effects. SYN exists in the synaptic vesicle membranes of presynaptic components, and indirectly reflects the number, distribution, and density of synapses, meaning that it is often used as a marker of synaptic density. As well as being a marker of synapse density, SYN can also reflect the efficiency of synaptic transmission. Furthermore, BDNF and SYN, as markers of presynaptic and postsynaptic components,are also markers of synaptic plasticity. They are involved in the formation and reconstruction of synapses, and their decreased expression can be taken to mean synaptic loss.Both BDNF and SYN are important for the structure, function,and plasticity of synapses. As the brain ages, synaptic plasticity changes, resulting in reductions in learning and memory abilities. In the present study, the immunofluorescence and western blotting results indicated that the protein expression levels of SYN and BDNF in the hippocampus of the Aβ group were decreased, which may have led to the decreased learning, memory, and cognitive abilities of this group. AST has been shown to improve oxidative stress-induced learning,memory and cognitive function during the brain aging process in our previous study (Zhang et al., 2019).
The SIRT1/PGC-1α signaling pathway plays a role in the regulation of oxidative stress. This signaling pathway has also been reported to be associated with synaptic plasticity (Harris and Winder, 2018; Wang et al., 2018; Li et al., 2019; Yan et al., 2019). NAM is an inhibitor of the SIRT1/PGC-1α signaling pathway, and can negatively regulate this pathway (Hwang and Song, 2017; Orlandi et al., 2017; Shen et al., 2017;Chandrasekaran et al., 2019; Pan et al., 2020). The results of the present study revealed that the hippocampal protein expression of SYN was significantly reduced in the Aβ25-35model of aging, and BDNF was also considerably reduced in this model. These results indicate that this signaling pathway may affect the spatial learning, memory, and cognitive functions of mice; this is similar to the findings of other studies (Liu et al., 2019; Wahl et al., 2019; Wang et al.,2019a; Zhong et al., 2019; Liang et al., 2020). Furthermore,the immunofluorescence and western blotting results in the current study indicated that the expression of PGC-1α, a pivotal protein of this signaling pathway, was decreased in the mouse hippocampus in the Aβ and Aβ + AST + NAM groups.This finding indicates that the expression of the SIRT1/PGC-1α signaling pathway decreased significantly in these groups,which may be related to alterations in the expression of synaptic-related proteins and the spatial memory, learning,and cognitive capacities of mice. This research therefore provides a further experimental basis for the previously reported correlations between the SIRT1/PGC-1α signaling pathway and learning, memory, cognitive, and antioxidant abilities (Sun et al., 2018; Wang et al., 2019b, c).
I told him he was not the only one who had lost a leg, even if mine was still attached to me. I showed him newspaper clippings of my accident. ‘So if you think I m going to let you feel sorry for yourself for the rest of your life, think again. There is a whole life waiting for us out there! I don t intend to be sorry for myself. But I have enough on my plate as it is, so you d better snap out of it too. And I am not going to carry you-you are going to walk yourself. Grandma giggled12, a surprisingly girlish sound coming from an old lady with white hair.
AST plays a vital role in antioxidative and anti-aging processes,and protects synaptic proteins; however, the precise underlying molecular mechanisms remain to be explored.As can be seen from our experimental results, the spatial learning, memory, and cognitive functions of mice can be decreased by injecting Aβ25-35. Through the SIRT1/PGC-1α signaling pathway, AST can protect synaptic proteins, increase the expression of SYN and BDNF proteins, and improve learning, memory, and cognitive abilities (El-Agamy et al.,2018; Han et al., 2019; Kanazashi et al., 2019; Zhou et al.,2019).
In conclusion, the SIRT1/PGC-1α signaling pathway is closely related to learning, memory, cognitive, and antioxidant abilities, as well as the increased expression of SYN and BDNF,which are synapse-associated proteins. AST can increase the expression of the SIRT1/PGC-1α signaling pathway. These experimental results indicate that AST influences the SIRT1/PGC-1α signaling pathway to regulate the expression of synaptic proteins in the hippocampus, such as SYN and BDNF,thus improving learning, memory, and cognition in aging mice and inhibiting oxidative stress-induced brain aging.The mechanisms of brain aging remain unclear; however,the results of the present study demonstrate that AST acts through the SIRT1/PGC-1 signaling pathway to regulate the expression of SYN and BDNF. Further studies are needed to fully elucidate the cellular and molecular mechanisms mediating the functional improvements in relation to oxidative stress. For example, the roles of SYN, BDNF, SIRT1, and PGC-1α in the aging process can be examined usingin vitroexperiments. We will continue to investigate whether AST upregulates the expression of other synaptic proteins through the SIRT1/PGC-1 signaling pathway, and explore relevant trials to select the appropriate dose of AST for clinical applications.This study may provide a reliable theoretical basis for the prevention and treatment of brain aging and senile dementia,as well as for the further development and clinical application of AST.
Author contributions:Study design and manuscript writing: NL, HL;experiment implementation: NL, LZ, YMZ; data analysis: NL, WP. All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare that there are no conflicts of interest.
Financial support:This study was supported by the National Natural Science Foundation of China, No. 8177051488 (to HL). The funder had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement:The study was approved by Institutional Animal Care and Use Committee, China Medical University,China (approval No. CMU2019294) on January 15, 2019.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Evandro Fei Fang, University of Oslo and Akershus University, Norway.
Additional file:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Entacapone promotes hippocampal neurogenesis in mice
- Electroacupuncture improves learning and memory functions in a rat cerebral ischemia/reperfusion injury model through PI3K/Akt signaling pathway activation
- Normobaric oxygen therapy attenuates hyperglycolysis in ischemic stroke
- MicroRNA-670 aggravates cerebral ischemia/reperfusion injury via the Yap pathway
- Corticospinal excitability during motor imagery is diminished by continuous repetition-induced fatigue
- TP53-induced glycolysis and apoptosis regulator alleviates hypoxia/ischemia-induced microglial pyroptosis and ischemic brain damage
