Single-Molecule Field-Effect Transistors with Graphene Electrodes and Covalent Pyrazine Linkers
2021-11-22HantaoSunJianhuiLiaoShiminHou
Hantao Sun , Jianhui Liao , Shimin Hou ,*
1 Key Laboratory for the Physics and Chemistry of Nanodevices, Department of Electronics, Peking University,Beijing 100871, China.
2 Centre for Nanoscale Science and Technology, Peking University, Beijing 100871, China.
Abstract:In single-molecule junctions, anchoring groups that connect the central molecule to the electrodes have profound effects on the mechanical and electrical properties of devices. The mechanical strength of the anchoring groups affects the device stability, while their electronic coupling strength influences the junction conductance and the conduction polarity. To design and fabricate high-performance single-molecule devices with graphene electrodes,it is highly desirable to explore robust anchoring groups that bond the central molecule to the graphene electrodes. Condensation of ortho-phenylenediamine terminated molecules with ortho-quinone moieties at the edges of graphene generates graphene-conjugated pyrazine units that can be employed as anchoring groups for the construction of molecular junctions with graphene electrodes. In this study, we investigated the fabrication and electrical characterization of single-molecule field-effect transistors (FETs)with graphene as the electrodes,pyrazine as the anchoring groups, and a heavily doped silicon substrate as the back-gate electrode. Graphene nano-gaps were fabricated by a high-speed feedback-controlled electro-burning method, and their edges were fully oxidized; thus,there were many ortho-quinone moieties at the edges. After the deposition of phenazine molecules with orthophenylenediamine terminals at both ends, a large current increase was observed, indicating that molecular junctions were formed with covalent pyrazine anchoring groups. The yield of the single-molecule devices was as high as 26%,demonstrating the feasibility of pyrazine as an effective anchoring group for graphene electrodes. Our electrical measurements show that the ten fabricated devices exhibited a distinct gating effect when a back-gate voltage was applied.However, the gate dependence of the conductance varied considerably from device to device, and three types of different gate modulation behaviors, including p-type, ambipolar, and n-type conduction, were observed. Our observations can be understood using a modified single-level model that takes into account the linear dispersion of graphene near the Dirac point; the unique band structure of graphene and the coupling strength of pyrazine with the graphene electrode both crucially affect the conduction polarity of single-molecule FETs. When the coupling strength of pyrazine with the graphene electrode is weak, the highest occupied molecular orbital (HOMO)of the central molecule dominates charge transport.Depending on the gating efficiencies of the HOMO level and the graphene states, devices can exhibit p-type or ambipolar conduction. In contrast, when the coupling is strong, the redistribution of electrons around the central molecule and the graphene electrodes leads to a realignment of the molecular levels, resulting in the lowest unoccupied molecular orbital(LUMO)-dominated n-type conduction. The high yield and versatility of the pyrazine anchoring groups are beneficial for the construction of single-molecule devices with graphene electrodes.
Key Words:Molecular electronics; Graphene electrode; Pyrazine anchoring group; Single-molecule field-effect transistor; Coupling strength
1 Introduction
Anchoring groups between the central molecule and the electrodes, a key component affecting the electrical properties of single-molecule devices, have attracted extensive interest in the field of molecular electronics1,2. Target molecules are connected to the electrodes through anchoring groups both mechanically and electrically3. On the one hand, the mechanical strength of the anchoring groups directly affects the stability of molecular devices; on the other hand, the electronic coupling strength influences the energy shift and the broadening of frontier molecular orbitals of the central molecule, which is an important factor in determining the conducting channel and the conductance of molecular devices2,4. As a typical noble metal,gold is the most commonly used electrode material for the construction of molecular devices. In order to realize robust linkage between molecules and gold electrodes, in-depth research has been conducted on anchoring groups for gold electrodes5,6. Typical anchoring groups for gold electrodes can be classified into two types: dative anchors involving the electron donation from aπdonor or a lone pair donor to a Lewis acidic Au atom and covalent anchors resulting from covalent bonding between molecular radicals and the gold electrode surface. Studies have found that there is a clear dependence between the functionality of molecular devices and the structure of the anchoring groups2. For example, the conduction polarity of single-molecule field-effect transistors (FETs)is directly related to the types of anchoring groups, and the conductance values of molecular junctions have an obvious dependence on the coupling strength. However, due to the high mobility of gold surface atoms at room temperature, molecule-gold bonds are not stable in ambient conditions7,8; moreover, bulk gold electrodes have strong proximity shielding effects on the gate electric field,which weakens the modulation of the gate voltage on the central molecule. As a result, single-molecule devices with gold electrodes cannot meet practical demands in both the electrical performance and the device stability9.
Recently many advances have been made in the fabrication of graphene nano-gaps6,10. For example, both the feedbackcontrolled electro-burning method11–14and the dash-line lithographic plasma etching method15can efficiently fabricate graphene nano-gaps that are suitable for molecule connection,providing an opportunity for the study of charge transport in single-molecule FETs with graphene electrodes11,16–18. However,compared with systematic studies on anchoring groups used in molecular devices with gold electrodes, investigations on the anchoring groups connecting the central molecule to the graphene electrodes are still in the infancy. At present experimental studies on the anchoring groups for graphene electrodes are limited to two types6,10,19:π–πcoupling and the covalent amide bond. Theπ–πcoupling makes use of van der Waals interaction between the conjugated groups at both sides of the target molecule and the graphene electrodes, and the electronic coupling is usually very weak16,20. As a result, most of the devices constructed withπ–πcoupling display singleelectron tunneling transport properties, and the stability of the devices is also not good. Moreover, the atomic structures of the molecule-graphene interfaces formed byπ–πcoupling are different leading to large variations in the junction conductance.The amide bond is formed from the condensation of the carboxyl group at the edge of the graphene electrode and the amino group of the target molecule. The mechanical performance of this linkage is very good, which can facilitate the stability of singlemolecule devices operating at room temperature21–23. However,single-molecule FETs constructed with the amide groups mostly behave ap-type conduction, in which charge transport is dominated by the highest occupied molecular orbital (HOMO)of the central molecule14,18. It is very difficult to tune the conduction polarity of single-molecule FETs only by changing the structures of the central molecules2.
In order to meet the requirement for applications of singlemolecule FETs in future CMOS integration and enrich the types of anchoring groups for graphene electrodes, it is highly desirable to explore an experimentally feasible anchoring group suitable for constructingn-type single-molecule FETs with graphene electrodes24,25. Recently we have noticed several reports on modifying glassy carbon electrodesviacovalent pyrazine bonding in electrochemical catalysis experiments26–29.Surendranath and coworkers26,27performed surface modification of glassy carbon electrodes and high surface area graphitic carbon (Monarch 1300)usingortho-phenylenediamine terminated molecules; through condensation of the target molecules with theortho-quinone groups at the edges of graphene on the glassy carbon electrodes the pyrazine conjugation was formed. They find the obtained pyrazine conjugation has the following characteristics: (1)the condensation reaction only occurs at the edges of graphene. (2)the reaction condition is very mild, under which derivative reactions involving other groups such as carboxyl groups can be ignored; and at the same time this has little impact on the original structures of graphene electrodes. (3)covalent pyrazine bonding brings good stability and electronic coupling between the target molecule and the graphene electrodes. All these characteristics imply that pyrazine may be an ideal anchoring group for singlemolecule devices with graphene electrodes. Previous experimental results14show that the edges of graphene electrodes prepared by the feedback-controlled electro-burning method are in full oxidation state, so we speculate that there may exist manyortho-quinone moieties at the edges of graphene electrodes. If we choose a molecule withorthophenylenediamine terminals at both ends, it is reasonable to expect that theortho-phenylenediamine terminals will condensate with theortho-quinone groups at the edges of the graphene electrodes forming molecular junctions with pyrazine as the linkers.
In order to test the feasibility of pyrazine as an anchoring group for single-molecule devices with graphene electrodes, we synthesized phenazine molecules withortho-phenylenediamine terminals at both ends, and deposited them onto graphene nanogaps fabricated with the feedback-controlled electro-burning method. Large current increase was observed, indicating that molecular junctions were formed with covalent pyrazine anchoring groups. Then we investigated the electrical properties of these single-molecule FETs with the silicon back-gate, and found that the conduction polarity of these single-molecule FETs can be eitherp-type orn-type depending on the coupling strength of pyrazines.
2 Experimental
2.1 Device fabrication and molecule connection
Graphene nano-gaps were fabricated by the fast-speed feedback-controlled electro-burning method14described in the Supplementary Materials. After the fabrication process of electrodes (see Figs. S1–S3 in Supporting Information (SI)), the freshly prepared graphene nano-gaps were immersed in a N2-saturated ethanol solution (99.7%, MOS grade)with 0.2 mmol·L−1phenazine-2,3,7,8-tetraamine (> 95%). The reaction vessel was subsequently heated under N2for 10 h at 60 °C. Upon cooling, devices were washed with copious amounts of pure ethanol and subsequently treated with 0.1 mol·L−1HClO4 (70%,99.999% trace metals basis)for 1 h to hydrolyze adventitious imide linkages that might be formed in derivative reactions.Following this acid treatment, devices were rinsed with water and then soaked in CH3CN (ACS grade, Aldrich)for 1 h to remove physisorbed organic species. Following the CH3CN soak, devices were rinsed with water and then dried with N2flow.
2.2 Electrical characterization
Devices were measured in a cryogenic vacuum probe station(LakeShore TTP4). The feedback-controlled electro-burning process was performed using a data acquisition board (National Instruments, PCI 6281)together with a home-made LabVIEW program. A low-noise current amplifier (Ithaco 1211)was used to convert current to voltage. The preliminary electrical characterization was performed by using a Keithley 4200 semiconductor parameter system.
3 Results and discussion
Specifically, ortho-phenylenediamine terminated phenazine derivative (phenazine-2,3,7,8-tetraamine)was synthesized in a one-step reaction starting with 1,2,4,5-tetraamino-benzene30,31,following the synthetic route shown in Scheme S1 (SI). After graphene nano-gaps were fabricated using the feedbackcontrolled electro-burning method described in detail elsewhere,samples with graphene nano-gaps were soaked in an ethanol solution with phenazine molecules at 60 °C for 10 h. The condensation reaction of the phenazine molecules at the oxidized edges of graphene electrodes was shown in Scheme 1. Then, the devices were rinsed with copious amounts of pure ethanol, water,and 0.1 mol∙L−1HClO4. It should be noted that the skeleton of the phenazine molecule is composed of three conjugated sixmembered rings, however after connection, the central molecule in the junction can be regarded as one dipyrazine-phenazine(dipyrazino [2,3-b:2’,3’-i] phenazine, DPP)molecule which is composed of five six-membered rings. Therefore, in a certain extent the pyrazine linkage enhances the conjugation of the central molecule.
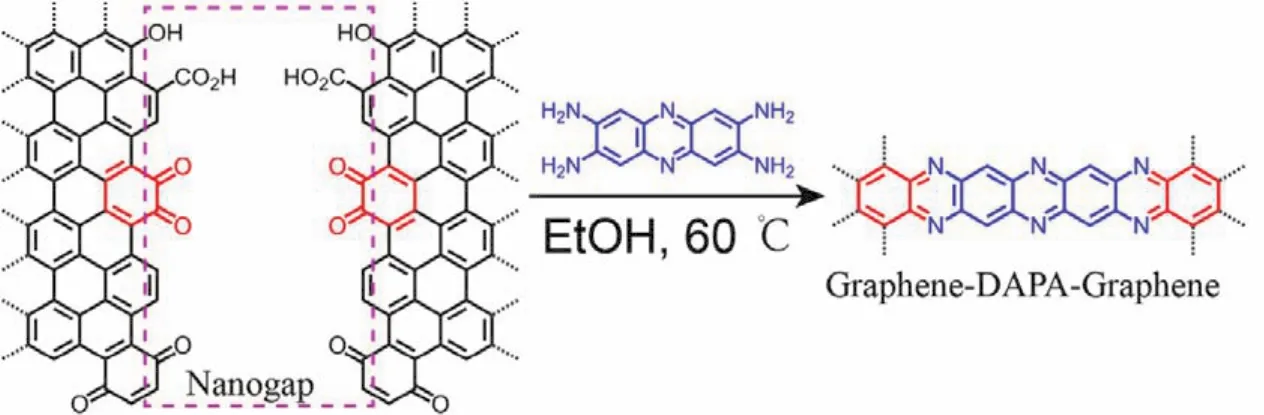
Scheme 1 Condensation of ortho-phenylenediamine terminated phenazine molecules with ortho-quinone edge sites of graphene nano-gaps, leading to the formation of graphene-DPP-graphene single-molecule junctions with covalent pyrazine contacts.
The schematic of DPP single-molecule FETs is shown in Fig.1a, the heavily doped silicon substrate with 300 nm SiO2serves as the back-gate electrode. One phenazine molecule bridges the graphene nano-gap forming a graphene-DPP-graphene singlemolecule junction with pyrazine connections. In our experiments, the formation of molecular junctions was confirmed by the direct comparison of the current–voltage (I–V)curves before and after molecule connection. A distinct current increase may indicate that one or a few molecules have interlinked the graphene nano-gap. Fig. 1b shows such a comparison of a representative device before (blue line)and after(black line)phenazine molecule connection. We fabricated 140 graphene nano-gaps, out of which 37 devices showed distinct current increase after molecule deposition, so that the yield of molecular junctions is up to 26%. And 10 devices showed an obvious gating effect when a back-gate voltage was applied.These devices showed good stability at room temperature. The inset in Fig. 1b is the scanning electron microscopy (SEM)image of a representative device in the nano-gap area, the graphene nano-gap is located between the source and drain Au electrodes. We then extracted the low-bias conductance from the least square linear fitting to theI–Vcharacteristics in the lowbias range from −0.1 to 0.1 V. The low-bias conductance histogram of these 37 devices is shown in Fig. 1c, which can be fitted with a Gaussian distribution. The most probable conductance value is determined to be (4.5 ± 1.5)× 10−5G0from the Gaussian fitting (the blue line in Fig. 1(c)), whereG0= 2e2/his the quantum of conductance.

Fig. 1 Structure and characterization of DPP single-molecule FET with pyrazine connections.
We investigated the electrical properties of these singlemolecule FETs by characterizing the dependence of the conductance on the back-gate voltage. In order to reduce the influence of the thermal noise, we performed the gate modulation measurements at 77 K. Interestingly, the ten fabricated devices exhibit three different gate modulation behaviors including thep-type, ambipolar andn-type conductions. Taking typical devices as examples, we discuss and analyze these conducting behaviors one by one.
Firstly, six of these 10 devices exhibitp-type conduction, of which the conductance always decreases when the gate voltage is varied from negative to positive values. Therefore, it is the HOMO of the central molecule that dominates the charge transport in these devices. The electrical characterization of a representativep-type Device A is shown in Fig. 2. Note that the Dirac point of the graphene electrodes is close toVg= 0, as confirmed by the electrical characterization (Supplementary Fig.S2 (SI)). So we use a modified single-level model32to fit theI–Vcharacteristics of these devices. In this model the linear density of states (DOS)of graphene near the Dirac point is included in order to take into account the influence of the intrinsic graphene electrodes. Fig. 2a shows the current–voltage characteristics of Device A before (blue solid line)and after (black dotted line)phenazine molecule connection at 77 K. The red dashed line is fitted to the modified single-level model. The HOMO is determined to beε0 = −0.56 eV, and the coupling strengths to the source and drain electrodes areΓS= 1.24 meV andΓD= 1.05 meV. We also turn to the transition voltage spectroscopy (TVS)analysis of Device A, its Fowler-Nordheim (F-N)plot is shown in Fig. 2b. The transition voltages at positive and negative polarities are +0.21 and −0.23 V, respectively. The energy values of the transition voltages are much less than the energy separation (0.56 eV)of the HOMO from the Fermi level, in good agreement with previous studies of single-molecule FETs with graphene electrodes32.

Fig. 2 DPP single-molecule FETs with p-type conduction.
Fig. 2c shows the current–voltage curves of Device A measured at 77 K for different gate voltages. The electric current is regularly modulated by the gate voltages. The corresponding transfer curve measured at the fixed bias voltageVds = 0.4 V is shown in Fig. 2d, the current decreases monotonically as the gate voltage sweeps from negative to positive, and the current can be modulated by over one order of magnitude. The electrical characteristics of other 5 devices withp-type conduction are shown in Fig. S4 (SI). Similar HOMO-dominated electronic transport properties are observed.
Then, two devices exhibit ambipolar conduction. For a representative Device B, the electrical characterization is shown in Fig. 3. The current–voltage curves of Device B for different gate voltages are given in Fig. 3c, and the corresponding transfer curve measured at the fixed bias voltageVds= 0.4 V is shown in Fig. 3d. The lowest conductance is located near the zero gate voltage, and the conductance keeps increasing at both positive and negative gate voltage polarities. Note that the current increase with the gate voltage is faster at the negative polarity than that at the positive one, so we infer that charge transport is also dominated by the HOMO. Besides this the ambipolar conduction is also related with the gating effects of the back-gate voltage on the graphene electrodes, as previously discussed elsewhere32. The current–voltage characteristics before (blue solid line)and after (black dotted line)phenazine molecule connection, and the fitting to the modified single-level model(the red dashed line)are shown in Fig. 3a. The HOMO is determined to beε0= −0.56 eV, and the coupling strengths to the source and drain electrodes areΓS= 1.34 meV andΓD= 1.32 meV. From the TVS analysis shown in Fig. 3b, the transition voltages at positive and negative polarities are respectively determined to +0.16 and −0.16 V. These values are close to those of Device A, indicating it is reasonable to conclude that the charge transport in Device B is also dominated by the HOMO.
The other device with ambipolar conduction, Device C,exhibits a behavior near the critical condition for the gating effect. The electrical characterization of Device C is shown in Fig. S5 (SI). In the negative gate voltage regime, the electric current exhibits a monotonicp-type conduction; however, at the positive gate voltage polarity the electric current behaves almost independently on the gate voltages. This is because the gate modulation of the graphene electrodes and that of the HOMO cancel each other at the positive gate polarity but still enhance each other at the negative polarity. Known from the single-level model fitting and the TVS analysis, the determined energy position of the HOMO and its coupling strengths with the source and drain electrodes are in good agreement with those of other HOMO-dominated devices.

Fig. 3 DPP single-molecule FETs with ambipolar conduction.
Finally, we observen-type conduction in the last two devices in which the lowest unoccupied molecular orbital (LUMO)of the central molecule dominates their charge transport. Fig. 4 presents the electrical characterization of a representative Device D. The current–voltage characteristics of Device D before (blue solid line)and after (black dotted line)phenazine molecule connection and the modified single-level model fitting (red dashed line)are shown in Fig. 4a. The LUMO is determined to beε0= 1.0 eV, and its coupling strengths to the source and drain electrodes areΓS= 32 meV andΓD= 36 meV. The transition voltages at positive and negative polarities are +0.20 and −0.24 V, respectively (Fig. 4b). As can be seen from Fig. 4c, d, Device D exhibits an obviousn-type conduction and the conductance keeps increasing when the back-gate voltage is swept from −40 to +40 V. Note that the absolute values of the energy level (ε0)and its coupling strengths determined for Device D are significantly larger than those of the devices withp-type conduction discussed above. Similarn-type conduction was observed in Device E, as is shown in Fig. S6 (SI).

Fig. 4 DPP single-molecule FETs with n-type conduction.
So, how do we understand the experimental results that bothp-type andn-type conduction appear in these single-molecule FETs? One possible explanation is that these devices may not be formed only by pyrazine connections and there may exist amide connections that are formed by the condensation of the amino terminals of the target molecule and the carboxyl groups at the edges of the graphene electrodes. Previous studies have demonstrated that molecular devices with amide connections are mainly HOMO-dominatedp-type conduction, and that the coupling strengths are usually in the meV range14,18,32. This indicates that those devices withp-type conduction may be constructed with amide connections. However, the mild reaction condition used in our experiments cannot support the amide formation reaction, and the HClO4treatment process also makes the adventitious amide connections hydrolysis. Furthermore,characterization of N peaks by nitrogenK-edge X-ray absorption near edge structure (XANES)spectroscopy at the edge of high surface area graphitic carbon (Monarch 1300)did not show any amide or imidazole nitrides26,27. This directly proves that the amide connections can be neglected under these reaction conditions. As a result, the existence of a large amount of amide connections in our experiments are nearly impossible and it is unreasonable to attribute these devices withp-type conduction to the amide connections.
In Table 1 we list the energy position of the dominating molecular level, the coupling strengths and the transition voltages of representative devices. It can be seen that the greatest difference between these two conduction types is the coupling strength. The coupling strengths in the devices with LUMO-dominatedn-type conduction are about 10 times larger than those in the devices with HOMO-dominatedp-type conduction.The possible band diagrams of single-molecule FETs corresponding to the two conduction types caused by different coupling strengths are plotted in Fig. 5. The Fermi level of the gold electrodes is assumed to be located near the middle of the HOMO-LUMO gap of the DPP molecules. The pyrazine rings containing N atoms in the DPP molecule has a much stronger ability to attract electrons than that of the C-6 rings of the graphene electrodes, which has been fully demonstrated in the studies of single-molecule diodes33,34. When the coupling is weak (Fig. 5a), the energy barrier is high and charge transfer between the central molecule and the graphene electrodes is difficult. The HOMO level is closer to the Fermi level and the molecular junctions behave HOMO-dominated charge transport,leading top-type FETs. In contrast, following the increase of the coupling strength the energy barrier decreases and more electrons transfer to the central molecule from the graphene electrodes (Fig. 5b), which results in the downshift in energy of the molecular energy levels. As a result, the LUMO level is located closer to the Fermi level, and the molecule junctions mainly behave LUMO-dominated charge transport, leading tontype FETs. The difference of the coupling strength between these two types may arise from the different edge configurations of the graphene electrodes. Further studies are needed to clarify this important issue and improve the control of the device functionality.

Table 1 Statistics of the fitting parameters and the measured transition voltages.

Fig. 5 Band diagram of DPP single-molecule FETs with pyrazine connections.
4 Conclusions
Here we explore a new anchoring group to fabricate singlemolecule devices with graphene electrodes. We have demonstrated thatortho-phenylenediamine terminated molecules can be selectively ligated toortho-quinone groups at the edges of graphene nano-gaps forming graphene-moleculegraphene junctions with covalent pyrazine connections. The yield of molecular junctions is up to 26%, which suggests that pyrazine can be a robust anchoring group for molecular devices with graphene electrodes. As prepared, DPP single-molecule FETs show different transport properties depending on the coupling strength between the central molecule and the graphene electrodes. Specifically, the HOMO level dominates charge transport in weak coupling, bringing aboutp-type conduction; in strong coupling, the redistribution of electrons among the central molecule and the graphene electrodes leads to a realignment of the molecular levels, resulting in LUMO-dominatedn-type conduction. This suggests a new approach that the conduction polarity of molecular junctions can be regulated by adjusting the coupling strength. The high yield and the versatility of pyrazine connections are helpful for the construction of single-molecule devices with graphene electrodes.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
杂志排行
物理化学学报的其它文章
- Hollow Nitrogen-Rich Carbon Nanoworms with High Activity for Metal-Free Selective Aerobic Oxidation of Benzyl Alcohol
- Photocrosslinking-Immobilized Polymer Vesicles for Lowering Temperature Triggered Drug Release
- CO Hydrogenation to Ethanol over Supported Rh-Based Catalyst:Effect of the Support
- CdTeSe合金幻数团簇的室温合成和形成机理研究
- 体相界面导通的复合快离子导体
- 异氰酸苯酯诱导的类胶原多肽自组装