In-situ construction of amorphous/crystalline contact Bi2S3/Bi4O7 heterostructures for enhanced visible-light photocatalysis
2021-11-19FeihuMuBenlinDiWeiZhoXiofnYngXiolongZhoXujingGuo
Feihu Mu,Benlin Di ,Wei Zho,Xiofn Yng,Xiolong Zho,Xujing Guo*
a Jiangsu Key Laboratory for Chemistry of Low-Dimensional Materials, Jiangsu Collaborative Innovation Center of Regional Modern Agriculture and Environmental Protection, School of Chemistry and Chemical Engineering, Huaiyin Normal University, Huaian 223300, China
b Department of Mechanical Engineering, The University of Hong Kong, Hong Kong, China
c College of Resources and Environment, Chengdu University of Information Technology, Chengdu 610225, China
1 These authors contributed equally to this work.
ABSTRACT Constructing a heterojunction photocatalyst is a significant method to enhance photocatalytic activity because it can promote the separation of photogenerated carriers.Herein,amorphous/crystalline contact Bi2S3/Bi4O7 heterostructure was successfully synthesized by in-situ sulfidation of Bi4O7.The amorphous Bi2S3 is diffused on the surface of Bi4O7 rod, enhancing the visible light response and improving the transport of photogenerated carriers.Various characterizations confirm that the rapid separation of photogenerated carriers leads to increased photocatalytic performance.The optimized Bi2S3/Bi4O7 heterostructure photocatalyst (BiS-0.15) exhibits the highest Cr(VI) reduction(0.01350 min-1) and RhB oxidation(0.08011 min-1)activity,which is much higher than that of pure Bi4O7 and Bi2S3/Bi4O7 mixture under visible light irradiation.This work provides new insights into the construction of efficient novel photocatalysts.
Keywords:Photocatalysis Cr(VI) reduction Heterojunction Bismuth-based oxide Bismuth-based sulfide
Nowadays,the discharge of wastewater containing heavy metal ions(such as Cr(VI),Hg(II),and Pb(II))and organic pollutants(such as toxic dyes,phenol,and naphthalene)is causing more and more environmental problems [1,2].Therefore, it is particularly important to remove these pollutants from wastewater.In the past few years, photocatalytic technology has been increasingly used in wastewater treatment [3-8].However, many semiconductor photocatalysts with wide bandgap (Eg) only absorb and utilize the ultraviolet region, which contributes 3%-5% to the solar spectrum[9-11].To use sunlight more efficiently,it is required to develop narrow Eg photocatalysts with a visible light response,because the visible light region accounts for about 43%of sunlight[12].Therefore,various new photocatalysts that can make full use of solar energy have been designed and explored [13-16].
As a versatile photocatalyst, bismuth-based semiconductor materials, such as BiVO4, Bi2MoO6, BiOCl, BiOBr, BiOI, Bi2O2CO3,BiOIO3, and bismuth-based oxides, are widely used in photocatalytic processes [17-25].In particular, bismuth-based oxides(e.g.,α-Bi2O3,β-Bi2O3,γ-Bi2O3,δ-Bi2O3,Bi2O4,Bi2O2-x,Bi4O7,Bi6O7)have a strong visible light response, because the Bi-O system contains intermediate phases and the 6 s orbital of Bi3+can overlap the O 2p orbital [26-28].However, the recombination rate of carriers in these bismuth-based oxides is relatively fast, which hinders their photocatalytic activity.To solve this problem,constructing bismuth-based oxide heterojunction materials is an effective method [29-32].For example, Deng et al.synthesized BiOCl/Bi2O3/rGO heterojunction for photocatalytic treatment of real industrial wastewater.Due to the reasonable band gap match between BiOCl and Bi2O3, this Z-scheme BiOCl/Bi/Bi2O3/rGO heterojunction exhibited excellent photocatalytic performance[33].Hong et al.synthesized β-Bi2O3@g-C3N4heterojunction by self-assembly method.This Z-scheme heterojunction photocatalyst can significantly extend the lifetime of carriers and promote the separation of carriers.As a result,the sample containing 5 wt%g-C3N4(5% CN@BO) can efficiently degrade tetracycline (k =0.0311 min-1)[34].However,there are only a few studies on Bi4O7heterojunction [26,35-38], which need further exploration.
Herein,amorphous/crystalline contact Bi2S3/Bi4O7heterostructure was constructed by in-situ sulfidation of Bi4O7(Fig.1a).Briefly,1.20 g NaBiO3•2H2O was dispersed into 40 mL water and put into a 50 mL autoclave, which was then heated at 180°C for 6 h.The resulting powders were collected, and taken into a crucible and heated at 250 ℃for 2 h with a heating rate of 2°C/min.After cooling,the Bi4O7product was prepared.Then,0.1 mmol Bi4O7was dispersed into 25 mL water and sonicated for 15 min.Subsequently, 10 mL thioacetamide solution (0.010 mmol) was added dropwise and reacted at 50°C for 5 h with stirring.Finally,the resulting Bi2S3/Bi4O7product was collected by centrifugation and labeled as BiS-0.10.In the case of other conditions unchanged,change the amount of thioacetamide solution to 15 mL(0.015 mmol) and 20 mL (0.020 mmol), the resulting products were labeled as BiS-0.15 and BiS-0.20, respectively.In this heterostructure, the amorphous Bi2S3boost the visible light response of Bi4O7and accelerate the photogenerated carrier transport.Under visible light irradiation, the amorphous/crystalline contact Bi2S3/Bi4O7exhibits excellent photocatalytic Cr(VI)reduction and rhodamine B (RhB) oxidation activity.
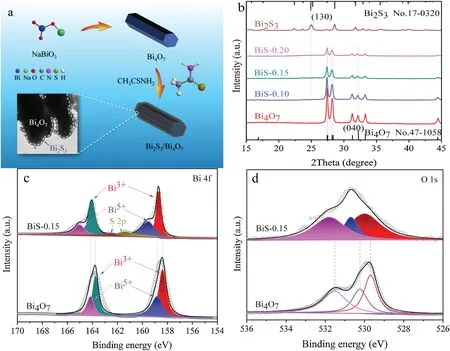
Fig.1.(a) Schematic diagram of Bi2S3/Bi4O7 preparation.(b) XRD patterns of different photocatalysts.XPS spectra of Bi4O7 and BiS-0.15: (c) Bi 4f, (d) O 1s.
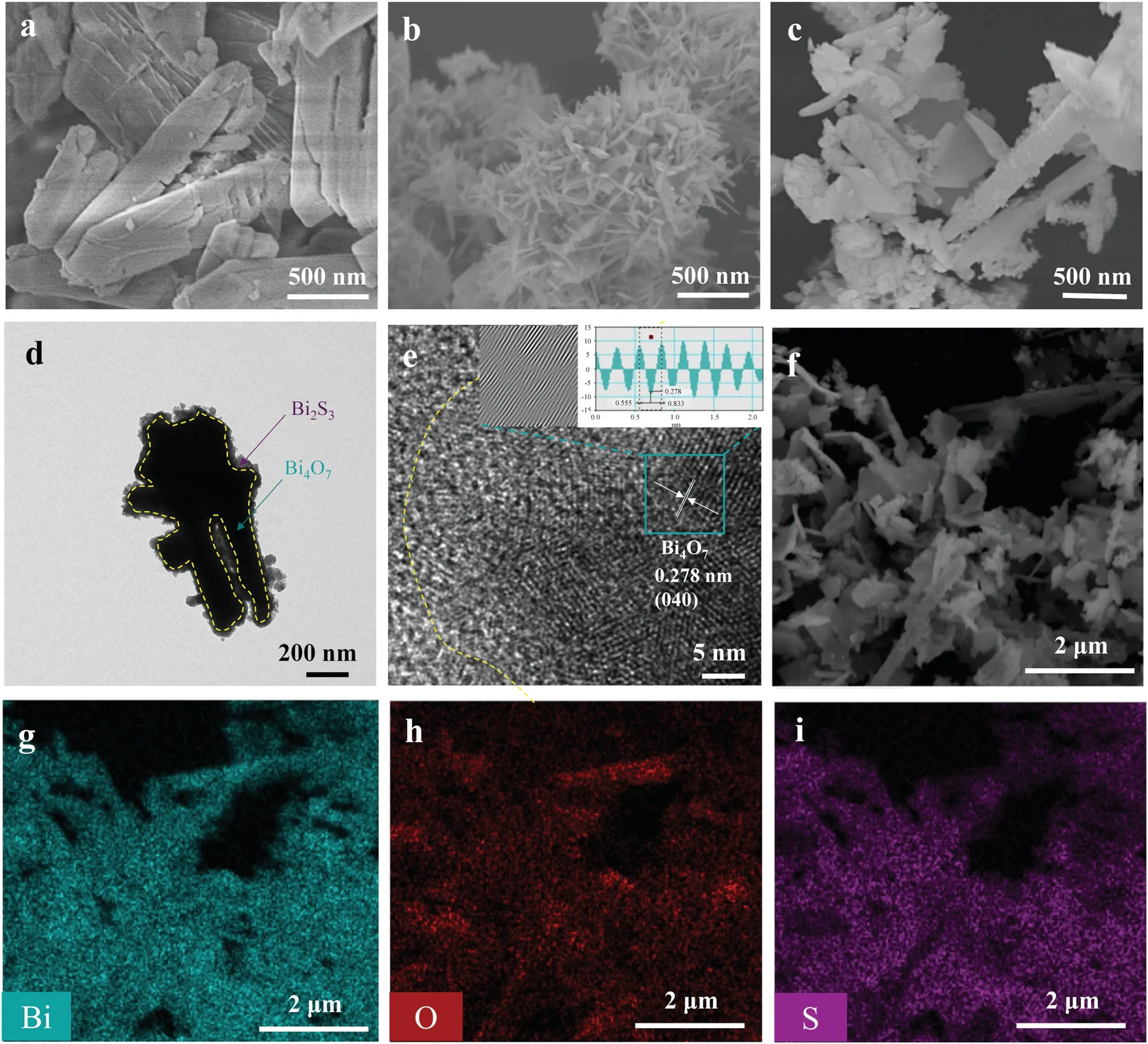
Fig.2.FESEM images of(a)Bi4O7,(b)Bi2S3,(c)BiS-0.15,TEM(d)and HRTEM(e)images of BiS-0.15,(f)FESEM images of BiS-0.15 and SEM-EDS elemental mapping of(g)Bi element, (h) O element, and (i) S element.
The crystal structures of different photocatalysts were studied by XRD.In Fig.1b,Bi4O7exhibits five characteristic XRD diffraction peaks at 27.4°,27.5°,28.1°,28.3°and 32.2°,which can be indexed to(22),(2),(22),(222)and(040)crystal planes of triclinic Bi4O7phase(JCPDS No.47-1058) [26,39], respectively.For Bi2S3, all peaks correspond to the orthorhombic Bi2S3phase (JCPDS No.17-0320)[40-42].In particular, the XRD patterns of the BiS-0.10, BiS-0.15,and BiS-0.20 composites show no characteristic peaks of Bi2S3,because Bi2S3is amorphous in these composites.Moreover, with the increase of Bi2S3content, the relative intensity of the Bi4O7diffraction peak in Bi2S3/Bi4O7composite decreases, and the relative intensity of the Bi4O7diffraction peak in BiS-0.20 is the lowest.
The surface chemical state and composition of different photocatalysts were further detected by XPS.The survey spectra(Fig.S1 in Support information) indicate that only Bi and O elements are present in Bi4O7,while BiS-0.15 is composed of Bi,O,and S elements.In Fig.1c (Bi 4f spectra),both Bi 4f5/2and Bi 4f7/2can be divided into double peaks.For Bi4O7,the peaks at 158.3 and 163.7 eV are assigned to Bi3+,while the peaks at 158.8 and 164.2 eV are ascribed to Bi5+[36,43].However, the characteristic peaks of Bi3+are 158.7 and 164.1 eV, and the peaks of Bi5+are 159.5 and 164.9 eV over BiS-0.15.Noticeably,the peaks at 161.3 and 162.5 eV in BiS-0.15 are attributed to S 2p [44,45], which confirms the presence of Bi2S3in BiS-0.15.In Fig.1d,the O 1s peak of Bi4O7can be divided into three peaks, located at 529.7, 530.4, and 531.5 eV,respectively.The XPS peak at 529.7 eV belongs to lattice oxygen,while the peaks at 530.4 and 531.5 eV are ascribed to chemically adsorbed oxygen and physically adsorbed oxygen, respectively[26,46].However,these O 1s peaks are located at 529.9,530.6,and 531.8 eV in BiS-0.15,respectively.Therefore,compared with Bi4O7,the peaks of Bi 4f and O 1s in BiS-0.15 are shifted to high values,which demonstrates that there is a strong interaction between Bi4O7and Bi2S3.
The morphologies of Bi4O7,Bi2S3,and BiS-0.15 were explored by field emission scanning electron microscopy (FESEM).Bi4O7exhibits a rod-like morphology (Fig.2a), while Bi2S3displays a needle-like morphology(Fig.2b).Fig.2c shows the typical FESEM image for BiS-0.15.Compared with the smooth surface of pure Bi4O7, the surface of BiS-0.15 becomes very rough, further indicating that Bi2S3is formed on the surface of Bi4O7after reaction with thioacetamide.Moreover,the microstructure of BiS-0.15 was further researched by TEM.In Fig.2d,a layer of Bi2S3is insitu formed on the surface of rod-like Bi4O7.In the HRTEM image of BiS-0.15 (Fig.2e), the fringe with a lattice spacing of 0.278 nm is characteristic to the(040)crystal plane of Bi4O7.This HRTEM image also confirms that Bi2S3is amorphous,which is in accordance with the XRD pattern.Additionally, in the scanning electron microscopy-energy dispersive X-ray spectrometry (SEM-EDS) elemental mapping(Figs.2f-i),the Bi,O,and S elements in BiS-0.15 are evenly dispersed,demonstrating that Bi2S3is uniformly dispersed on the surface of Bi4O7.
The surface area of the obtained samples was evaluated by N2adsorption-desorption.In Fig.3a, all the samples exhibit type IV isotherms.The BET surface areas of Bi4O7, BiS-0.10, BiS-0.15, and BiS-0.20 are 2.94,7.95,8.76,and 9.32 m2/g,respectively.Therefore,the surface area of the Bi2S3/Bi4O7heterojunction is greater than that of Bi4O7, which is beneficial to the photocatalytic reaction.Moreover, the optical characteristic of as-prepared samples was studied by UV-vis diffuse reflectance spectroscopy(DRS)(Fig.3b).In the visible light region,Bi4O7has strong light absorption,and its absorption edge is estimated to be about 700 nm, while Bi2S3has stronger light absorption than that of Bi4O7.The light absorption properties of BiS-0.10,BiS-0.15,and BiS-0.20 are between Bi4O7and Bi2S3.Besides, the colors of Bi4O7and Bi2S3are brownish-red and black (Fig.3b, inset), respectively.With the increase of Bi2S3content,the color of Bi2S3/Bi4O7heterojunction gradually becomes darker.According to the curve of Fig.3c((αhv)2versus energy),the band gaps (Eg) of Bi4O7and Bi2S3are estimated to be 1.82 and 1.35 eV, respectively.
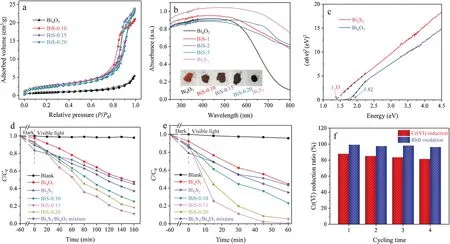
Fig.3.(a)Nitrogen adsorption-desorption isotherms,(b)UV-vis DRS(the inset is the digital pictures),(c)the curve of(αhv)2 against energy(hv),the result of photocatalytic(d) Cr(VI) reduction and (e) RhB oxidation, (f) four recycling runs of BiS-0.15 for Cr(VI) reduction and RhB oxidation.
The photocatalytic performance of Bi4O7, Bi2S3, BiS-0.10, BiS-0.15, BiS-0.20, and Bi2S3/Bi4O7physical mixture was studied by Cr(VI) reduction and RhB oxidation in water.To confirm the important roles of photocatalyst, blank experiments (in the absence of photocatalysts, self-degradation) were also performed under identical conditions.From Fig.3d,it can be observed that the self-degradation process of Cr(VI) can be neglected.Meanwhile,the Cr(VI) reduction rates of pristine Bi4O7and pristine Bi2S3are 51.3%and 45.8%,respectively.After the incorporation of Bi2S3,the degradation activity of BiS-0.10 increases significantly to 72.5%.Further increasing the amount of Bi2S3, the Cr(VI) reduction rates of BiS-0.15 and BIS-0.20 are 87.9% and 79.7%, respectively.In particular,BiS-0.15 exhibits the highest activity of Cr(VI)reduction.Noticeably, the photocatalytic activity of the Bi2S3/Bi4O7heterojunction is much higher than that of the Bi2S3/Bi4O7physical mixture (58.4%).These experimental results demonstrate that loading Bi2S3on the surface of Bi4O7can promote Cr(VI)reduction,and the optimal dosage of Bi2S3is 15%.In addition, this Cr(VI)reduction results conform to the pseudo-first-order dynamics model: ln(C0/Ct) = kappt [47].In Fig.S2 (Supporting information),the apparent reaction rate constants(kapp)of Bi4O7,Bi2S3,BiS-0.10,BiS-0.15, BiS-0.20 and Bi2S3/Bi4O7mixture are 0.00467, 0.00396,0.00819, 0.01350, 0.01023 and 0.00550 min-1, respectively.
In Fig.3e,the self-degradation rate of RhB was only 4.3%,while the RhB oxidation rates of pristine Bi4O7and pristine Bi2S3are 51.8%and 45.5%,respectively.After the incorporation of Bi2S3,the RhB oxidation rate of BiS-0.10 increases significantly to 73.7%.In particular, BiS-0.15 exhibits the highest activity of photocatalytic RhB oxidation(99.2%).Further increasing the amount of Bi2S3,the oxidation rate of BIS-0.20 is reduced to 93.7%, which is similar to the result of Cr(VI) reduction.Noticeably, the photocatalytic RhB oxidation activity of the Bi2S3/Bi4O7heterojunction is much higher than that of the Bi2S3/Bi4O7physical mixture (59.1%).Moreover,these RhB oxidation results also conform to the pseudo-first-order dynamics model.In Fig.S3(Supporting information),the apparent reaction rate constants kappof Bi4O7, Bi2S3, BiS-0.10, BiS-0.15, BiS-0.20 and Bi2S3/Bi4O7mixture are 0.01154, 0.01009, 0.02124,0.08011, 0.04768 and 0.01531 min-1, respectively.
In addition, the stability of the photocatalytic reaction on BiS-0.15 was evaluated.As shown in Fig.3f, after four cycles, the photocatalytic Cr(VI) reduction rate of BiS-0.15 is 81.4%, and the rate is 92.6%of the initial rate.This indicates that BiS-0.15 has good stability in photocatalytic Cr(VI)reduction.Moreover,BiS-0.15 also shows high stability in photocatalytic RhB oxidation since the oxidation rate is still up to 96.3%after four cycles.Besides,the XRD pattern (Fig.S4 in Supporting information) of BiS-0.15 shows no significant difference, demonstrating that the BiS-0.15 photocatalyst is structurally stable after photocatalytic cycles.
The recombination rate of photogenerated carriers is an important parameter that affects the photocatalytic performance[48].The PL spectra can provide information about carrier recombination.Fig.4a exhibits the PL spectra of Bi4O7, BiS-0.10,BiS-0.15, and BiS-0.20 excited at 360 nm.The PL intensity of BiS-0.10, BiS-0.15, and BiS-0.20 is lower than that of Bi4O7, and BiS-0.15 has the lowest PL intensity.Therefore, the photogenerated carriers rapidly recombine over Bi4O7, while the photogenerated carriers over the Bi2S3/Bi4O7heterostructures are effectively separated due to interfacial charge transfer.
In addition, photoelectrochemical techniques were employed to further study the interface charge separation of photocatalysts.As illustrated in Fig.4b (transient photocurrent responses), the photocurrent density of Bi4O7is low because of the rapid recombination of photogenerated carriers.The photocurrent intensity over BiS-0.15 is much higher than that over Bi4O7, BiS-0.10, and BiS-0.20, which indicates that the photogenerated carriers over BiS-0.15 can be separated rapidly.Fig.4c describes the electrochemical impedance spectroscopy (EIS) Nyquist plots for Bi4O7,BiS-0.10,BiS-0.15,and BiS-0.20.As an observation,the arc radius of BiS-0.15 is smaller than that of Bi4O7, BiS-0.10, and BiS-0.20,demonstrating that BiS-0.15 possesses a lower charge carrier transfer resistance.Noticeably, these EIS characterization results are consistent with the PL and transient photocurrent responses characterization results.In addition,these characterization results also agree with the results of photocatalytic Cr(VI) reduction experiments.
Generally, reactive species are very important in the photocatalytic reaction process and directly determine the reaction mechanism.To study the reaction species of RhB oxidation, some trapping agents were added during the BiS-0.15 photocatalytic oxidation of RhB.Ammonium oxalate (AO), Benzoquinone (BQ),and isopropanol (IPA) were employed to trap h+, O2•-, and•OH,respectively,and the results are shown in Fig.4d.After the trapping agent is added,the photocatalytic oxidation is partially suppressed,and the photocatalytic oxidation efficiency value is reduced.The lower the photocatalytic oxidation efficiency value caused by the trapping agent,the more important the role of the corresponding reactive species in the photocatalytic oxidation.From Fig.4d,it is observed that with the addition of AO and BQ, the photocatalytic oxidation efficiency value of RhB is significantly decreased,indicating that h+and O2•-are the main reactive species.However,IPA has almost no effect on the photocatalytic oxidation efficiency value, demonstrating that•OH is not the main reactive species.

Fig.4.(a)PL spectra,(b)transient photocurrent responses,(c)EIS Nyquist plots of Bi4O7,BiS-0.10,BiS-0.15,and BiS-0.20,(d)trapping experiments,(e)Mott-Schottky plots,(f)the proposed photocatalytic mechanism.
Furthermore, the band structures of Bi4O7and Bi2S3were measured by Mott-Schottky plots.In Fig.4e, the flat band potentials (EFB) of Bi4O7and Bi2S3are 0.77 and -0.55 V vs.Ag/AgCl,that is,0.97 and-0.35 V vs.NHE,respectively.In general,the EFBof an n-type semiconductor is close to the conduction band potential(ECB)[49],so the conduction band potentials of Bi4O7and Bi2S3are estimated to be 0.97 and -0.35 V vs.NHE, respectively.Furthermore, the valence band potentials (EVB) of Bi4O7and Bi2S3can be calculated to be 2.79 and 1.00 V vs.NHE, respectively,because the Eg of Bi4O7and Bi2S3is 1.82 and 1.35 eV (Fig.3c),respectively.
If the electrons are transported from the CB of Bi2S3to the CB of Bi4O7,while the holes are transported from the VB of Bi4O7to the VB of Bi2S3.Since the ECBof Bi4O7(0.97 V vs.NHE)is more positive than the potential of O2/O2•-(-0.33 V vs.NHE), the CB of Bi4O7cannot generate O2•-radicals, conflicting the above reactive species trapping experiments.Therefore, it is concluded that electrons are transferred from the CB of Bi4O7to the VB of Bi2S3.Based on the above analysis,a probable photocatalytic mechanism is proposed(Fig.4f).Under visible light irradiation,both Bi2S3and Bi4O7are excited,thereby generating photogenerated electrons in their CB and photogenerated holes in their VB.The electrons on the CB of Bi4O7tend to be transmitted to the VB of Bi2S3.The electrons in the CB of Bi2S3react with O2to form O2•-,while the holes in the VB of the Bi4O7react with the H2O to produce the•OH.Then, the formed O2•-,•OH,e-,and h+participate in the photocatalytic Cr(VI)reduction and RhB oxidation.
In summary,amorphous/crystalline contact Bi2S3/Bi4O7photocatalyst was successfully constructed by the in-situ reaction of thioacetamide with Bi4O7.Compared with pure Bi4O7and Bi2S3/Bi4O7mixture, the amorphous/crystalline contact Bi2S3/Bi4O7heterostructure shows superior photocatalytic Cr(VI) reduction and RhB oxidation activity.In particular, BiS-0.15 exhibits the highest photocatalytic activity,and the kappof Cr(VI)reduction and RhB oxidation are 0.01350 and 0.08011 min-1, respectively.The boosted photocatalytic activity is attributed to the effective separation of photogenerated carriers.The photocatalytic process is further supported by PL spectra, transient photocurrent responses, and EIS experimental results.This work is expected to provide valuable insights into the preparation of effective novel photocatalysts.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.51808250, 51676082), the Natural Science Foundation of Jiangsu Province of China(Nos.BK20160430,BK20181070), the Jiangsu Planned Projects for Postdoctoral Research Funds of China (No.1601179C), the Project Funded by China Postdoctoral Science Foundation (Nos.2016M591757,2017M610336), and Jiangsu Key Research and Development(R&D) Projects (Social Development, No.BE2020772).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the on line version, at doi:https://doi.org/10.1016/j.cclet.2020.12.016.
杂志排行
Chinese Chemical Letters的其它文章
- Challenges in cell membrane-camouflaged drug delivery systems:Development strategies and future prospects
- Visible and near-infrared light activated azo dyes
- Development of bioorthogonal SERS imaging probe in biological and biomedical applications
- A H2S-triggered two-photon ratiometric fluorescent theranostic prodrug for bio-imaging
- Light-up lipid droplets for the visualization of lipophagy and atherosclerosis by coumarin-derived bioprobe
- The density of surface ligands regulates the luminescence of thiolated gold nanoclusters and their metal ion response
