Mn2+-doped ZrO2@PDA nanocomposite for multimodal imaging-guided chemo-photothermal combination therapy
2021-11-19NingChenWenhuiFuJieZhouLinqingMeiJiminYngYngTinQingWngWenynYin
Ning Chen,Wenhui Fu,Jie Zhou,Linqing Mei,Jimin Yng,Yng Tin,Qing Wng,*,Wenyn Yin*
a Laboratory for Micro-sized Functional Materials, Department of Chemistry and College of Elementary Education, Capital Normal University, Beijing 100048,China
b CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, CAS-HKU Joint Laboratory of Metallomics on Health and Environment, Beijing Metallomics Facility, National Consortium for Excellence in Metallomics, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China
c School of Chemistry and Chemical Engineering, Linyi University, Linyi 276005, China
d Laboratory of Nano-Bio Interface, Suzhou Institute of NanoTech and Nano-Bionics, Chinese Academy of Sciences, Suzhou 215123, China
1These authors contributed equally to this work.
ABSTRACT Developing low toxicity and multifunctional theranostic nanoplatform is the key for precise cancer diagnosis and treatment.Herein,an inorganic-organic hybrid nanocomposite is designed by modifying zirconium dioxide (ZrO2) with polydopamine (PDA) followed by doping Mn2+ ions and functionalizing with Tween 20 (Tween-ZrO2@PDA-Mn2+) for multimodal imaging and chemo-photothermal combination therapy.The as-prepared nanocomposite exhibits good biocompatibility in vitro and in vivo.Specifically, it can be employed as a multifunctional platform not only for computed tomography (CT)imaging and T1-weighted magnetic resonance (MR) imaging, but also for efficient chemotherapeutic drug doxorubicin hydrochloride (DOX) loading.Importantly, because of the pronounced photothermal conversion performance and controllable DOX release ability triggered by the near-infrared (NIR)irradiation and acidic pH,the synergistic effect between photothermal therapy and chemotherapy results in an enhanced cancer treatment efficacy in vivo.Our work provides a high-performance inorganicorganic hybrid nanotheranostic platform for chemo-photothermal cancer therapy guided by CT and MR imaging.
Keywords:ZrO2 nanoparticles Polydopamine Multimodal imaging Chemo-photothermal therapy
As one of the most traditional therapeutic treatment of cancer,chemotherapy is widely used, while the severe side effects to normal tissues greatly limit its further application [1-4].To overcome the defect, photothermal therapy (PTT) has recently attracted widespread attention owing to its minimal invasiveness and high specific spatial-temporal selectivity, which utilizes the heat energy generated by photoabsorbing nanoagents under nearinfrared (NIR) laser radiation to kill cancer cells [5-7].However,because of the limited heat energy distribution and depthdependent decline of laser intensity, PTT alone may not ensure a satisfactory therapeutic outcome [8,9].A combined PTT and chemotherapy treatment is herein developed as a promising strategy to couple the advantages of each therapy and work cooperatively for effective supression of cancer growth [10,11].
Over the past decades, many inorganic nanomaterials have been cultivated as photothermal nanoagents or chemotherapeutic drug nanocarriers [12-15].Nevertheless, the long-term safety of some inorganic nanoagents without proper surface modification is undesirable,which may exert adverse effect on living body,further limiliting their application towards the clinical target [16-18].To solve this issue,considerable effort has been devoted to modify the inorganic nanoparticles (NPs) using biocompatible organic polymers [19,20].Although amphiphilic polymer are widely used for reducing the toxicity of some inorganic nanoagents, such whole process is performed in organic solvents, which may lead to potential risk[6,21,22].Therefore,it is worthwhile to choose a kind of polymer with a green modification procedure.Recently,dopamine (DA) has gained much attention due to its good biocompatibility and self-polymerization property in water solution under weakly alkaline pH value [23,24].Moreover, the generated polydopamine(PDA)has a strong absorbance capability in NIR region (650-950 nm) and high photothermal conversion efficiency [23,25,26], which has been verified as an efficient PTT nanoagent.PDA can also serve as a multifunctional platform by grasping many functional moieties such as drugs,photosensitizers,contrast agents because of their inner π-conjugated structure and strong hydrogen bonding [27,28].Therefore, developing PDAcoated inorganic-organic hybrid nanomaterial is highly worth considering.Zirconium dioxide nanoparticles (ZrO2NPs),employed as bone tissue engineering material,are widely reported in anticancer drugs nanocarrier and microwave thermal therapy field because of the superior biocompatibility and enhanced computed tomography (CT) imaging efficiency [29-32].Taken together, if ZrO2NPs were modified by PDA layer, the formed nanocomposite could demonstrate the following advantages: (i)Facile integration of ZrO2and PDA could produce a highly biocompatible nanosystem without involvement of any heavy metal components and harsh organic solvents.(ii) The nanocomposite could act as a multifunctional platform not only as CT imaging contrast agent and PTT nanoagent,but also as nanocarrier for loading chemotherapeutic drugs and other contrast agents to achieve a precise imaging guided chemo-photothermal combination cancer treatment efficacy.
Herein, we fabricate an inorganic-organic hybrid nanocomposite by modifying ZrO2NPs with PDA to form ZrO2@PDA NPs through a green and facile route.As illustrated in Fig.1, the ZrO2NPs was firstly synthesized via a simple hydrothermal method[33].After coating with PDA, the color of ZrO2solution changed from white to dark black, which implies that ZrO2NPs has been coated by PDA(Fig.S1 in Supporting information).The ZrO2@PDA NPs exhibits good photothermal conversion efficiency under 808 nm laser irradiation and effective CT imaging performance.Furthermore,Mn2+ions were directly chelated by the coordination effect of catechol or carboxyl groups in PDA for manganeseenhanced T1-weighted magnetic resonance(MR)imaging,and the amphiphilic nontoxic surfactant Tween 20 was applied to functionalize the nanocomposite for achieving good dispersibility and stability.In addition,after loading antitumor drug doxorubicin hydrochloride(DOX)to obtain Tween-ZrO2@PDA-Mn2+/DOX nanoplatform, the DOX can effectively release from the nanoplatform under multiple stimuli including NIR irradiation and acidic pH value of tumor microenvironment, presenting remarkable synergistic chemo-photothermal therapy both in vitro and in vivo.We believe that the Tween-ZrO2@PDA-Mn2+nanocomposite will provide a powerful nanotheranostic platform for multimodality imaging-guided chemo-photothermal cancer therapy.
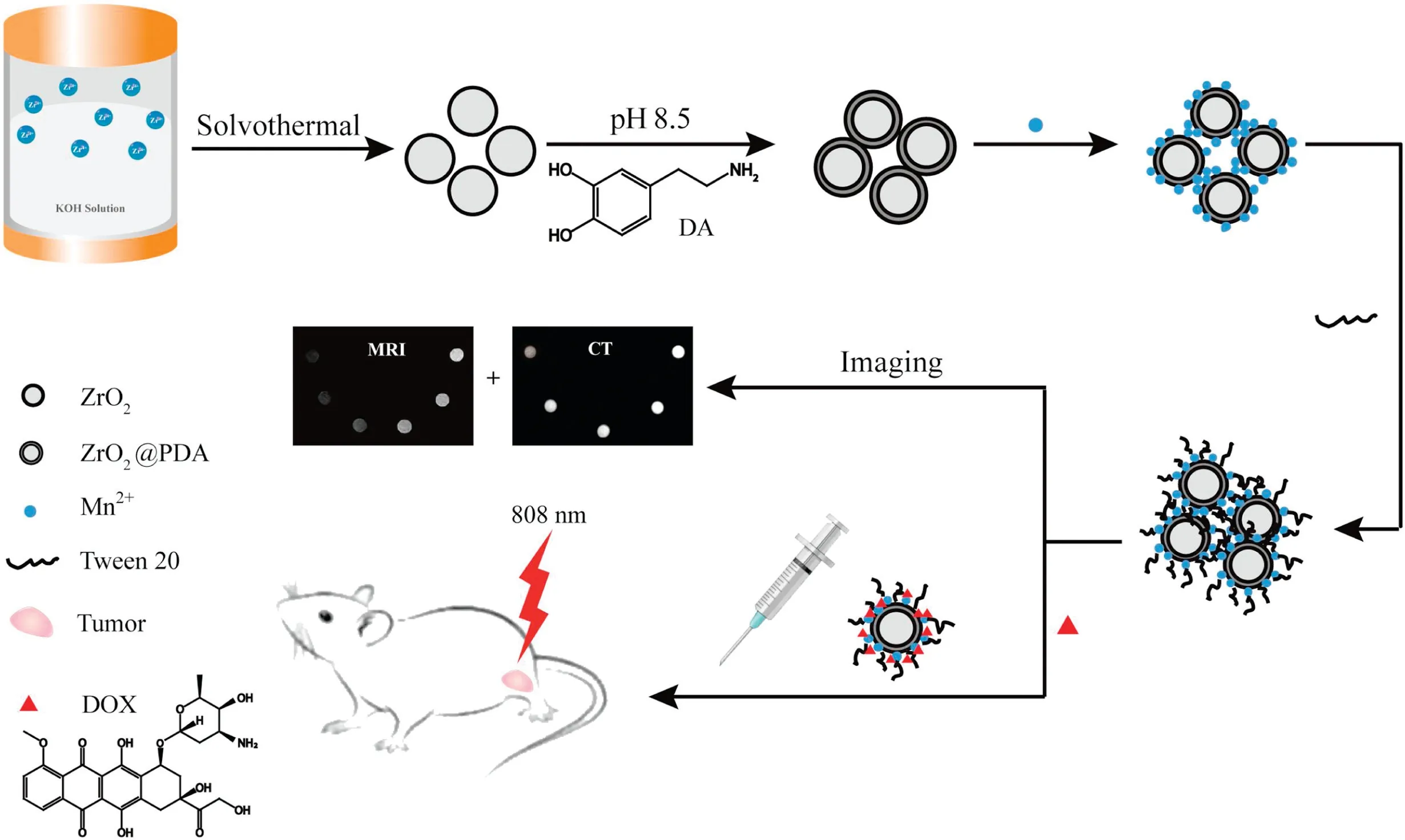
Fig.1.Schematic synthetic process of Tween-ZrO2@PDA-Mn2+/DOX nanocomposite and its CT/MR imaging guided chemo-photothermal therapy.
The size, morphology and composition of the samples were confirmed.The powder X-ray diffraction (PXRD) patterns of asprepared ZrO2(Fig.S2 in Supporting information) matches well with the standard pattern(JCPDS card No.37-1484),suggesting the formation of monoclinic phase ZrO2.Transmission electron microscopes (TEM) image indicates that the ZrO2NPs (Fig.2a)has round baking-like structure with the single particle size of around 70 nm and PDA shell was formed on the ZrO2core with the thickness of ZrO2@PDA increasing by ~4 nm (Fig.2b).The hydrodynamic size distribution before and after PDA coating was obtained by dynamic light scattering (DLS) measurement(Fig.S3 in Supporting information).After mixing with MnSO4solution, the corresponding element mapping analysis of ZrO2@PDA-Mn2+in high-angle annular dark field scanning transmission electron microscopy (HADDF-STEM-EDS) illustrates the averaged element distribution of Zr,Mn,O,and N on the surface of the synthesized nanocomposite (Figs.2c-h), and the X-ray photoelectron spectroscopy (XPS) spectrum of ZrO2@PDA-Mn2+reveals the main constitution element ratio of Zr (10.56 at%), C(43.49 at%),N(15.12 at%),O(30.36 at%),and Mn(0.48 at%)(Fig.3a and Fig.S4 in Supporting information).Moreover,the comparison of Zr 3d3/2and Zr 3d5/2peaks of ZrO2and ZrO2@PDA-Mn2+(Fig.S5 in Supporting information)shows that there are no binding energy shifts on those two typical peaks, suggesting the formation of ZrO2@PDA-Mn2+was merely through the physical interaction between ZrO2and PDA.By using inductively coupled plasma mass spectrometry (ICP-MS) technique, the doping concentration of Mn2+ions was determined to be 11.2 ng in pristine 100 ng ZrO2@PDA-Mn2+solution.
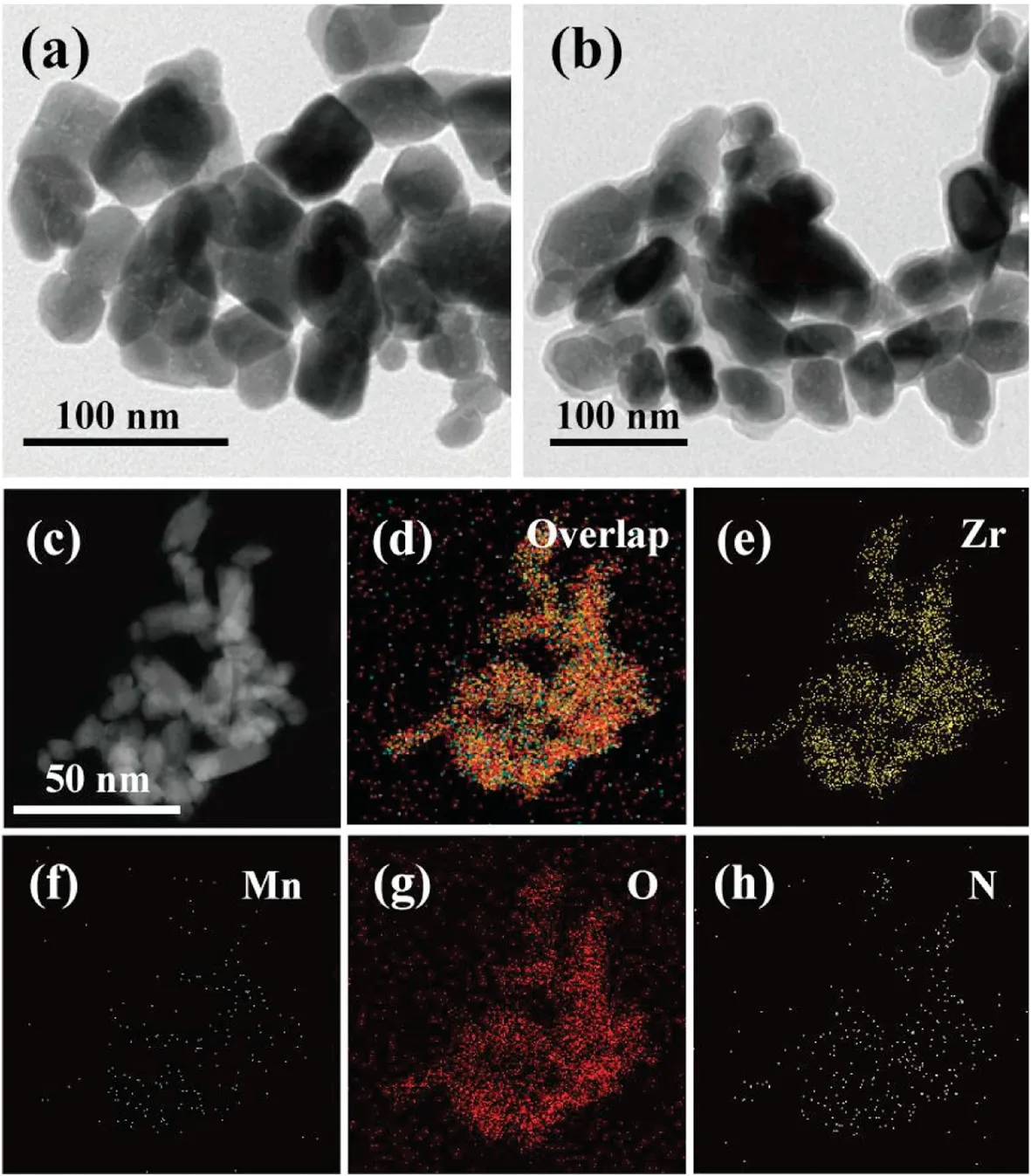
Fig.2.Typical TEM images of the(a)ZrO2 and(b)ZrO2@PDA NPs.(c)HAADF-STEM imaging and (d-h) the corresponding elements mapping of the ZrO2@PDA-Mn2+nanocomposite.
Nest, Fourier transform infrared (FT-IR) spectra demonstrates that the ZrO2@PDA-Mn2+nanocomposite had peaks at 1485,1619,and 3420 cm-1, denoting as the typical stretch vibrations of aromatic rings and catechol -OH groups of PDA, respectively(Fig.S6 in Supporting information) [34].In addition, there is a stronger characteristic peak at 1727 cm-1belonging to keto carbonyl group C=O than that on ZrO2@PDA-Mn2+after functionalizated by Tween 20,proving the successful interaction between ZrO2@PDA-Mn2+and Tween 20 (Fig.S7a in Supporting information) [35].And the Tween 20 functionalized ZrO2@PDA-Mn2+(Tween-ZrO2@PDA-Mn2+) nanocomposite exhibited pronounced stability in the water system within 6 h (Fig.S7b in Supporting information).
As one of the potential photothermal conversion agents, the optical absorption and photothermal conversion effect under the NIR region of Tween-ZrO2@PDA-Mn2+nanocomposite were investigated.As shown in Fig.3b, Tween-ZrO2@PDA-Mn2+had an obviously improved absorption character from 400-900 nm comparing with ZrO2NPs.With the relatively strong absorbance,the photothermal conversion effect of Tween-ZrO2@PDA-Mn2+nanocomposite solution at different concentrations were measured under NIR irradiation (1 W/cm2).As expected, all the aqueous solution containing Tween-ZrO2@PDA-Mn2+nanocomposite exhibited dramatic heating effect(Fig.3c).To be specific,the temperature of 1 mg/mL Tween-ZrO2@PDA-Mn2+aqueous solution had rapid temperature increase from 26.8°C to 56.9°C with ΔT =30.1°C at the mere 10 min(Fig.3d),while the distilled water shows negligible temperature change at the same operated condition.The photothermal conversion efficiency (η) of the Tween-ZrO2@PDAMn2+nanocomposite solution was calculated up to 29.5%.The calculating details were listed in Supporting information and Fig.S8 (Supporting information).Such value is superior to many recently reported PDA based nanocomposites (Table S1 in Supporting information) and Au nanorods (22%) [36].Fig.3e suggests that Tween-ZrO2@PDA-Mn2+solution had a negligible temperature change during eight cycles of irradiation/cooling test.Altogether, these results proved that the Tween-ZrO2@PDA-Mn2+could be favorably used as a promising photothermal conversion nanoagent towards efficient cancer treatment.
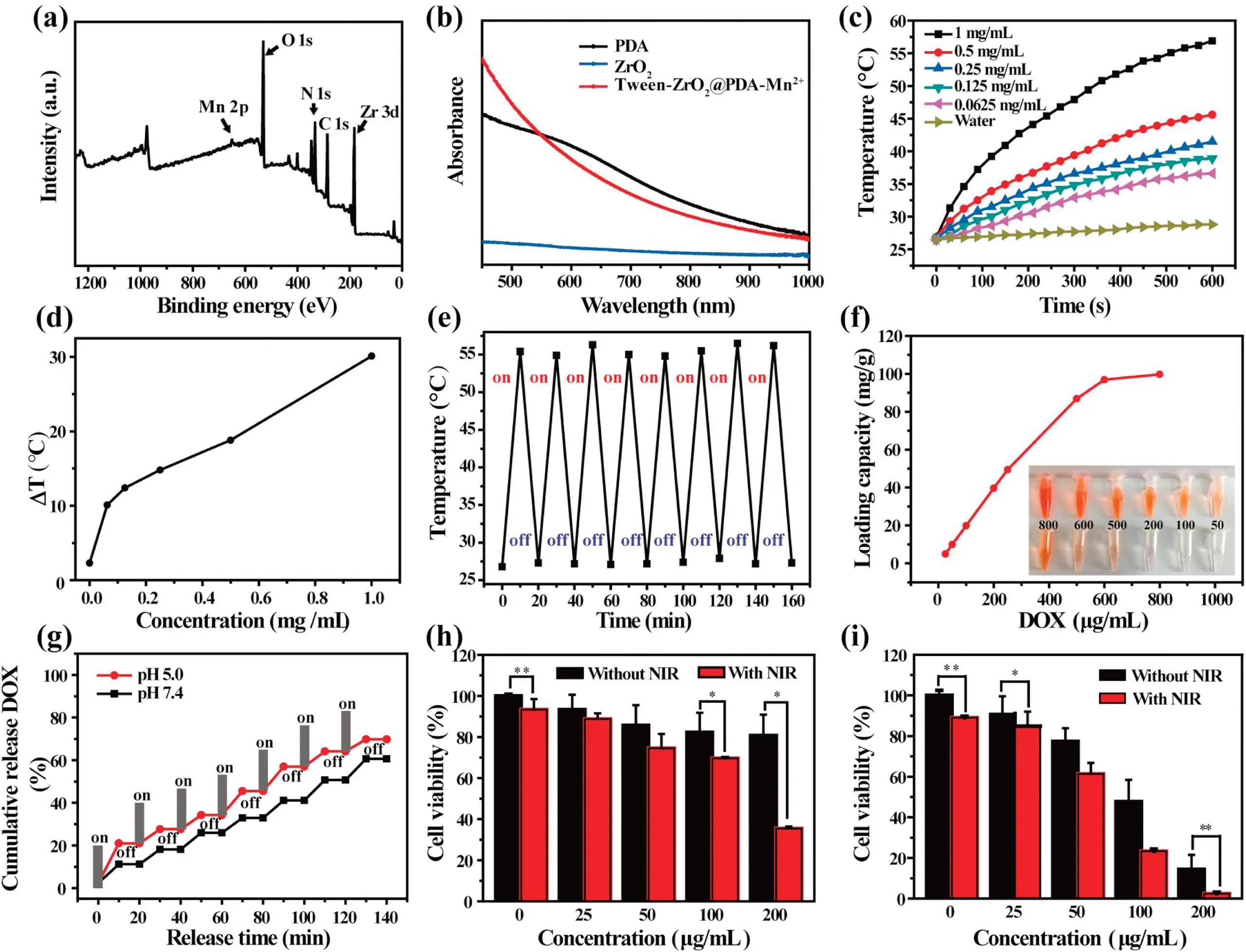
Fig.3.(a)Full XPS spectrum of ZrO2@PDA-Mn2+.(b)UV-vis-NIR curves of the ZrO2,PDA,and Tween-ZrO2@PDA-Mn2+solutions.(c)Photothermal conversion profiles of pure water and aqueous dispersions of Tween-ZrO2@PDA-Mn2+nanocomposite with different concentrations under 808 nm laser irradiation(1 W/cm2).(d)Plot of temperature change(ΔT)of Tween-ZrO2@PDA-Mn2+nanocomposite solution of 1 mg/mL over a period of 10 min.(e)Stability curve under 8 cycles irradiation/cooling process.(f)Loading capacity of the DOX on Tween-ZrO2@PDA-Mn2+nanocomposite.The inset photograph shows an obvious color change of DOX supernatant after loading on the nanocomposite.(g)DOX release in PBS(pH 5.0 and 7.4)with or without an 808 NIR laser irradiation(1 W/cm2,10 min).The effect of synergistic chemo-photothermal therapy on SMMC-7721 cells with(h)Tween-ZrO2@PDA-Mn2+and(i)Tween-ZrO2@PDA-Mn2+/DOX with different concentrations.The concentrations of Tween-ZrO2@PDA-Mn2+/DOX correspond to the concentrations of Tween-ZrO2@PDA-Mn2+.Data are presented as mean±SD (n=3), *P< 0.05, **P< 0.01.
In order to overcome the limitation of radiation power and penetrated depth of NIR laser,the chemotherapeutic drug DOX was introduced to load on the Tween-ZrO2@PDA-Mn2+nanocomposite by the π-π stacking and hydrogen bonding interaction of PDA[27].The color change of DOX supernatant and Tween-ZrO2@PDA-Mn2+nanocomposite dispersion proves that DOX was successfully loaded on Tween-ZrO2@PDA-Mn2+nanocomposite (Fig.3f and Fig.S9a in Supporting information).Because of the characteristic chromophore with an aromatic structure, DOX exhibits the main peak at 490 nm.And the Tween-ZrO2@PDA-Mn2+/DOX shows a similar absorption around 490 nm,which further verified the DOX have been successfully loaded on the surface (Fig.S9b in Supporting information).Furthermore, UV-vis absorption spectrum of the unloaded DOX supernatant after centrifugation shows that the DOX loading capacity on Tween-ZrO2@PDA-Mn2+had the nearly positive linear relationship with increased DOX amount at the concentration range of 0-600 μg/mL and then remained steady at 800 μg/mL(Fig.3f).The saturated loading amount of DOX can reach up to ~102.36 mg/g.
Next,the DOX release process under multiple stimulis including pH and NIR laser was investigated.As shown in Fig.3g, the total DOX release from Tween-ZrO2@PDA-Mn2+/DOX reached to 70%at simulated tumor microenvironment of pH 5.0 when exposure to 808 nm laser for 140 min (1 W/cm2), which was significantly higher than the condition of no 808 nm laser irradiation or pH 7.4(Fig.S9c in Supporting information).The result suggests that the NIR-laser-induced local hyperthermia exploited as the ideal on-off control for DOX release under tumor microenvironment.Therefore,the Tween-ZrO2@PDA-Mn2+/DOX provides a smart nanoplatform for highly killing efficacy toward tumor cells.
Biocompatibility as a prerequisite factor for biological application was also investigated[37,38].The CCK-8 assay was performed to determine cell viabilities after 24 h of co-incubating with the Tween-ZrO2@PDA-Mn2+.As shown in Fig.S10 (Supporting information),the Tween-ZrO2@PDA-Mn2+maintained significantly lower cytotoxicity even at higher concentration (400 μg/mL)with survival rate of SMMC-7721 cells (human hepatocellular carcinoma) and glioma cells (neuroglioma cell line in the human body) maintaining above 85%.
Furthermore, SMMC-7721 cells were used to evaluate the synergistic chemo-photothermal treatment of Tween-ZrO2@PDAMn2+nanoplatform by the CCK-8 assay.After being incubated with various treatments,the viability of cells had significant difference.First, the cell viability was not influenced by the Tween-ZrO2@PDA-Mn2+nanocomposite even at the high concentration of 200 μg/mL,verifying the low cytotoxicity of Tween-ZrO2@PDAMn2+nanocomposite(Fig.3h,black column).In contrast,as seen in Fig.3i, Tween-ZrO2@PDA-Mn2+/DOX +808 nm irradiation possessed the highest killing ability to SMMC-7721 cells at all tested concentrations than the PTT(Fig.3h,red column)or chemotherapy alone (Fig.S11 in Supporting information).The concentration range of free DOX used in Fig.S11 was calculated based on the loading ratio of DOX on the Tween-ZrO2@PDA-Mn2+surface.Almost no cells remained alive under Tween-ZrO2@PDAMn2+/DOX incubation at the concentration of 200 μg/mL plus 808 nm laser irradiation, indicating the significantly synergistic therapeutic effect.
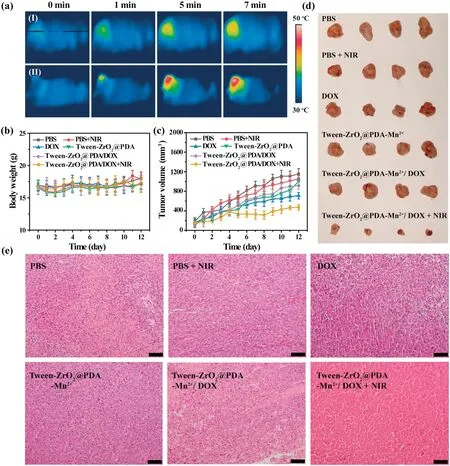
Fig.4.In vivo synergistic chemo-photothermal therapy of Tween-ZrO2@PDA-Mn2+/DOX.(a)IR thermal images of mice injected intratumorally with(I)PBS and(II)Tween-ZrO2@PDA-Mn2+/DOX(10 mg/kg).(b)Body weights of mice after various treatments.(c)Tumor volume changes and(d) images of excised tumor after 14-day therapy in every group (n=4).(e) Representative H&E staining images of tumors for each treated group (scale bar: 50 μm).
Encouraged by the above results in vitro,the synergistic chemophotothermal therapeutic efficacy was next studied in vivo.The animal experiments were approved by the Animal Study Committee of the Ministry of Science and Technology of the People’s Republic of China.Firstly,the in vivo photothermal effect of Tween-ZrO2@PDA-Mn2+/DOX was recorded by monitoring the tumor temperature with a thermal camera during NIR 808-nm laser irradiation(Fig.4a).The local tumor temperature was detected to be 38°C in the PBS group,suggesting that the NIR laser irradiation was safe for tissue.In contrast, for Tween-ZrO2@PDA-Mn2+/DOXtreated mice, the corresponding temperature at the tumor site elevated to over 50°C under NIR laser irradiation for 7 min,inducing that the NIR irradiation in the Tween-ZrO2@PDAMn2+/DOX-treated groups could induce localized hyperthermia to eliminate tumor cells.Then, the synergistic curative effect of PBS, PBS+NIR, free DOX, Tween-ZrO2@PDA-Mn2+, Tween-ZrO2@PDA-Mn2+/DOX, and Tween-ZrO2@PDA-Mn2+/DOX+NIR was investigated (Fig.S12 in Supporting information).Tumor volumes were recorded in every group during treatment(Fig.4c).The results showed that all the tumor size in the PBS+NIR irradiation, free DOX, Tween-ZrO2@PDA-Mn2+, and Tween-ZrO2@PDA-Mn2+/DOX groups revealed a slightly decrease compared with the control group (PBS), while the desired antitumor effect was not reached.As anticipated,the Tween-ZrO2@PDA-Mn2+/DOX+NIR group realized a stronger inhibition than any other group.Moreover, the body weight had no abvious loss during treatment (Fig.4b), which suggested that there were no detrimental effects to mice during the treatment.After 14-day treatment,all animals were sacrificed.Tumors(Fig.4d) and main organs (heart, liver, spleen, lung, kidney) of tested mice were collected for histological examination (H&E staining) (Fig.4e,Figs.S13 and S14 in Supporting information).It was illustrated that the best antitumor efficacy was achieved for the Tween-ZrO2@PDA-Mn2+/DOX+NIR group and the tumor growth was efficiently suppressed with the antitumor rate up to 87.92%.Also,the tissue of the Tween-ZrO2@PDA-Mn2+/DOX+NIR showed a large area of necrosis compared with other groups.Besides that,neither inflammation nor obvious damage could be observed in other main organs because of the negligible toxicity (Fig.S14).These results indicated that Tween-ZrO2@PDA-Mn2+/DOX could achieve efficient chemo-photothermal therapy.
To further trace tumor, the diagnosis technique of CT and MR imaging is introduced.The in vitro CT and MR imaging effects were demonstrated in Fig.5.For CT imaging, it was found that the CT imaging captured on Tween-ZrO2@PDA-Mn2+nanocomposite became brighter as the concentration of ZrO2increasing, and the obtained HU values suggest a linear relationship with the arising ZrO2concentration, which demonstrates enhancive CT response capability of the Tween-ZrO2@PDA-Mn2+(Fig.5a).For MR imaging, Fig.5b indicates a clear change from dark to the significantly white for the Tween-ZrO2@PDA-Mn2+with the increased Mn2+and the r1relaxivity value is 4.17 L mmol-1s-1,such value was larger than that of commercially available Gd-DTPA contrast agent (Magnevist, 3.217 L mmol-1s-1), which signifies that Tween-ZrO2@PDA-Mn2+nanocomposite possessed enhanced MR imaging property [39,40].Then, BALB/c nude mice bearing SMMC-7721 cells were subsequently utilized to probe the feasibility of Tween-ZrO2@PDA-Mn2+nanocomposite as a CT and MR imaging contrast agent in vivo.In Fig.5c, an obvious contrast enhancement was emerged from the tumor location (black line circle) after the injection of Tween-ZrO2@PDA-Mn2+for 4 h.Additionally, MR images obtained from tumor-bearing-mice at different time point were displayed in Fig.5d.The enhancement effect was clearly observed after 2 h of post-injection and the tumor region showed the brightest imaging quality after injection of Tween-ZrO2@PDA-Mn2+for 12 h with the corresponding signalto-noise ratio(SNR)arisen from 33.36 of the control group to 63.18.All these results favorably support that Tween-ZrO2@PDA-Mn2+nanocomposites could be used as an ideal CT and MR contrast agent both in vitro and in vivo.
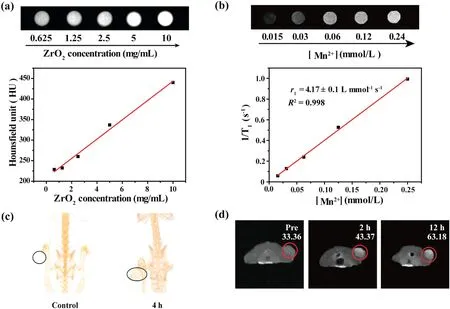
Fig.5.(a) In vitro CT images and the corresponding HU values of the Tween-ZrO2@PDA-Mn2+ nanocomposite at different ZrO2 concentrations.(b) T1-weighted phantom images with different Mn2+concentrations(mmol/L)at 0.5 T and the linear fitting of longitudinal relaxation rates versus Mn2+concentrations for Tween-ZrO2@PDA-Mn2+.(c)CT and (d) MR images in vivo before and after intravenous injecting Tween-ZrO2@PDA-Mn2+ nanocomposite.
In summary, multifunctional inorganic-organic hybrid nanocomposite named as Tween-ZrO2@PDA-Mn2+has been successfully fabricated by a simple,effective,and green route.The as-synthesized nanocomposite was demonstrated to have good biocompatibility and ignorable toxicity in vitro and in vivo.As expected, the nanocomposite can serve as multimodal contrast agents for efficient CT and T1-weighted MR imaging.Importantly,due to the pronounced photothermal conversion performance and controllable DOX release triggered by the NIR irradiation and acidic pH, the synergistic chemo-photothermal therapeutic efficacy mediated by nanocomposite is superior to that of either monotherapy alone, as shown both in vitro and in vivo.This inorganic-organic hybrid nanotheranostic platform may have substantial potential for cancer diagnosis and therapy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowedgments
This work was supported by the National Natural Science Foundation of China (Nos.51772293, U1932112, and 21471103),Beijing Natural Science Foundation (No.2202064), Science and Technology Innovation Service Ability Construction Project of the Beijing Municipal Commission of Education (No.19530050182),and CAS Key Laboratory of Nano-Bio Interface (No.20NBI01).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the on line version,at doi:https://doi.org/10.1016/j.cclet.2021.02.030.
杂志排行
Chinese Chemical Letters的其它文章
- Challenges in cell membrane-camouflaged drug delivery systems:Development strategies and future prospects
- Visible and near-infrared light activated azo dyes
- Development of bioorthogonal SERS imaging probe in biological and biomedical applications
- A H2S-triggered two-photon ratiometric fluorescent theranostic prodrug for bio-imaging
- Light-up lipid droplets for the visualization of lipophagy and atherosclerosis by coumarin-derived bioprobe
- The density of surface ligands regulates the luminescence of thiolated gold nanoclusters and their metal ion response
