Surface microstructure-controlled ZrO2for highly sensitive room-temperature NO2sensors
2021-11-16YuhuYnZongtoJingyoSunMiomioBuYnmingHuoZiyingWngYunfeiLiNingHu
Yuhu Yn,Zongto M,Jingyo Sun,Miomio Bu,Ynming Huo,Ziying Wng,*,Yunfei Li,d,e,**,Ning Hu
a Tianjin Key Laboratory of Electronic Materials and Devices,School of Electronics and Information Engineering,Hebei University of Technology,5340Xiping Road,Tianjin,300401,PR China
b State Key Laboratory of Reliability and Intelligence Electrical Equipment,Hebei University of Technology,Tianjin,300130,PR China
c National Engineering Research Center for Technological Innovation Method and Tool,And School of Mechanical Engineering,Hebei University of Technology,Tianjin,300401,PR China
d Center for Advanced Laser Technology,Hebei University of Technology,Tianjin,300401,PR China
e Hebei Key Laboratory of Advanced Laser Technology and Equipment,Tianjin,300401,PR China
Keywords:ZrO2 Room temperature Microstructure Chemisorbed adsorbed oxygen NO2
ABSTRACT
1.Introduction
Air pollution not only has a serious impact on the natural environment but also does great harm to human beings,animals and plants.Nitrogen oxide(NOx)is one of the main pollutants leading to air deterioration,including nitrogen dioxide(NO2),nitric oxide(NO)and nitrous oxide(N2O),etc[1].Among them,NO2is a highly toxic gas and a major contributor to acid rain,ozone and photochemical smog,which primarily comes from high-temperature combustion of industrial fuels and exhaust emissions from automobiles[2].When the concentration of NO2is higher than200g/m3,human respiratory system will be seriously damaged[3].Therefore,it is of great scientific significance to develop NO2gas sensors with low power consumption and high sensitivity.
In recent years,many researchers have developed NO2sensors based on different nanomaterials(metal oxide[4–8],transition metal sulfide[9,10],carbon materials[11,12],and polymers[13]).Metal oxide is one of the superior sensing materials for fabricating gas sensors because of their high sensitivity,good stability and ease of fabrication.Besides,metal oxide can be prepared with unique structures like nanoparticles[4],nanosheets[5],nanowires[6]and nanocubes[7,14]and so on[15].These materials exhibit excellent gas sensitive properties due to their high specific surface area and enhanced surface reactivity[16].However,high operating temperature of some metal oxide-based sensors can lead to the increase of power consumption and reduce of the lifetime of sensors.Therefore,some researchers devote themselves to develop room-temperature gas sensors.Zhang et al.have prepared the sensors based on metal-organic frameworks-derived In2O3hollow microtubes/MoS2(In2O3/MoS2)nanocomposites,which exhibited excellent room-temperature gas sensing performance[9].Cho et al.have investigated room temperature NO2and NH3sensing performance of atomic-layered MoS2[10].Density functional theory and in-situ PL characterization are used to elucidate the gas sensing mechanisms of charge transfer between the MoS2and gas molecules.In addition,we have prepared three SnO2NPs-RGO nanocomposites via three different assembly methods and investigated the effect of material microstructures on NO2sensing at room temperature in our previous works[11].Wu et al.have fabricated3D SnS2/RGO through hydrothermal method,which presents high sensitivity(6.1ppm-1)and low theoretical limit of detection(8.7ppb)[12].Tai et al.have successfully prepared polypyrrole/nitrogen-doped multi-walled carbon nanotube(ppy/N-MWCNT),the response of which to5ppm NO2at room temperature was24.82%[13].These methods can realize to detect NO2at room temperature.Nevertheless,the sensitivity is lower than metal oxide based NO2sensors.
ZrO2is a low-cost and environmental and metal oxide with wide band gap(3.25–5.1eV)[17,18],which exhibites p-type semiconductor[19–21].ZrO2has oxidized or reduced sites on its surface with high ionic conductivity and outstanding capability of manipulating its morphology[17].These unique physical and chemical properties make it a good candidate material for the construction of gas sensors.However,most reported NO2gas sensors based on zirconia are yttrium stabilized zirconia(YSZ)sensors operating at high temperature(400–700°C)[22–24].In addition,there are a few reports of NO2sensors based on ZrO2composite material[25,26].Myasoedova et al.have prepared SiO2/ZrO2composite film by sol-gel method[25].The sensitivity of the sensor to1060ppm high concentration of NO2was low at25°C,and the response was only44%.Mohammadi et al.have exhibited a remarkable response towards low concentrations of NO2gases at150°C[26].Herein,we have successfully synthesized a series of ZrO2with different morphologies by hydrothermal and solvothermal methods,which are designated as ZrO2-HS,ZrO2–S and ZrO2-R,respectively.Compared with some other NO2sensors based on carbon materials,transition metal sulfides,polymers,metal oxides and ZrO2composites[13,25–29],the three sensors based on the synthesized ZrO2with different morphologies show excellent room-temperature NO2gas properties,as shown in the Table S1.Moreover,we intend to discover the effect of material morphologies on sensing performances.Through a series of material characterization techniques,such as X-ray diffraction(XRD),transmission electron microscopy(TEM),Brunauer Emmett and Teller(BET),the relationship between the surface morphology of the ZrO2materials and their gas sensitivity characteristics of NO2are studied.The present work provides an effective strategy for fabricating room-temperature NO2sensors.
2.Experimental section
2.1.Preparation of ZrO2-HS,ZrO2–S and ZrO2-R
(1)Synthesis of ZrO2-HS:11.2mM ZrOCl2⋅8H2O and20mM urea were dissolved in80mL ethanol.After that,20mL hydrogen chloride solution(36.5wt%)was slowly added into above solution under stirring at room temperature.The mixed solution was poured into 150mL Teflon-lined autoclave and maintained at160°C for24h.The precipitate was washed with distilled water and then was freeze-dried at-45°C for24h.Finally,the obtained precursor was calcined at450°C for4h.
(2)Synthesis of ZrO2–S:3.6mM ZrOCl2⋅8H2O and0.6mM CH3COONa were dissolved in12mL distilled water under stirring at room temperature.Then,the mixed solution was transferred to30mL Teflon-lined autoclave and reacted at240°C for6h.The processesof washing,freeze-drying and calcine are the same as that of ZrO2-HS.
(3)Synthesis of ZrO2-R:1150μL NH3⋅H2O(25%)was dropwise added into40mL ZrOCl2⋅8H2O solution(7.2mM).Then,12mL NaOH solution(0.1mM)was added into the above solution drop by drop.The mixture was placed in Teflon-lined autoclave and heated at250°C for48h.The product was collected,washed freeze-dried and then calcined by the same method in the above synthetic process of ZrO2-HS and ZrO2–S.The preparation condition of the three ZrO2samples is listed in Table1and Fig.1.
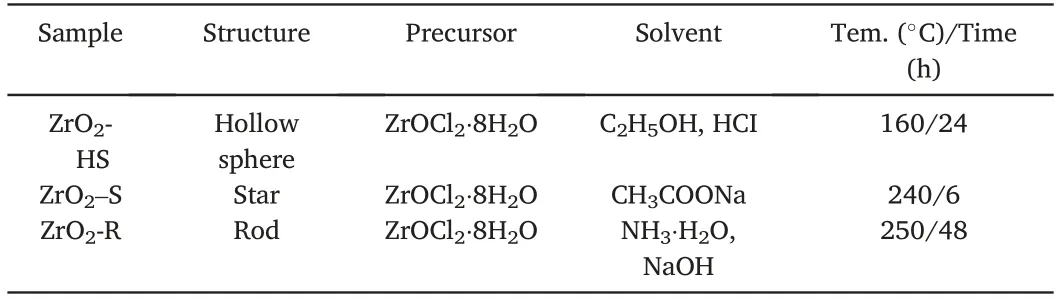
Table1 The detailed experimental parameters for the preparation of ZrO2-HS,ZrO2–S and ZrO2-R.
2.2.Characterization of microstructure
The X-ray Diffraction(XRD)data of the three samples are characterized on a Rigaku Smartlab(9KW)diffractometer with Cu Kα radiation(λ=1.5406Å)at a scanning rate of8°min-1and XRD patterns are collected from2θ=10–80°at4kw.The surface morphologies of the samples are characterized on scanning electron microscopy(SEM,JSM-7610F)and transmission electron microscopy(TEM,FEI Talos F200X).The elementary composition of samples is analyzed by X-ray photoelectron spectroscopy(XPS,ThermoFischer ESCALAB250Xi).The N2gas adsorption and desorption isotherm is obtained by BSD-PS/PM specific surface area analyzer,which is performed to analyze the specific surface area and pore structure of the materials.The band gap of the samples is analyzed by the UV–visible diffuse reflectance spectrum(UV–Vis,PerkinElmer Lambda950/PE).
2.3.Gas sensing measurement
The gas sensors consist of sensing films and substrates.Firstly,the ceramic substrates were washed in alcohol solution by ultrasonic treatment.The same amount of three ZrO2samples powder was mixed with a certain amount of deionized water and ground to form paste.Then,all the paste was spin-coated onto the surface of ceramic substrates to form homogeneous sensing films.The fabrication method of three gas sensors is similar to that in our reported works[11,30].
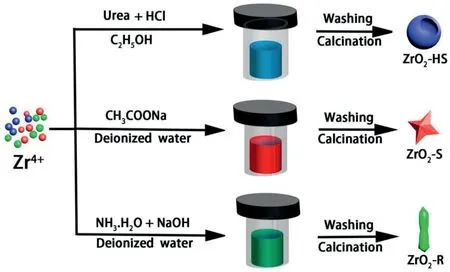
Fig.1.Schematic diagram of the preparation of ZrO2-HS,ZrO2–S and ZrO2-R.
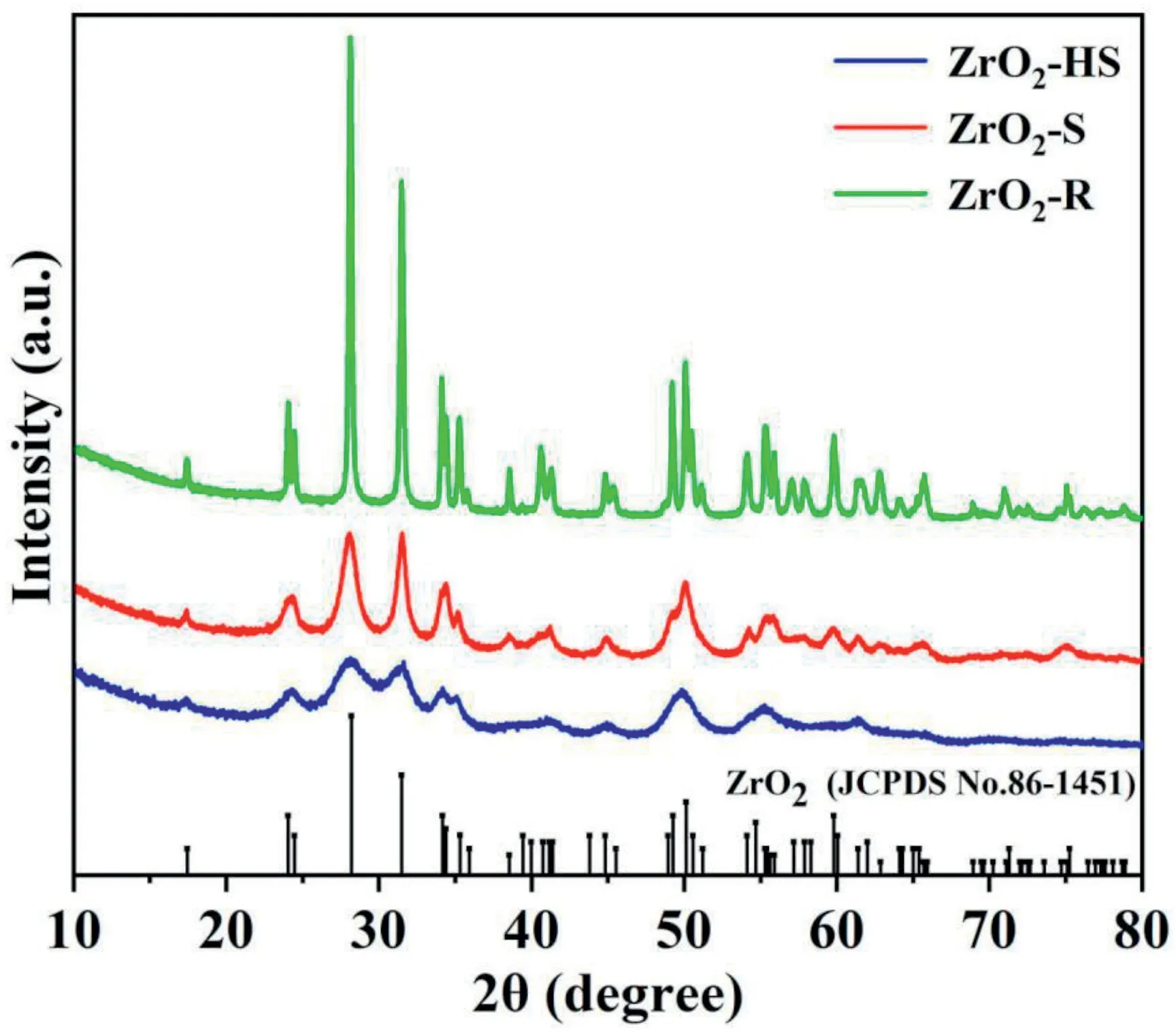
Fig.2.XRD pattern of ZrO2-HS,ZrO2–S and ZrO2-R.
3.Results and discussion
3.1.Structure and morphology characterization
The three ZrO2samples with different morphologies are prepared by simple and facile hydrothermal reactions without templates.The synthesis process is shown in Fig.1.By changing the experiment condition(solvent,pH value,reaction temperature and time),three kinds of ZrO2nanostructures with hollow sphere,star and rod morphology are prepared.Subsequently,a series of material characterization techniques are performed to analyze the microstructure and surface morphology of the materials.The crystalline structure of the three samples is characterized via XRD measurement.Several obvious diffraction peaks of ZrO2-HS,ZrO2–S and ZrO2-R mainly appearing at24.14°,28.08°,31.48°,34.05°,34.96°,41.03°,44.98°,49.19°,50.12°,54.90°and61.32°can be observed in Fig.2,which are assigned to the(011),(111),(111),(002),(200),(102),(112),(022),(220),(122)and(113)planes.Obviously,among the three samples,the diffraction peaks of the ZrO2-R are the narrowest and the highest in intensity as compared to the other two samples,which indicates that the ZrO2-R has perfect crystallinity.According to the Scherrer equation[31],the average particle sizes of the three samples are calculated and the corresponding results are listed in Table S2.It is found that the grain size of the three samples followed the order of:ZrO2-R(122.65nm)>ZrO2–S(17.70nm)>ZrO2-HS(12.85 nm).
The surface morphology and microstructure of the three samples are characterized by SEM and TEM.As can be seen from Fig.S1,three different morphologies for the ZrO2are present,which exhibits hollow sphere,stellate or rod-shaped nanostructures,respectively.Furthermore,the size of the hollow spheres for the ZrO2-HS is about2μm(Fig.3a)and the surface of the hollow spheres is rough(Fig.3a1).Interestingly,the ZrO2–S exhibits regular tetragonal star-like morphology,the size of which is ranges from30to180nm(Fig.3b and b1).The ZrO2-R is shaped like lotus roots having the size of~100nm in width and~300nm in length(Fig.3c and c1).In addition,clear lattice striations are seen in HRTEM,which reveals the high crystallinity of the ZrO2samples(Fig.3a3-3c3).After measuring,the neighboring spacing of these lattice fringes corresponding to ZrO2-HS,ZrO2–S and ZrO2-R is about0.290nm,0.258nm and0.257nm,belonging to(111),(200)and(200)planes,respectively.The diffraction rings in the selected area electron diffraction(SAED)figures have showed the polycrystalline and monoclinal structure of the three samples(Fig.3a4-c4).Among them,the ZrO2-R has the best crystallinity,which agrees with the results of XRD.Finally,EDS element mapping is performed on carbon membrane.It is clearly observed that the zirconium and oxygen elements in the three samples are evenly distributed according to the shape of hollow sphere,stellate and rod in Fig.3a5-c5and3a6-c6.
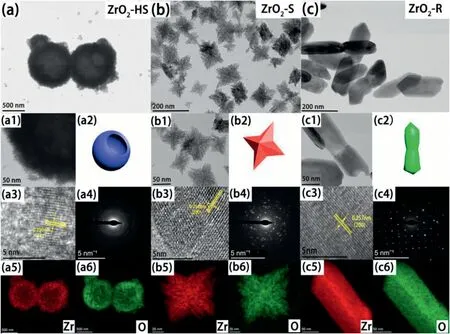
Fig.3.(a–c)The low magnification TEM images,(a1-c1)high magnification TEM images,(a2-c2)schematic illustration,(a3-c3)HRTEM images,(a4-c4)SAED patterns,(a5,a6-c5,c6)the typical EDS elemental mapping of ZrO2-HS,ZrO2–S and ZrO2-R.
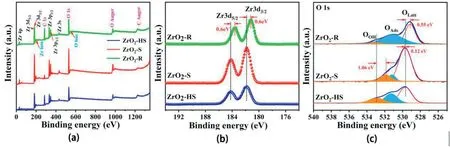
Fig.4.(a)XPS spectra(b)Zr3d XPS spectra(c)O1s XPS spectra of ZrO2-HS,ZrO2–S and ZrO2-R.
XPS technologies are used to analyze the surface chemical composition and the valence of the samples.The XPS spectra of the three ZrO2samples illustrates the same elemental compositions of Zr and O in Fig.4a.The high-resolution XPS spectrum of Zr3d(Fig.4b)shows that two prominent peaks appear at approximately184.21eV and181.90eV,which are assigned to Zr3d5/2and Zr3d3/2,respectively.The Zr of ZrO2in the three samples presents a single Zn4+state[32].In contrast to the ZrO2-R,the position of the Zr3d peaks in ZrO2-HS and ZrO2–S are shifted 0.6eV(Fig.3b),indicating that the binding energy between Zr nucleus and inner electrons is changed[34].Fig.4c shows the original peak value and the post-fitting XPS spectra of O1s for the three samples.The position of peak at~529.1–529.8eV is attributed to lattice oxygen(Olatt.)[33].The peak shift of Olatt.may be affected by electrostatic repulsive interaction in the lattice of O[11].In addition,another fitting O1s is located at~531.1–531.8eV,which is ascribed to adsorbed oxygen(OAds)[34].Generally,the adsorbed oxygens are produced by the adsorption of oxygen on oxygen vacancies of metal oxide[30].
The final fitting O1s is relative to the adsorption of hydroxyl(-OH)[35].As shown in Table S3,the relative atomic content of O1s in ZrO2-HS,ZrO2-S and ZrO2-R is illustrated.It is found that the content of OAdsin ZrO2-R is48.9%,which is obviously higher than that of ZrO2-HS and ZrO2-S.The OAdsis considered to be a key factor in improving the gas sensitive properties of materials[36].
Fig.5a–c illustrate the N2adsorption-desorption isotherms and Fig.5d–f show the pore size distribution of three samples with different morphologies.The valuable information of the BET sur face area,pore volume and average pore size is shown in Table2.Although the value of BET surface area and pore volume of ZrO2-R is relatively lower than that of the other two samples,the value of average pore size of ZrO2-R(43.24 nm)is the largest among the three samples,demonstrating that the ZrO2-R exhibites a highly gas-accessible structure.
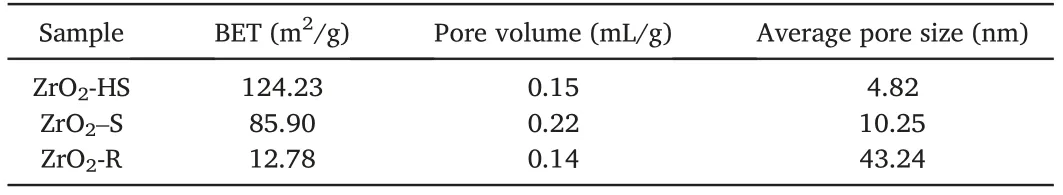
Table2 The results of BET specific surface area,pore volume and average pore size of ZrO2-HS,ZrO2–S,ZrO2-R.
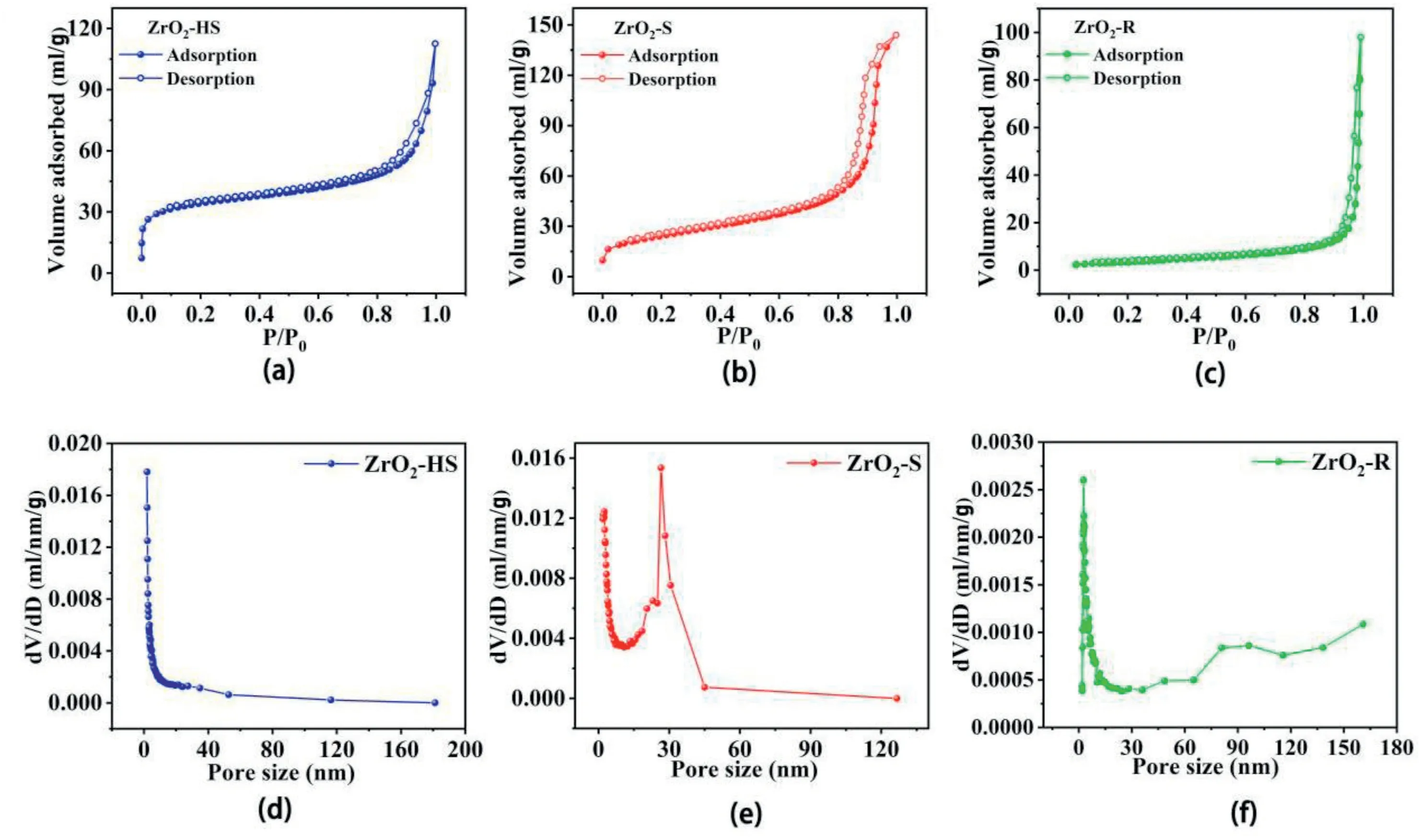
Fig.5.(a–c)N2adsorption-desorption isotherms and(d–f)pore size distribution of ZrO2-HS,ZrO2–S and ZrO2-R.
3.2.Gas sensing properties and sensing mechanism
In order to investigate the effect of the surface structure of the ZrO2on the room-temperature gas sensitive properties of ZrO2sensors with different morphologies,the room temperature selectivity for the three ZrO2-sensors is shown in Fig.6a,including20ppm NH3,20ppm NO2and 200ppm volatile organic compounds(VOCs)gases(C6H6,CH3COCH3,CH3CH2OH and HCHO).Compared with other gases,the three ZrO2-based sensors have obvious response to NO2.Moreover,the ZrO2-R-based sensor exhibits the highest response to20ppm NO2,confirming that the sensor possesses the high selectivity toward NO2.Fig.6b–d show the gas measurement curves for the ZrO2-HS,ZrO2–S and ZrO2-R-based sensors to30ppm NO2at room temperature,respectively.When NO2was injected into each of the three sensors’chambers,the three sensors responded immediately and recovered quickly after the sensors were put into the air.The ZrO2-R-based sensor shows the highest response with 423.8%toward30ppm NO2,which can be related to the largest pore size and the highest content of OAdsin ZrO2-R.However,ZrO2-HS and ZrO2–S-based sensors show the lower response towards30ppm NO2(232.9% and245.1%,respectively).The adsorption amount of NO2molecules on the surface of ZrO2-R is increased.Besides,the ZrO2–Sbased sensor exhibits the shortest response time and recovery time(4s and3s)towards30ppm NO2,which is caused by the large pore volume(0.22mL/g)of ZrO2–S.The large pore volume is beneficial to fast adsorption and desorption of gases.
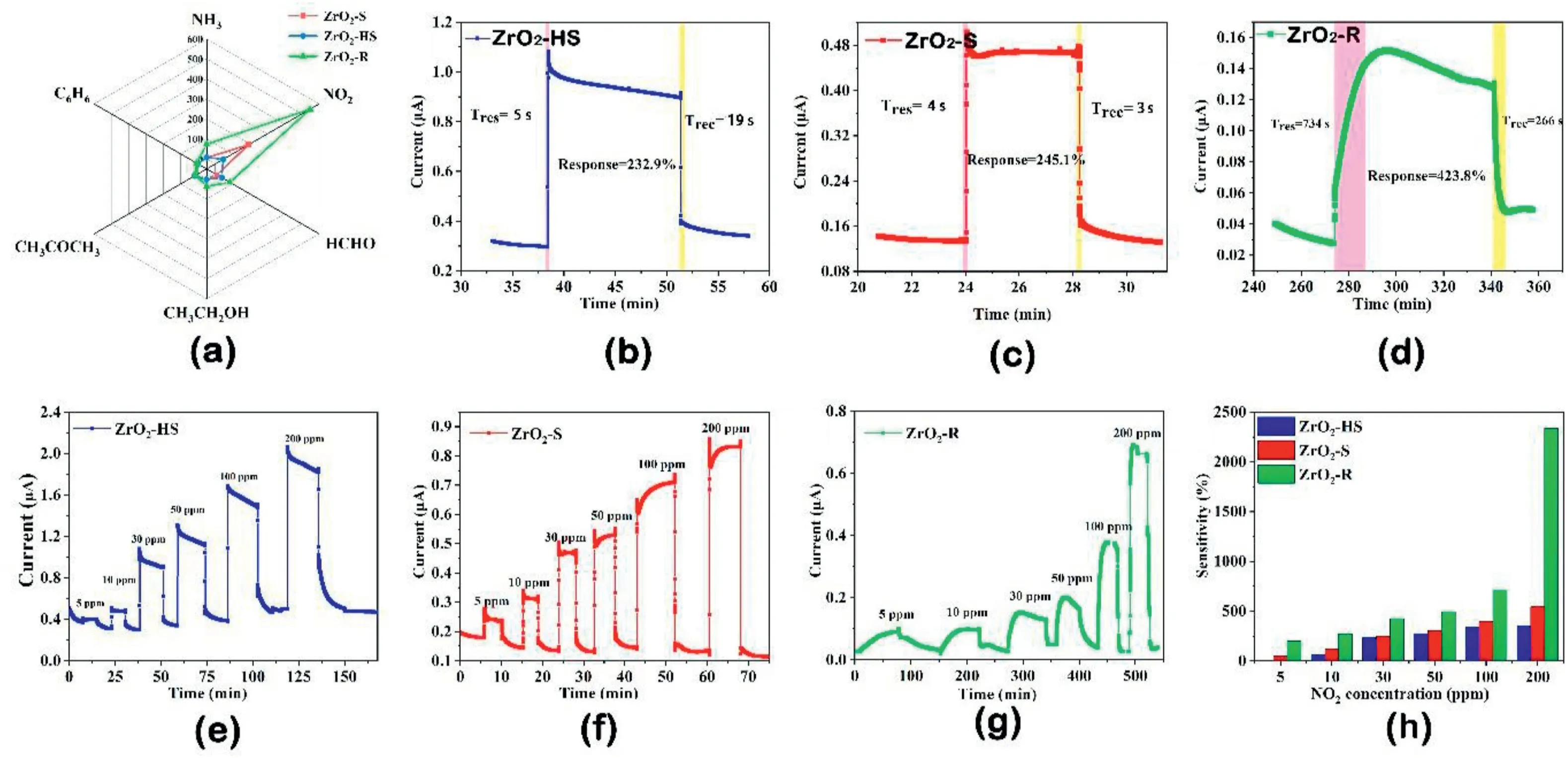
Fig.6.(a)polar graphs of ZrO2-HS,ZrO2–S,ZrO2-R-based sensors to different target gases;(b–d)the response and recovery curves of ZrO2-HS,ZrO2–S,ZrO2-R-based sensors toward30ppm NO2at room temperature;(e–g)the response and recovery curves of ZrO2-HS,ZrO2–S,ZrO2-R-based sensors to different concentration of NO2(5–200ppm)at room temperature and(h)the relationship between sensitivity and NO2concentrations of ZrO2-HS,ZrO2–S,ZrO2-R-based sensors.
The dynamic response and recovery curves for the sensors based on ZrO2-HS,ZrO2–S and ZrO2-R to5–200ppm NO2are shown in Fig.6e–g,respectively.The current value of the sensors increased significantly in NO2and then quickly recovered to its initial current after eliminating NO2in the air.The response of the three sensors increases with the increase of NO2concentration.Fig.6h shows the bar graph of responses for three ZrO2-based sensors towards different NO2concentrations.Obviously,among the three sensors,the ZrO2-R-based sensor shows extremely high response at various NO2concentration.In order to investigate the effect of relative humidity on the NO2sensing properties,the response and recovery curves of ZrO2-HS,ZrO2–S and ZrO2-R-based sensors under different RH(0%,30%,60%,90%)to15ppm NO2are shown in Fig.7.It is surprising that the sensitivity of the three sensors to15ppm NO2will increase as the humidity increases,which indicates the humidity promotes the NO2sensing reactions on the surface of materials at room temperature.Similar reaction will take place(NO2-+H+↔HNO2and 3NO2+H2O↔2H++2NO3-+NO).The findings are consistent with our previous work[37].In addition,the response and recovery curves of ZrO2-HS,ZrO2–S and ZrO2-R-based sensors toward60% humidity at room temperature are also shown in Fig.S2.Among them,the ZrO2-R--based sensor shows a higher sensitivity to humidity,while the ZrO2-HS-based sensor can not recover to its original state after the adsorption of humidity.
In order to examine the stability,sensitivities of ZrO2-based sensors are further measured and presented in Fig.S3.It is obvious that the response of the ZrO2sensors based on three different morphologies fluctuate within12% of the initial value within17days.The response and recovery curves of three sensors based on ZrO2to3ppm NO2on the 1st day and17th day are shown in inset of Fig.S3.The above results suggest that the ZrO2-based sensors have good stability at room temperature.
The structure,size and morphology of the sensing materials make a great difference on the sensing performance[38].The excellent response of ZrO2-R-based sensor to NO2at room temperature is mainly affected by four factors:crystallinity,pore size,band gap and chemisorption oxygen.The possible reasons for these three different sensing characteristics may be explained as follows:
(1)Crystallinity:the crystallinity has a certain effect on the gas sensitive performance of the gas sensors[39,40].The number of metal oxide carriers with better crystallinity is lesser[40].This will lead to more obvious changes in the resistance of ZrO2-R when adsorbing and desorbing NO2gases.As characterized by XRD and TEM,ZrO2-R-based sensor has better crystallinity.
(2)Pore size:the larger pore size is conducive to the diffusion of the target gas into the sensing materials and the increase of the contact between target gas and the surface adsorbed oxygen[41].As can be seen from Table2,the average pore size of ZrO2-R sample is up to43.24nm.The typical mesoporous structure can facilitate gas adsorption and transportation[42],which is the key factor to improve the response to NO2.
(3)Band gap:the UV–vis diffuse reflectance spectra of three materials are shown in Fig.7.The tangent interception between(ahv)2and the curve of photonic energy are used to estimate the band gap energy[43].As shown in Fig.8a–c,all the ZrO2samples exhibit strong absorption ranging from200to300nm,attributed to the characteristic absorption peak of ZrO2crystals.The band gap energy of the ZrO2-HS,ZrO2–S and ZrO2-R are at5.07eV,5.02eV and4.97eV,respectively.Obviously,the band gap of ZrO2-R is the narrowest.Narrower band gap will lead to lower required energy for electronic transition[44],which is another reason for the high sensitivity of the ZrO2-R-based sensor to NO2.
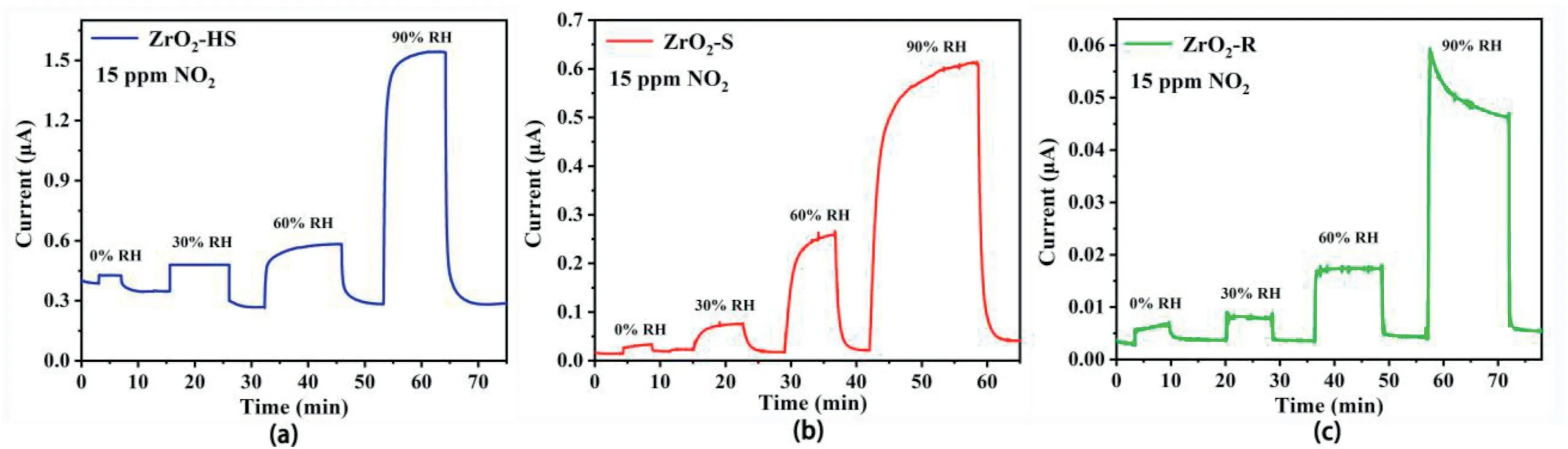
Fig.7.The response and recovery curves of(a)ZrO2-HS,(b)ZrO2–S and(c)ZrO2-R-based sensors at room temperature under different RH(0%,30%,60%,90%)to 15ppm NO2.
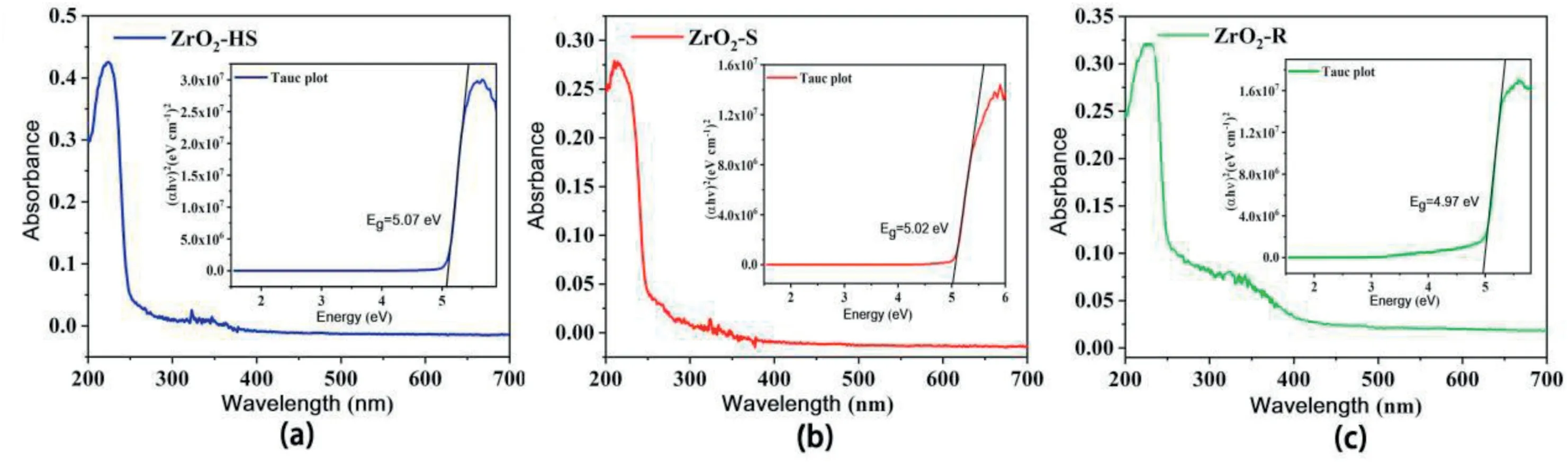
Fig.8.(a–c)UV–vis diffuse reflectance spectra of ZrO2-HS,ZrO2–S and ZrO2-R.The insets show the plots of(αhν)2versus photo energy for the band gap energies(Eg)of ZrO2-HS,ZrO2–S and ZrO2-R.
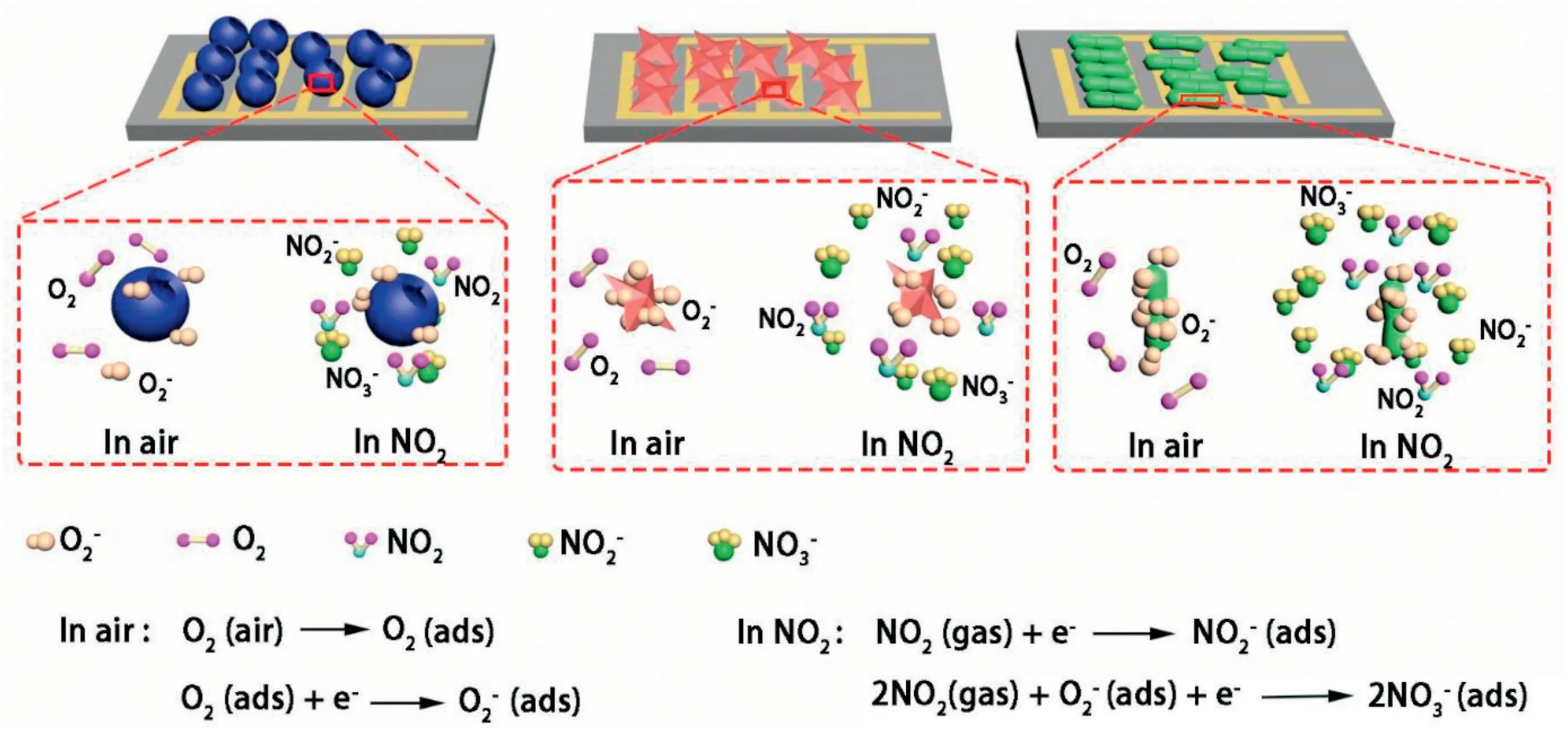
Fig.9.The schematic illustration of proposed gas sensing mechanism of ZrO2-HS,ZrO2–S nd ZrO2-R in air and in NO2gas.
(4)Chemisorption oxygen:OAdsis one of the important factors to improve the gas sensitive properties of materials[36].As shown in Fig.9,when three ZrO2samples with different morphologies are exposed to the air,oxygen molecules in the air capture free electrons in the conduction band forming oxygen ions(O2-,O-,O2-).Oxygen ions mainly exist in the form of O2-at room temperature[30].Electrons are captured during the reaction of NO2with O2-(2NO2+O2-+e-→2NO3-)[11].As shown in Fig.4c and Table S3,the ZrO2-R contains the largest proportion of adsorbed oxygen.Therefore,more adsorbed oxygen reacts with NO2,resulting in high response of the ZrO2-R-based sensor to NO2.
4.Conclusions
In conclusion,three kinds of ZrO2nanomaterials were successfully prepared by a hydrothermal method to explore their responses to NO2at room temperature.In order to investigate the effect of their morphology on room-temperature gas sensing properties,a series of characterizations and their gas-sensitive properties were compared.The response of ZrO2-R-based sensor is the highest.Crystallinity,average pore size,band gap and OAdsare the main factors that affect the gas-sensitive performance of sensors.The developed room-temperature NO2sensors resolve the issues of high power consumption and provide a direction for detecting NO2at room temperature.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgments
This work was supported by the Natural Science Foundation of Hebei Province(Project No.F2020202050)and the National Natural Science Foundation of China(Grant No.62004059,11632004and U1864208),the Key Program for International Science and Technology Cooperation Projects of the Ministry of Science and Technology of China(No.2016YFE0125900),National Science and Technology Major Project(2017-VII-0011-0106),Science and Technology Planning Project of Tianjin(20ZYJDJC00030),the Key Program of Research and Development of Hebei Province(202030507040009),the Fund for Innovative Research Groups of Natural Science Foundation of Hebei Province(A2020202002)and the Key Project of Natural Science Foundation of Tianjin(S20ZDF077).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nanoms.2021.02.001.
杂志排行
Namo Materials Science的其它文章
- Low-cost fabrication of highly dispersed atomically-thin MoS2nanosheets with abundant active Mo-terminated edges
- Hierarchically electrospun nanofibers and their applications:A review
- RTV silicone rubber composites reinforced with carbon nanotubes,titanium-di-oxide and their hybrid:Mechanical and piezoelectric actuation performance
- Advanced carbon materials with different spatial dimensions for supercapacitors
- Applications of carbon nanomaterials in perovskite solar cells for solar energy conversion
- Synthesis of hexagonal boron nitrides by chemical vapor deposition and their use as single photon emitters
