Advanced carbon materials with different spatial dimensions for supercapacitors
2021-11-16XiolingWuRuonnLiuJingZhoZhungjunFn
Xioling Wu,Ruonn Liu,Jing Zho,Zhungjun Fn
a College of Chemistry,Chemical Engineering and Resource Utilization,Northeast Forestry University,26Hexing Road,Harbin,150040,China
b Department of Chemical and Biomolecular Engineering,National University of Singapore,117585,Singapore
c College of Materials Science and Engineering,State Key Laboratory of Heavy Oil Processing,China University of Petroleum,Qingdao,266580,China
Keywords:Carbon Supercapacitors Graphene
ABSTRACT
1.Introduction
With the increasing serious environmental problems,the rapid requirement of portable devices and hybrid vehicles,the low-cost and environmentally friendly energy storage devices are developing rapidly[1,2].Among various energy storage devices,supercapacitors(SCs)have attracted extensive attention in the last decade due to their ultrahigh power density,fast charging/discharging rate,excellent electrochemical stability and environmental friendliness[3–6].However,the interfacial energy storage mechanism of SCs causes much lower energy density(less than10W h kg-1)compared with batteries and fuel cells(Fig.1),which severely restrict their further practical application[4,7].With the rapid demand for high energy density devices,it is urgent to enhance the energy density of SCs without sacrificing their high power capability and electrochemical stability.Tremendous efforts have been made to solve this issue and great achievements have been obtained in recent years.The energy density(E)of SCs is given in follow:

where C is the total capacitance of SCs,while V is the voltage window of SCs.Both of the two parameters extensively depend on the properties of electrode materials,which are accordingly crucial to the final energy density of SCs.Compared with specific capacitance,energy density is proportional to the square of cell voltage.Hence,the construction of carbon-based high voltage supercapacitors is more conducive to improving the energy density,the systematic review on boosting the cell voltage of aqueous SC has been summarized by Li[8].
Based on the energy storage mechanism,SCs can be divided into two categories:(1)electric double layer capacitor(EDLC),which stores energy by charging accumulated at the electrode/electrolyte interfaces;(2)pseudocapacitor,which stores energy by generating reversible faraday reaction with electrolyte ions.Typically,the EDLCs electrode materials compose of carbon-based materials,while the pseudocapacitor electrode materials consist of transition metal oxides and conducting polymers.Although the pseudocapacitive electrode materials have higher specific capacitance and energy density than the double-layer electrode materials,their intrinsic low electric conductivity,sluggish electrochemical kinetics,and poor electrochemical stability limit the practical application.Thus,the current electrodes of most commercial SCs are made of carbon materials.
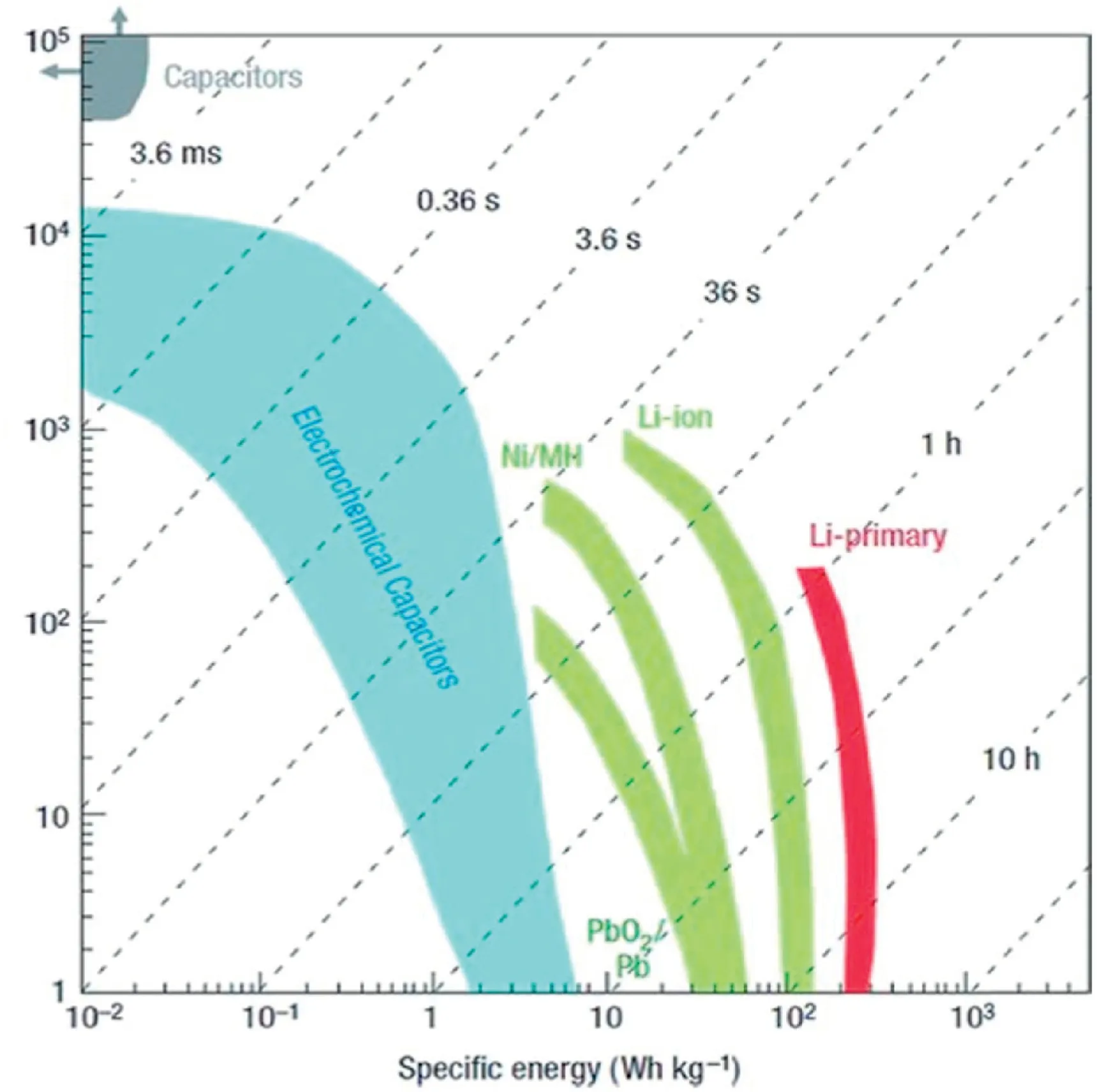
Fig.1.Ragone plots for different energy storage systems.Copyright2008,Nature Publication Group.
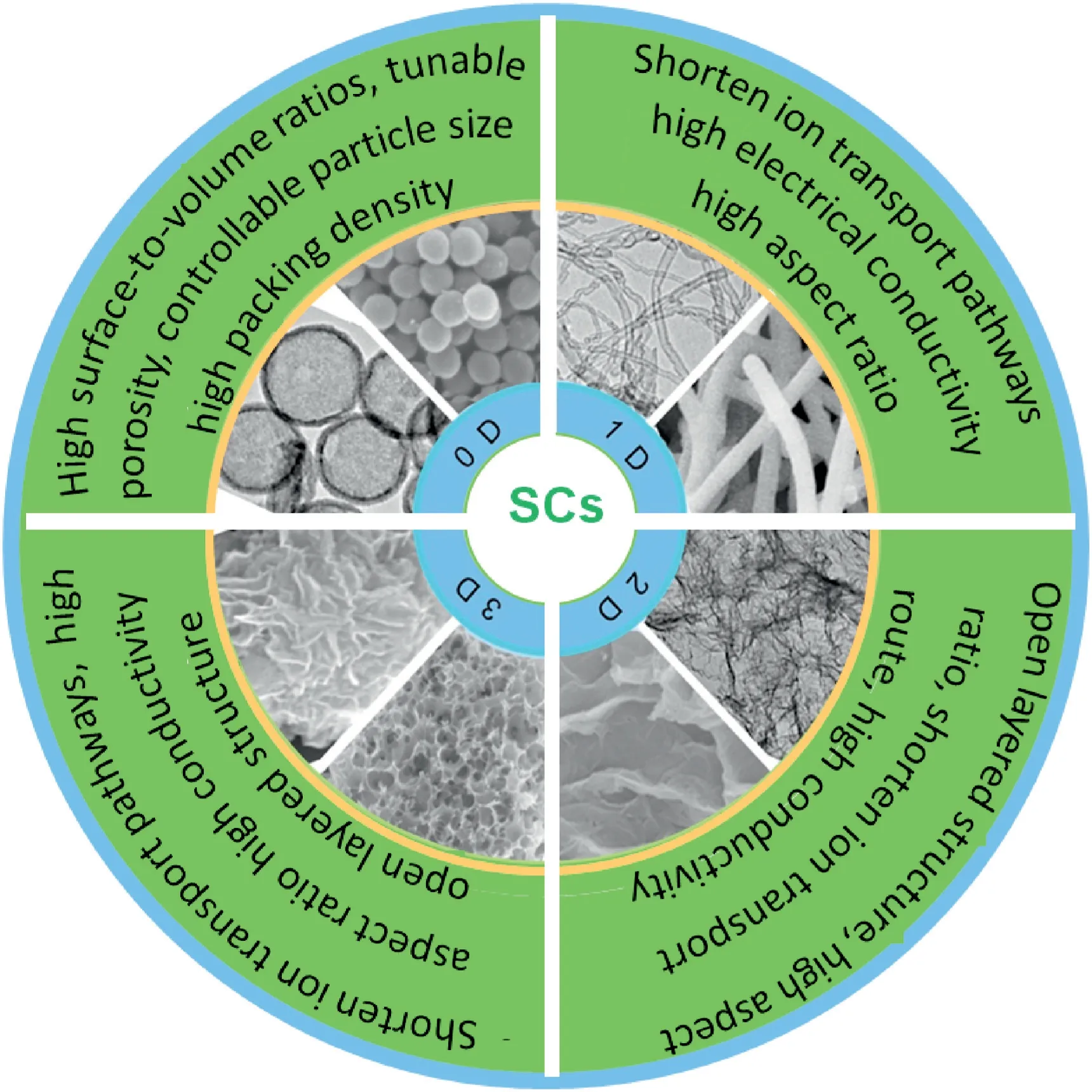
Fig.2.An overview of various spatial dimensional carbon materials and applications in SCs.
Various spatial dimensional carbon materials,including0D carbon materials(nanosphere,dots,etc.),1D carbon materials(nanotube,nanofiber,etc.),2D carbon materials(nanosheet)as well as3D carbon materials(Fig.2)have been employed for SCs in recent years.Carbon materials with different spatial dimensions have their unique advantages and disadvantages when used as electrode materials for SCs.Currently commercial carbon materials cannot satisfy the ever-increasing demands for high energy devices.Therefore,it is urgent for the design and preparation of advanced carbon electrode materials with both high gravimetric and volumetric performance without sacrificing their inherent good rate performance and excellent electrochemical stability[8].In this review,recent research progress on various spatial dimensional carbon materials and their advantages and disadvantages as electrode materials for SCs are summarized.Moreover,main affecting factors on the electrochemical properties of carbon materials were discussed.Finally,the new development trend of carbon materials has also been proposed.
2.0D carbons
0D carbon materials are in the range of nanoscale(1–100nm)in three dimensions or consist of them as basic units.For carbon materials,the0D structure used for SCs electrodes mainly includes carbon spheres(CSs),carbon quantum dots(CQDs)and graphene quantum dots(GQDs).
2.1.Carbon spheres(CSs)
CSs are emerged as a kind of promising carbon material for SCs due to their high surface-to-volume ratios,high packing density,uniform shape and size,tunable porosity and particle size[9–11].Numerous efforts have been devoted to prepare CSs with unique structure,tunable diameter and pore size to tailor the physicochemical property of CSs.Moon et al.[12]prepared nitrogen-doped carbon spheres(NCSs)by the carbonization of emulsion-polymerized polystyrene based colloidal spheres.The obtained NCSs samples contain a high nitrogen content of 20% and show a specific capacitance of191.9F g-1.Mai et al.[10]reported a facile aqueous self-catalyzed polymerization method for the large-mass production of N-doped carbon spheres using3-aminophenol and hexamethylenetetramine(HMTA)as precursors(Fig.3).The size of the N-doped carbon spheres can be tuned from1530to600nm by adjusting the HMTA/3-aminophenol mole ratio.The sample with the smallest size of600nm delivered the highest specific capacitance(282F g-1at0.5A g-1)and best rate performance(170F g-1at 20A g-1).
Most of the prepared polymer-based carbon spheres without template or further activation have solid sphere structure,which is not conducive to the transport and diffusion of electrolyte ions inside the electrode materials[13].The design of porous or hollow structure of CSs can provide more channels for ions rapid diffusion in electrode materials and thus greatly improve their electrochemical performance.Jaroniec et al.[14]developed a series of nitrogen-rich activated carbon spheres(NACS)with adjustable diameters in the range of50–1200nm using resorcinol/formaldehyde as carbon precursors and ethylenediamine as both catalyst and nitrogen precursor.Those materials show a high capacitance of388F g-1at1.0A g-1,outstanding rate capability with60% capacitance retention at100A g-1and good electrochemical stability.Gan et al.[15]reported a template-free and self-doping approach to fabricate N,O-enriched porous carbon spheres(PCSs)by one-step carbonization/activation of melamine-glyoxal polymer.The PCSs with interconnected spherical morphology generated stacking porosities as ion reservoirs for rapid ion diffusion and provided conductive networks to shorten the transport path for electron transfer.Besides,the PCSs exhibited high surface area(1302m2g-1),ultramicropores(0.54nm),rich micropores and mesopores with high N(7.97%)and O(10.16wt%)contents.Hence,the PCSs electrode showed a high specific capacitance up to344F g-1at1.0A g-1,remarkable rate capability,and long-term cycling stability.Notably,the PCS-based SCs exhibited an ultrahigh energy density of33.37Wh kg-1in Na2SO4electrolyte.Chen et al.[16]reported an encapsulated self-activation strategy to prepare highly ordered mesoporous carbon spheres(O-MCS-x)with precise tunable large pore sizes(Fig.4).The mesopore size distributed from3.1to10.0nm with specific surface area from696to1186m2g-1can be adjusted by simply increasing the amount of silica precursor.The long-range ordered mesoporous structure facilitated diffusion of guest molecules in the channels and exhibited great advantage in electrochemical applications.
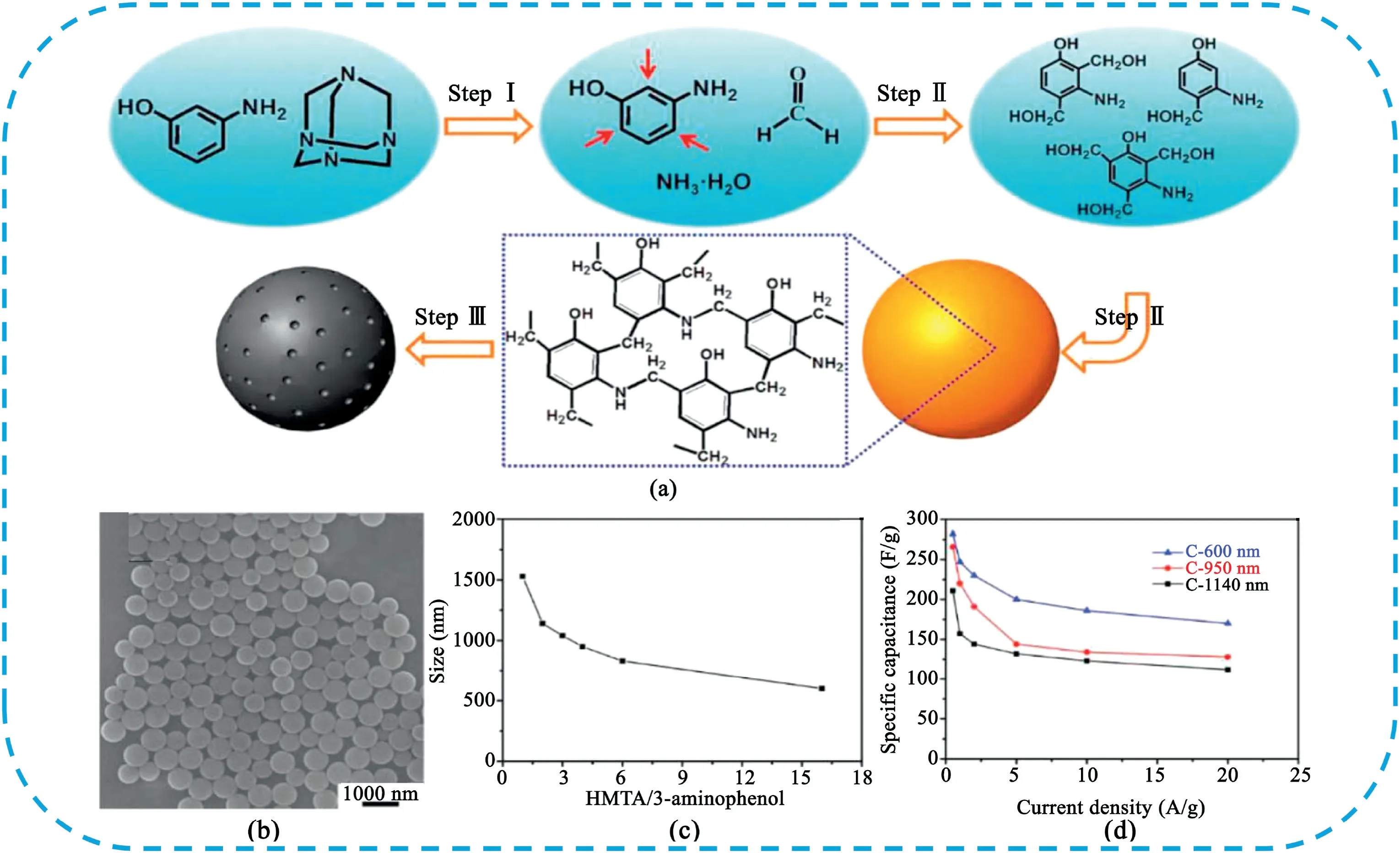
Fig.3.(a)Schematic illustration for the synthesis of monodisperse N-doped carbon spheres.(b)SEM image of N-doped carbon spheres.(c)Particle size as a function of HMTA/3-aminophenol ratio.(d)Specific capacitance of the N-doped carbon spheres as a function of current density.Copyright2017,WILEY-VCH Verlag GmbH&Co.KGaA.
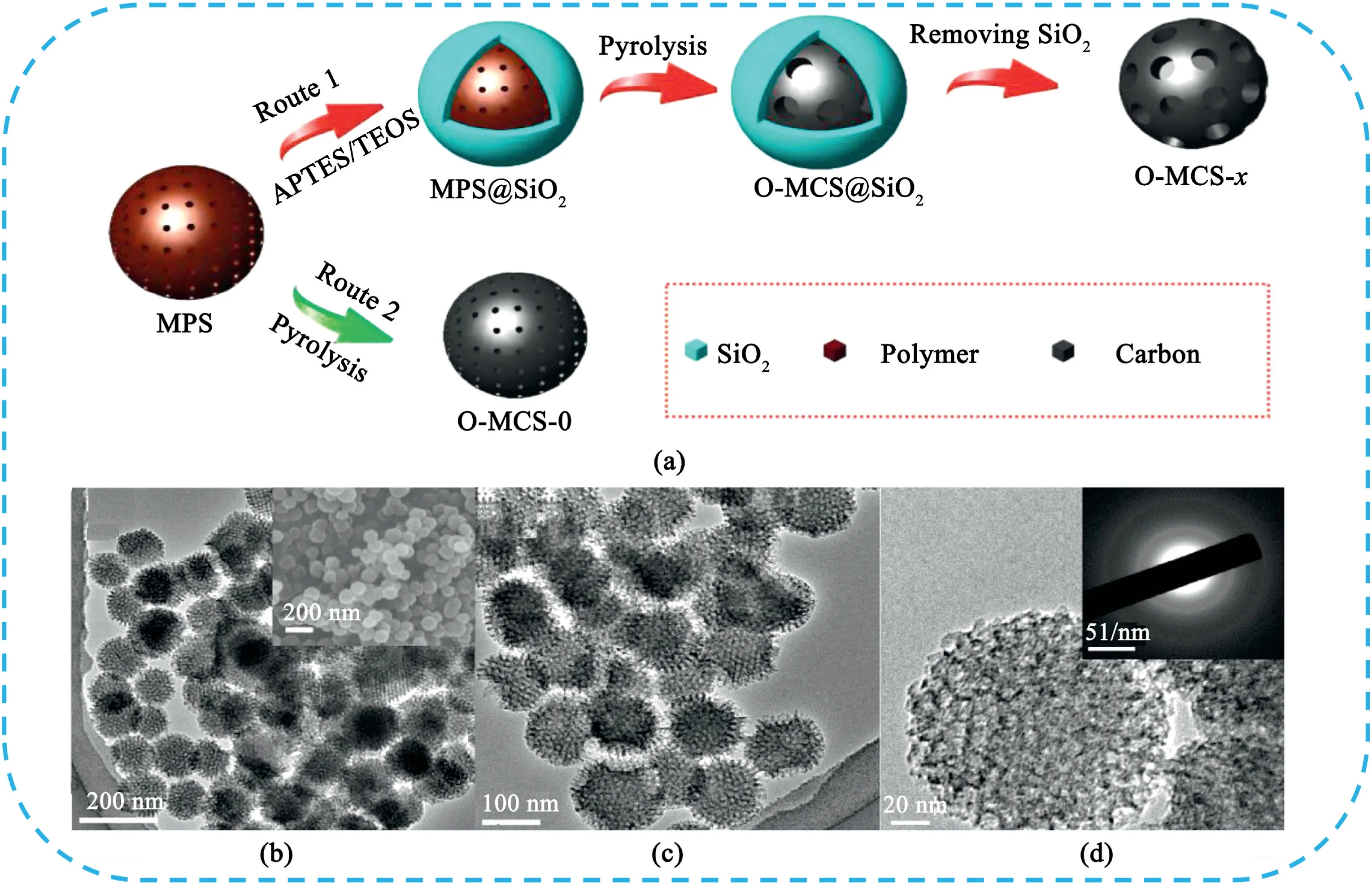
Fig.4.(a)Schematic illustrations of the synthesis of the O-MCS-x with tunable large mesoporous size.(b–d)TEM images of O-MCS-1,(inset of b)SEM image of OMCS-1,(inset of d)the selected area electron diffraction(SAED)patterns.Copyright2018,WILEY-VCH Verlag GmbH & Co.KGaA.
Hollow carbon spheres(HCSs)possess high surface-to-volume ratios and reduced transport path for both mass and charge transport and show promising applications as electrode materials for SCs[17–19].The porous carbon shell of carbon sphere enhances effective contact area between active material and electrolyte,which can reduce ions diffusion path and buffer the volume expansion/contraction during charging/discharging process.Yang et al.[20]used silica nanospheres as template and soluble phenol-formaldehyde resin as carbon source to prepare hollow core,mesoporous shell carbon nanospheres.The obtained samples had large mesopores and high surface area,resulting in high specific capacitance and long cyclic life.Chen et al.[21]prepared N-doped HCSs by pyrolysis of silica shell coated polystyrene/polyaniline composite.Regulating the pore size of silica shell can optimize the surface properties and structural parameters.The N-doped HCSs showed highly monodisperse hollow nanostructure with a diameter of410nm and a shell thickness of30nm(Fig.5).Benefiting from the thin shell,large mesopore size,high surface area and high nitrogen content,the obtained samples displayed a high specific capacitance of436.5F g-1at0.5Ag-1and good rate capability with capacitance retention of84.1% at10A g-1.Apart from template method,synthesis of hollow porous carbon by template-free method was also reported in the literature.For example,Du et al.[22]reported a template-free way to synthesize nitrogen-doped HCSs with tunable porous structure and nitrogen contents(from1.3%to7.4%)by the carbonization of homopolymer vesicle.The obtained sample exhibited high specific capacitance(266.9F g-1)and excellent rate performance(84%retention rate at20A g-1).
Besides,yolk-shell structured CSs(YSCSs)have attracted massive attention in recent years.The large void space between core and shell can act as a storage reservoir,and this unique structure can provide controlled pathways for fast mass transport[23–26].Zhao et al.[27]synthesized uniform YSCSs by a new gradient sol-gel process with surfactant-directing co-assembly using cationic surfactant cetyltrimethylammonium bromide(CTAB)as the template,resorcinolformaldehyde(RF)as carbon source and tetraethoxysilane(TEOS)as assistant pore-forming agent.The obtained YSCSs possessed microporous yolk,mesoporous shell and macroporous hollow cavity between the yolk and shell,high specific surface areas(887–1085m2g-1)and large pore volumes(1.4–2.8cm3g-1).Moreover,the yolk diameter can be well controlled from320to600nm,the shell thickness from140to250nm and the mesopore size from4.6to10.6nm by simply adjusting the amount of CTAB and TEOS.The YSCSs electrode exhibited high performance with high specific capacitance and good rate capability.Chen et al.[28]developed a facile confined pyrolysis strategy for the direct conversion of solid resin spheres into YSCSs.A silica shell with different degrees of openness was built on the surface of a solid resin sphere to form a confined nanospace and thus the volatile gases generated in the process of pyrolysis to form a tunable yolk-shell structure(Fig.6).As an electrode material for SCs,the YSCSs electrode exhibited a high specific capacitance of330F g-1at0.5A g-1and excellent stability with88.5%initial capacitance retention after10,000cycles.
The design of hollow and yolk-shell structure can reduce transport path for both mass and charge transport,improving the utilization efficiency of specific surface area,which can greatly improve the electrochemical performance.However,the complex preparation process limits their extensive applications.
2.2.Carbon quantum dots
Carbon quantum dots(CQDs)are the typical0D carbon nanomaterials with a particle size less than10nm in all3D direction.CQDs have attracted considerable attention in recent years due to their unique characteristics such as tunable band gap,ultra-small sizes,high electron mobility,abundant active edge sites and better amenability for hybrid with other nanomaterials[29].
Over the past few years,CQDs as electrode materials for SCs have shown attractive properties and attracted more and more attention[30–34].Zhu et al.[34]fabricated CQDs with a layer structure by reacting C60molecules with excess KOH.The as-obtained CQDs electrode displayed high volumetric capacitance of157.4F cm-3and high areal capacitance of0.66F cm-2.Qu et al.[31]used resorcinol and formaldehyde as organic precursors to build the basic polymer gel framework and assembled CQDs in a sol-gel polymerization,followed by pyrolyzing the wet CQD gels under argon.The obtained CQD aerogel had a high specific surface area of1027m2g-1,and the symmetrical SCs based on the CQD aerogel electrodes showed a high capacitance of294.7F g-1at 0.5A g-1,20times higher than that of the SCs based on the CQD-free aerogel.
Apart from as active material,CQDs can act as excellent conductive substrates combined with pseudocapacitive materials,such as NiCo2O4[35,36],MnO2[37–39],NiO[40],CuS[41],Ni(OH)2[42–44],to enhance the conductivity of composite material,thus resulting in enhanced electrochemical performance.Ji et al.[35]reported that CQDs decorated in porous NiCo2O4sphere and the composite electrode showed high specific capacitances and good rate capability with60.8%retention rate at100A g-1.Xia et al.[39]reported that CQDs serve as the bridge for connecting MnO2and graphene to synthesize the stable MnO2/CQDs/graphene composite aerogel.The composite electrode displayed high specific capacitance(721F g-1at1A g-1),good rate capability(89.2% at20A g-1)and remarkable cycle stability(92.3%after10,000cycles).
2.3.Graphene quantum dots
Graphene quantum dots(GQDs)are highly crystallized single or fewlayer graphene derivations with a0D structure.They have the unique properties of both graphene and quantum dots due to the pronounced quantum confinement and edge effects[45,46].Owing to their good conductivity,large surface area,and superior mechanical property,GQDs have been considered as a promising electrode material for SCs[47,48].Yan et al.[46]prepared GQDs by a simple electro-deposition method and used them as electrode materials to assemble symmetric micro-SCs(Fig.
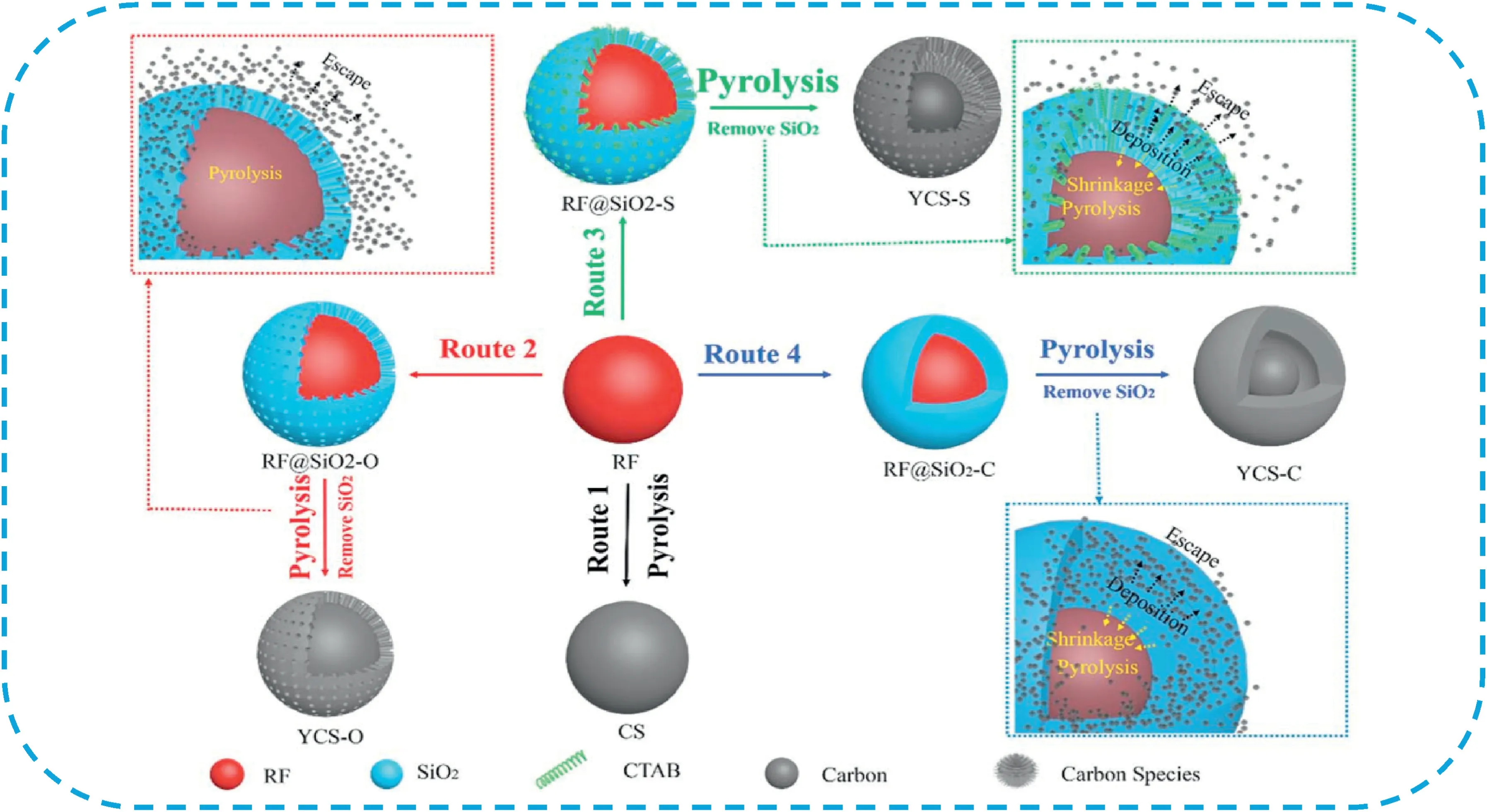
Fig.6.Schematic illustration of the fabrication processes of YSCSs under different confined conditions.Copyright2019,Royal Society of Chemistry.
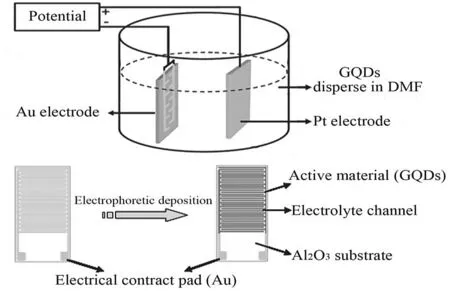
Fig.7.Schematic illustration of the electrophoretic deposition of GQDs on the interdigital finger electrodes to prepare a GQDs//GQDs symmetric microsupercapacitor
Fig.5.(a–c)TEM images of N-doped HCSs.Copyright2019,WILEY-VCH Verlag GmbH & Co.KGaA.7).The symmetric micro-SCs showed superior rate capability up to 1000V s-1,excellent power response and cycle stability.Zhang et al.[49]synthesized ultramicroporous carbons puzzled by GQDs as the building units by chemical welding and in situ activation.The resulted carbon had a unique ultramicroporous structure with both high surface area(1730m2g-1)and packing density(0.97g cm-3).The obtained electrodes achieved high gravimetric and volumetric capacitances of 270F g-1and262F cm-3at1A g-1,respectively.More importantly,such carbon achieved an ultrahigh areal capacitance of5.70F cm-2with a high mass loading of25mg cm-2at1A g-1.
Moreover,GQDs have highly defective oxygen-containing groups and thus make them play an important role in integrating with other active materials to improve the conductivity of composites.Accordingly,various composite materials such as GQDs/activated carbons[48],GQDs/MnO2[50],GQDs/carbon fibre[51],GQDs/Ni(OH)2[52],GQDs/NiCo2O4[53],GQDs/graphene[54]and GQDs/polypyrrole[55]have been prepared for high performance SCs electrodes.These materials performed improved capacitances and rate performances due to good conductive GQDs shells.
GQDs have rich edge sites and surface functional groups,which can provide a large number of active sites for the ion storage with high capacitance.However,the rich surface functional groups of GQDs make it very dispersive in electrolyte,and it is easy to fall off from the collector,resulting in the degradation of energy storage performance.Moreover,the complex preparation process and high production cost restrict the application of GQDs in supercapacitors.
3.1D carbons
1D carbon materials have a high aspect ratio and good electron transport.As the electrode materials for SCs,they can shorten the transmission/diffusion pathways of electrons and ions,provide large specific surface area,and effectively promote the kinetics of electrochemical reaction.Therefore,1D carbon materials become a promising candidate for achieving high rate and power density SCs.1D carbon materials mainly include carbon nanotubes(CNTs),carbon nanofibers(CNFs),graphene nanoribbons(GNRs)and carbon cloth(CC).
3.1.Carbon nanotubes
CNTs have attracted extensive interest as electrode materials for SCs due to their high conductivity,good mechanical properties,unique large accessible mesopore and high aspect ratio[56,57].Their specific capacitance can reach up to about50μF cm-2,which is higher than those of porous carbon(20–50μF cm-2)owing to the perfect electrolyte accessibility by the tube entanglement.Nevertheless,their gravimetric specific capacitances are still unsatisfactory due to the low surface area(less than500m2g-1)and hydrophobic property[58,59].
Several strategies increase the specific surface area or the surface functionality have been done to enhance the specific capacitance of CNTs[58,60–62].The treated CNTs can not only significantly increase the specific surface area with maintaining the nanotubes-like structure,but also induce oxygen functional groups to generate extra pseudocapacitance.Our groups[58]utilized KMnO4etching CNTs followed by low temperature treatment reduction to form functionalized graphene nanosheet/carbon nanotube networks(Fig.8).Benefiting from the unique hybrid structure consisting of1D carbon nanotubes as the continuous conductive networks and2D functionalized graphene nanosheets as pseudocapacitive materials,the optimized samples(G/CNTs-200)exhibited a high specific capacitance of202F g-1(five times higher than that of pure CNTs).Lee et al.[61]directly synthesized CNTs on the Ni substrates by plasma-enhanced chemical vapor deposition(CVD)of methane and hydrogen.The specific capacitance of the CNT electrode increased from38.7to207.3F g-1by surface treatment using ammonia plasma.Dai et al.[60]utilized plasma activation to open the end tips of aligned carbon nanotubes,introduce massive defects and obtained a higher specific surface area of400m2g-1.Due to the combined contribution from double layer capacitance and redox pseudocapacitance,the treated carbon nanotubes showed ultrahigh capacitance(440F g-1),high energy density(148Wh kg-1)in a wide voltage range(4V)in ionic liquids electrolyte.
Compared with randomly entangled CNTs,aligned CNTs(ACNTs)have attracted massive attention as electrode materials for SCs due to the relatively regular pore structures and conductive channels,high specific surface area,vertically aligned structures with well spacing,fast ion and electron transportation[60,63–68].Hata et al.[63]synthesized vertically ACNT forests with high specific surface area(1300m2g-1)by water-assisted CVD.As the durable electrodes for symmetric SCs,they could be operated at a high voltage(4V)while maintaining full charge/discharge cycle ability with an energy density(94Wh kg-1,47Wh L-1)and power density(210kW kg-1,105kW L-1).Fedorovskaya et al.[65]synthesized vertically ACNTs arrays on N-doped silicon substrates using an aerosol-assisted catalytic CVD.Electrochemical properties of the electrodes were significantly dependent on the array thickness.The electrode achieved large specific capacitance of180F g-1at2mV s-1.Peng et al.[69]developed a nitrogen-doped core-sheath CNTs(NCNTs)array as a flexible electrode for SCs(Fig.9).The NCNTs electrode demonstrated a high specific capacitance of31.1mF cm-2.The specific capacitance has been maintained98.9% after stretching to a strain of 400%and96%after stretching for1000cycles at a strain of200%.
Moreover,due to the unique structure,CNTs can be assembled into films for application in flexible and lightweight energy-storage systems to meet the demands for portable electronic device.CNTs printed on different substrates such as plastic films,cellulose fiber paper,sponges and cotton textiles to assemble thin and flexible SCs[70–74].Hu et al.[75]reported that SCs based on CNT conductive paper delivered a specific capacitance of200F g-1and an energy density of30–47Wh kg-1,a power energy of200kW kg-1,and a stable cycling life over40,000cycles.Peng et al.[74]developed ACNTs sheets wrapped on an elastic fiber as two electrodes.As a result,the fiber shaped SCs showed a high specific capacitance of18F g-1after stretched by75%for100cycles.Wu et al.[73]reported a compressible and deformation-tolerant SCs based on CNTs sponges consisting of highly porous conductive networks.In aqueous electrolyte,the sponges maintained a similar specific capacitance(>90%of original value)under a predefined compressive strain of 50%,and retained more than70% of original capacitance under80%strain while the volume normalized capacitance increases by3-fold.In spite of numerous great achievements have obtained,a number of problems still need to be addressed to further promote the industrial application of CNTs,including the limited SSA,highly variable purity,high production cost,and limited availability.
3.2.Carbon nanofibers
CNFs have attracted significant attention due to their good conductivity,high surface area,excellent flexibility,structural stability.CNFs with the efficient1D electron-transport path along the axial direction and short ionic-diffusion distance along the radial direction are particularly attractive,which can act as promising electrode materials for SCs[76–78].CNFs with various fiber diameters,porosities,and surface chemistries have been developed by different methods,such as,electrospinning,template directed hydrothermal carbonization and direct pyrolysis of biomass[79].
Electrospinning technology is a simple and general method to prepare CNFs[80,81].By controlling the experimental conditions,such as precursor solution concentration,spinning voltage,receiving distance,temperature and humidity,can obtain the polymer and its composites with adjustable size and composition.After the subsequent high-temperature heat treatment and thus people can obtain the CNFs.The commonly used precursor polymers are mainly including polyacrylonitrile(PAN),polyvinyl alcohol(PVA),polymethylmethacrylate(PMMA),phenolic resin,and cellulose.The conventional electrospinning CNFs is confronted with low conductivity and specific surface area,which results in low specific capacitance and poor rate capability.Currently,the main methods to improve the performance of CNFs based electrode materials mainly including enhance the graphitization degree,adjust pore structure and introduce heteroatoms[82–84].Xie et al.[85]fabricated a highly flexible porous CNFs using electrospinning technique combining with metal ion-assistant acid corrosion process.The graphitization degree of CNFs network can be effectively improved due to the catalysis of Co metal during the carbonization process.At the same time,the thermal decomposition of metal salts generally produced volatile gases and created porous structures,thus increased the specific surface area.The resultant CNFs electrode displayed a specific capacitance of 104.5F g-1at0.2A g-1,good rate capability and electrochemical stability.Through physical/chemical activation or template introduction to adjust the pore structure of CNFs and thus the specific surface area can be significantly increased.Hou et al.[86]prepared hollow CNFs with hierarchical porous shells by the pyrolysis of electrospinning polyureasilazane(PSN)/polyvinylpyrrolidone(PVP)precursor fibers,followed by carbonization treatment and further NaOH activation(Fig.10).The obtained samples possessed a high BET specific surface area of2628.10m2g-1,a large pore volume of2.32cm3g-1.As an electrode,it delivered a high specific capacitance of330.11F g-1at 1A g-1and still maintained65.3%of its original specific capacitance at 20A g-1.Jang et al.[83]fabricated nitrogen/fluorine co-doped mesoporous CNFs by facile electrospinning,hydrothermal and carbonization methods.The prepared electrode showed a superior specific capacitance of252.6F g-1at0.5A g-1.Moreover,different types of polymer precursors were used to improve the performance of CNFs electrodes.Different types of polymer precursors can also be selected to improve the electrochemical performance of CNFs.Moreover,construction of the hierarchical multi-level structure and the introduction of other electroactive materials can improve the specific surface area,pore structure,conductivity and surface chemistry of CNFs,and then greatly improve the electrochemical performance.
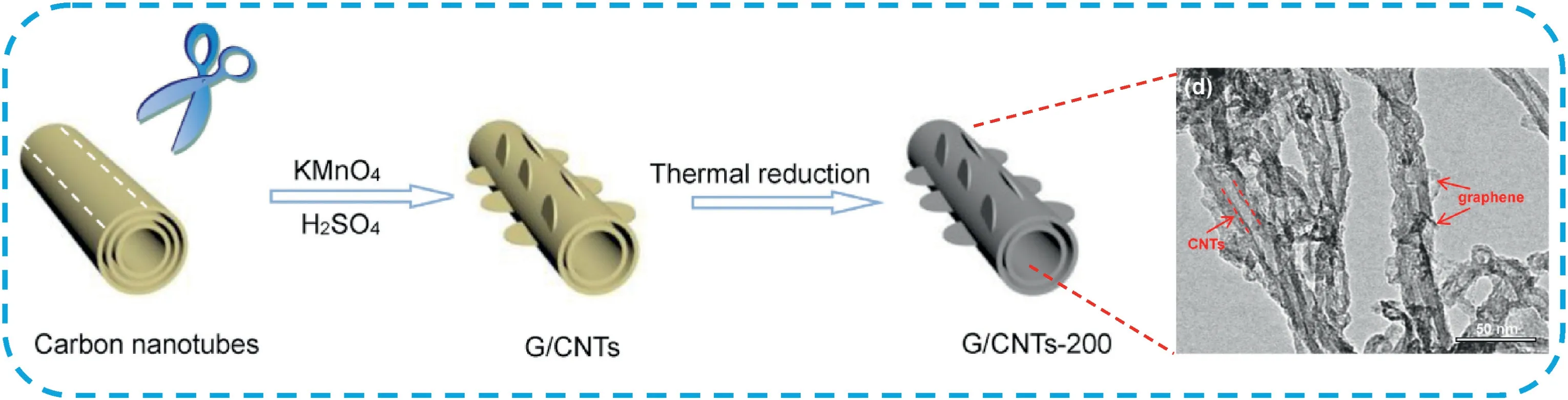
Fig.8.Schematic illustration of the synthetic route of G/CNTs-200and TEM image of G/CNTs-200.Copyright2018,Elsevier.
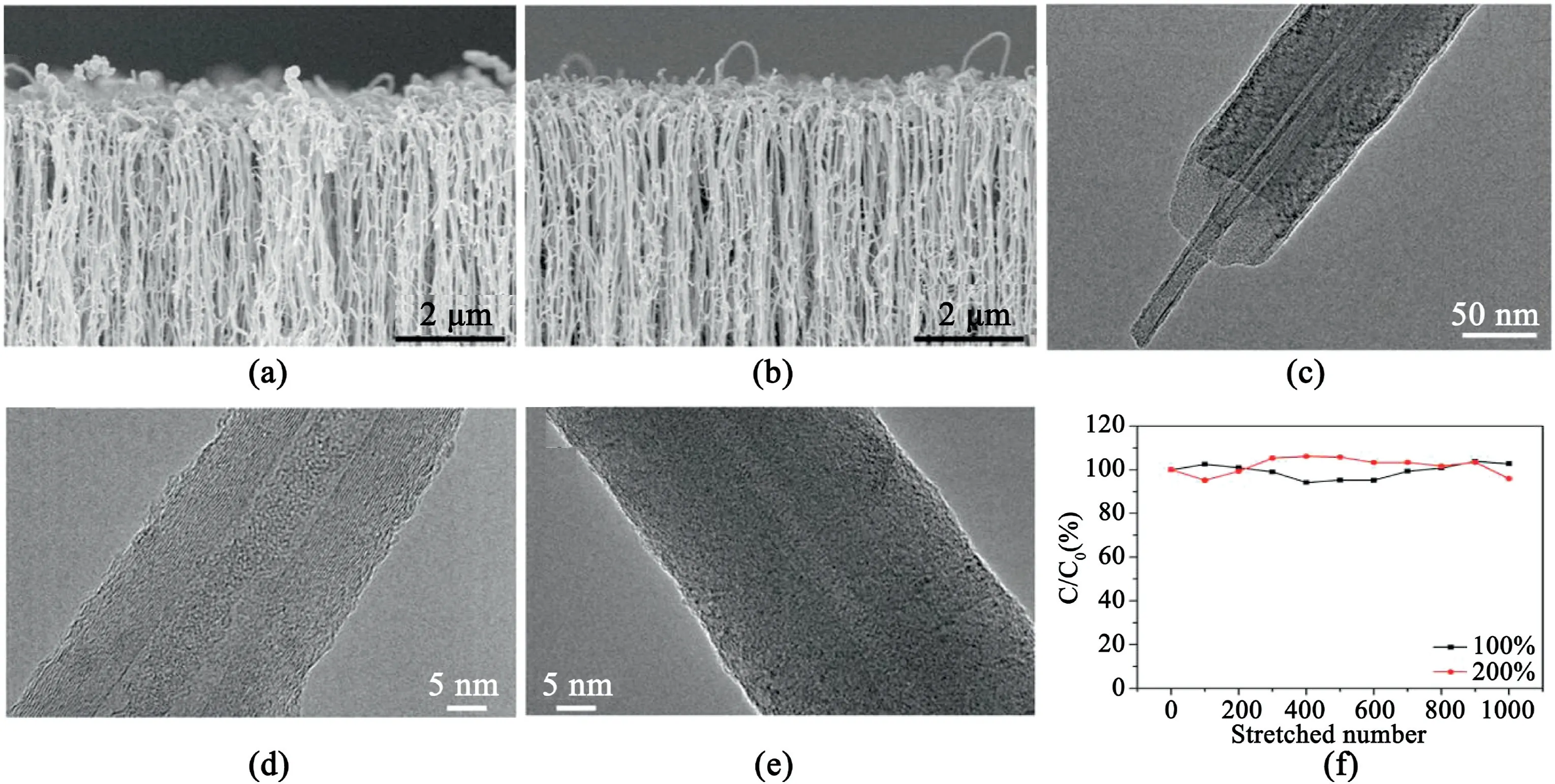
Fig.9.SEM images of NCNT re-grown for(a)15min,(b)20min TEM images of NCNTs re-grown for(c)20min,(d)15min,(e)20min,(f)Dependence of specific capacitance on stretched cycle number.C0and C correspond to specific capacitances before and after stretching,respectively.Copyright2017,WILEY-VCH Verlag GmbH & Co.KGaA.
Inspired by the confined synthesis in channels of anodic alumina(AAO),much efforts have been devoted to synthesize porous CNFs by carbonizing precursors within suitable templates[87–90].Zhi et al.[87]synthesized1D mesoporous CNFs by a simple precursor controlled thermolysis approach using AAO membranes as template and alkyl-substituted polyphenylene as carbon precursor.The obtained samples were composed of a highly porous carbonaceous network with large-sized mesopores(10–20nm)interconnected to each other,which formed a unique3D framework with a surface area of more than 1140m2g-1.The optimized samples showed a high specific capacitance of304F g-1at5m V s-1and good rate characteristic.Zhao et al.[88]reported a self-template way to synthesize mesoporous CNFs using ethylene glycol as the carbon precursor and Zn(CH3COO)2as template precursor.As the electrode materials,the specific capacitance was up to 280F g-1at0.5A g-1.Yu et al.[91]synthesized nitrogen-doped carbonaceous nanofibers(N-CNFs)aerogels by a template-directed hydrothermal carbonization process using ultrathin tellurium nanowires(TeNWs)as templates and nitrogen-containing carbohydrates as carbon source and followed by high-temperature treatment and CO2activation(Fig.11).The treated N-CNFs possessed high conductivity and porosity,resulting in an outstanding performance with a specific capacitance of 182.3F g-1at2A g-1.Yang et al.[90]prepared N-enriched mesoporous carbon nanofibers(NMCNFs)by an electrospinning technique using graphitic carbon nitride(g-C3N4)nanosheets as both sacrificial template and N-doping source.The obtained NMCNFs film had a high N-doping level of8.6wt%,a high specific surface area of554m2g-1with an improved specific capacitance of220F g-1.
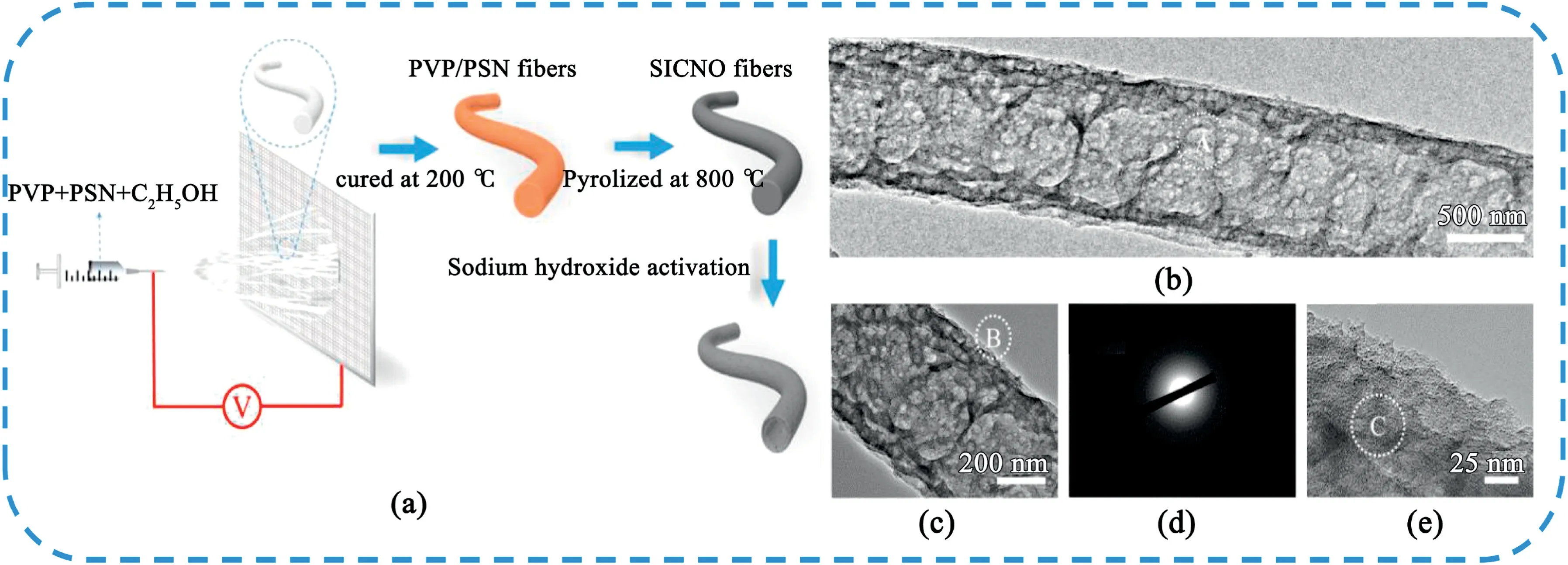
Fig.10.Schematic illustration of the preparation of porous hollow CNFs.(b,c)Typical TEM images of the hollow CNFs under different magnifications.(d)Typical SAED pattern recorded from the marked area of A in part a.(e)Typical HRTEM image obtained from the marked area of B in part b.Copyright2020,American Chemical Society.
Many natural biomass materials possess fibrous structures,for example,ramie[92],cotton[93],alginate[94],stalk[95],chitin[96],agar[97],bacterial cellulose(BC)[98–103]and lignin[104],and thus biomass-derived CNFs usually can be obtained by carbonization of biomass precursor.After further activation or heteroatom doping,the electrochemical performance of CNFs can be greatly enhanced.Lei et al.[93]developed a facile way to prepare CNFs aerogel by carbonization of cotton with followed KOH activation.The optimized CNFs aerogel had a specific surface area of2307m2g-1,good electrical conductivity(about 860S m-1).As an electrode material,it displays a high specific capacitance of283F g-1at1A g-1and excellent rate characteristic(224F g-1at100A g-1).Our groups[98]reported that the synthesis of nitrogen-doped carbon networks through the carbonization of polyaniline(PANI)coated BC.The obtained carbon nanofiber networks can act not only as support for obtaining high capacitance electrode materials such as activated carbon(AC)and carbon/MnO2hybrid material,but also as conductive networks to integrate active electrode materials(Fig.12).The assembled AC//carbon-MnO2asymmetric SCs displayed a high energy density of63Wh kg-1in1.0M Na2SO4aqueous solution.Xia et al.[97]reported that the residue of gelidium amansii after extraction from agar to fabricate nanofibrilated cellulose by a facile ultrasonication treatment.Then the N-doped CNF aerogel was developed by freeze-drying,pyrolysis in NH3and further activation.The obtained samples possessed hierarchically porous structure with high surface area(2290m2g-1),N-doping,and3D interconnected channels,which were beneficial to electrolyte ions and electron transportation.
3.3.Graphene nanoribbons
Compared with graphene,GNRs exhibits better electronic conduction and have more edge planes or defect sites.They are a kind of quasi-onedimensional carbon nanomaterials with excellent conductivity and thermal conductivity[105–107].Preparation methods of GNRs can be mainly divided into two categories;one is physical methods mainly includes micro-mechanical stripping,etching and so on.Another is chemical methods mainly including vapor deposition,organic synthesis and so on.Moreover,the preparation of GNRs from carbon nanotubes is also an interesting idea[108].Preparation methods,advantages and disadvantages have been systematically summarized in some previous literature[109].Sharma et al.[105]fabricated lacey reduced graphene oxide nanoribbon(GONR)by chemically unzipping the CNTs using strong oxidizing agent with further KOH activation and reduction process.The specific capacitances of GONR were1042,1272,and1324F g-1in H2SO4,TEABF4/AN and BMIMBF4electrolytes in a three-electrode system,respectively.Our groups[110]reported a strategy to synthesize free-standing graphene ribbon films(GRF)by blade-coating of interconnected graphene oxide ribbons and a subsequent thermal treatment process(Fig.13).Even with an ultrahigh mass loading of21mg cm-2,the compressed multilayer-folded GRF films delivered a high areal capacitance of6.7F cm-2at5mA cm-2,high gravimetric/volumetric capacitances(318F g-1,293F cm-3),and excellent rate characteristic(3.9F cm-2at105mA cm-2),as well as outstanding electrochemical stability(109%of capacitance retention after40,000cycles).
3.4.Carbon cloth(CC)
CC made up of numerous uniform carbon microfibers,is an inexpensive and highly conductive textile with good mechanical flexibility and strength[111–113].Nevertheless,pristine CC is confronted with low surface area(~5m2g-1)and poor electrochemical activity,which results in low specific capacitance(<5F g-1)[111,114].To improve the electrochemical properties of CC,chemically activated and electrochemical activated,as well as plasma functionalized were used to enhance its electrochemical activity,specific surface area and surface state[112,115,116].Li et al.[111]activated CC to improve the surface area by controlled exfoliation of carbon fibers using Hummers’method with followed hydrazine reduction and annealing.TEM image(Fig.14)revealed that each activated carbon fiber formed a core-shell structure with a high surface area of61.2m2g-1.The activated carbon fiber showed a higher specific capacitance than untreated CC and activated CC reduced by hydrazine.Compared with chemical activation,it needs a complex preparation process and consumes considerable time.Electrochemical activation can effectively improve the electrochemical performance of CC without complicated steps and severe conditions.Lu et al.[116]reported a one-step electrochemical activation strategy to oxidize CC in a mixed acid solution with a potential of3V for10min.The as-obtained electrode displayed a high areal capacitance of756mF cm-2with superior rate capability and outstanding cycling durability.Moreover,an asymmetric SC achieved a stable cell voltage of2.0V using the as-obtained electrode as anode and TiN@MnO2as cathode,which delivered an ultrahigh energy density of1.5mWh cm-3.Wang et al.[114]demonstrated a facile high-temperature annealing process can greatly improve the capacitance of CC.The improvement stems from ultramicropores effects in the range of0.46–0.64nm besides superhydrophilicity and high specific surface area.The assembled flexible solid-state device delivered an area capacitance of920mF cm-2and an energy density of128μW h cm-2.
4.2D carbons

Fig.11.(a)Schematic illustration of the synthetic steps.(b)SEM image of the N-CNFs aerogels.Copyright2015,Elsevier.
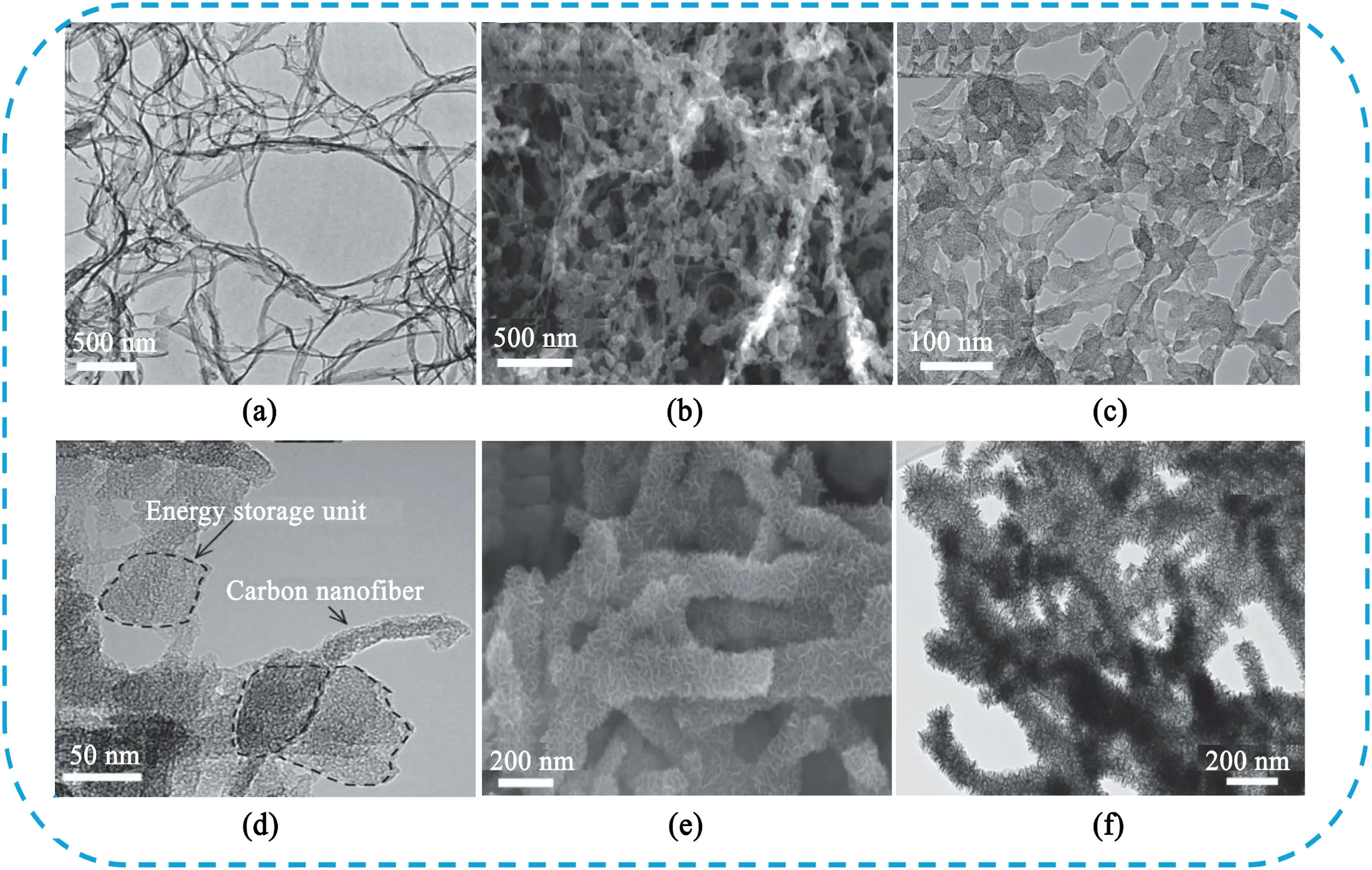
Fig.12.(a)TEM image of BC.(b)SEM image of carbonization of BC/PANI hybrid material.(c)TEM image of carbonization of BC/PANI hybrid material(c-BP).(d)TEM image of activation of c-BP.(e)SEM image of c-BP/MnO2hybrid material.(f)TEM image of c-BP/MnO2hybrid material.Copyright2014,WILEY-VCH Verlag GmbH & Co.KGaA.
2D carbon materials possess large open layer structure with high surface area,high aspect ratio,excellent electronic transportation properties,enabling them as promising candidates for SCs[117,118].More importantly,2D carbon materials of individual sheets do not depend on the distribution of pores in a solid support to give them their high surface areas,which is instrumental in the improvement of electrode kinetics by reducing ion-transport resistance and diffusion distance[119].2D carbon materials mainly include graphene and porous carbon nanosheets(PCNs).
4.1.Graphene
As a typical2D carbon material,graphene has drawn a great deal of attention as electrode materials for SCs due to its high theoretical specific surface area,excellent conductivity and high mechanical strength and chemical stability.Since Ruoff et al.[120]firstly reported that using chemically modified graphene as electrode materials for SCs.Graphene as electrode material for SCs has attracted extensive attention in recent ten years.Unfortunately,graphene suffers from the irreversible re-stacking or agglomeration in the bulk state due to the intensive mutual attraction of layer van der Waals force,which result in a significant loss of specific surface area and electrochemical activity.Currently,the main ways to improve the electrochemical characteristic of graphene mainly includes heteroatom doping,pore design and structure design.
Heteroatom doped graphene materials have been demonstrated to possess unique physical and chemical properties,resulting in superior capacitive performance by changing the electronic state of graphene layer or introducing pseudocapacitance[121].Up to now,the doping of different atoms,such as O[122,123],B[124],N[125–129]or polyatomic co-doping[130–133]into the graphene have been reported.In particular,oxygen and nitrogen doped graphene has attracted more attention.Our group[134]demonstrated that the synthesis of functionalized graphene nanosheets by low temperature(300°C)treatment of graphite oxide using Mg(OH)2nanosheets as template.Benefiting from the dented sheet with high surface area,suitable oxygen-containing groups,and low pore volume,the obtained graphene displayed both high specific gravimetric and volumetric capacitances of456F g-1and 470F cm-3,almost3.7times and3.3times higher than hydrazine reduced graphene(Fig.15).
Moreover,N doping is the most promising method due to the easy preparation methods.Four types of nitrogen-containing functional groups have been incorporated into graphene to enhance capacitive performance:pyridinic N(N-6),pyrrolic N/pyridine N(N-5),quaternary N(N-Q),and pyridine-N-oxide(N-X).It has been reported that N-6and N-5are mainly attributed to providing pseudocapacitance through redox reactions involving protons,whereas N-Q can improve the conductivity of carbon.Feng et al.[127]reported a facile method to synthesize highly crumpled nitrogen-doped graphene nanosheets(C-NGNSs)with a pore volume as high as3.42cm3g-1.The C-NGNSs exhibited significant improvement in terms of various performance parameters of SCs owing to the abundant wrinkled structures,high pore volume,nitrogen doping,and improved electrical conductivity.Qu et al.[135]fabricated a versatile,ultralight,nitrogen doped,3D graphene framework with a high capacitance of484Fg-1.Park et al.[129]synthesized3D,nitrogen doped reduced graphene oxide(N-RGO)monoliths using graphene oxide and melamine through an ice-templated assembly.The obtained N-RGO showed a specific capacitance of217F g-1,which is approximately three times higher than that of the pristine3D RGO due to the pseudocapacitive contribution of N-RGO.
Since B doping increases charge carrier concentration can enhance the intrinsic electric conductivity of the doped carbons,resulting in smaller equivalent series resistance[136].Park et al.[137]developed a novel B-doped nanoplatelet(borane-reduced graphene oxide,B-RGO)by the reduction of graphene oxide using a borane-tetrahydrofuran adduct under reflux.The B-RGO had a high specific surface area of466m2g-1and showed a high specific capacitance of200F g-1and good electrochemical stability.
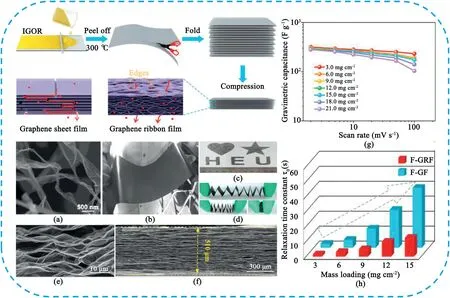
Fig.13.Preparation of multilayer-folded graphene ribbon film(F-GRF).(a)SEM image of the interconnected graphene oxide ribbons(IGORs).(b)A photograph showing a large-area IGOR film through blade-coating method.(c,d)Photographs of the GRF film exhibiting good flexible characteristic.The film can be repeatedly folded or cut into the desired shapes.(e,f)Cross-sectional SEM images of a GRF film and F-GRF after releasing pressure(10MPa).(g)Gravimetric capacitances of the F-GRF films with different mass loadings at different scan rates.(h)relaxation time constant(τ0)of the F-GRF and F-GF films with different mass loadings.Copyright 2018,WILEY-VCH Verlag GmbH & Co.KGaA.
Compared with single heteroatom doping that improves merely one aspect of properties,co-doping can enhance the overall performance of the materials owing to the synergetic effect[138].Feng et al.[139]demonstrated a simplified prototype device of high-performance all-solid-state SCs based on3D nitrogen and boron co-doped monolithic graphene aerogels(BN-GAs).Compared with the single boron(55F g-1)or nitrogen(52F g-1)doped monolithic graphene aerogels,the BN-GAs all-solid-state SCs delivered a higher specific capacitance of64F g-1.Jia et al.[140]reported that the synthesis of nitrogen and sulfur co-doped graphene(NSG)by hydrothermal way using methionine as doping agent and GO as precursor.When used as a free-standing electrode in SCs,the specific capacitance of NSG achieved431F g-1at 0.5A g-1owing to high specific surface area and pseudocapacitance provided by N and S functional groups.
Through the design of the porous structure can reduce the resistance of ion diffusionand increase the specific surface area,thus greatly improve the electrochemical performance of graphene[141–147].Due to the unique2D structure of graphene,there are two main methods to prepare porous graphene.One is the direct fabrication of porous graphene sheets,namely by introducing defective micropores,mesopores or macropores into the basal planes of graphene.On the other hand,the graphene framework with three-dimensional macropores structure by stacking and self-assembly of graphite nanosheets.Here,we classify it as structure design for a detailed review.Up to now,several ways have devoted to synthesize porous graphene,mainly including template approach and chemical activation.Lei et al.[148]reported a convenient CVD method to prepare highly porous graphene using flake-like MgO as template and ferrocene as carbon precursor.The graphene layers possessed a highly porous structure with small mesopores(4–8nm),large mesopores(10–20nm)and additional macropores(100–200nm).Benefiting from the unique structure,the electrode showed a specific capacitance of303F g-1and an areal capacitance up to17.3mF cm-2.Zhao et al.[149]synthesized continuously hierarchical nanoporous graphene(HPG)films by a combination of low-temperature CVD growth of hydrogenated graphite coating on nanoporous copper(NPC)and rapid catalytic pyrolysis of hydrogenated graphite(HG)at high temperature(Fig.16).The obtained HPG films possessed a high specific surface area of1160m2g-1with small pore size(1–150nm),resulting in fully accessible channels for ion transport.A specific capacitance of 38.2F cm-3and excellent rate performance based on the device was achieved.
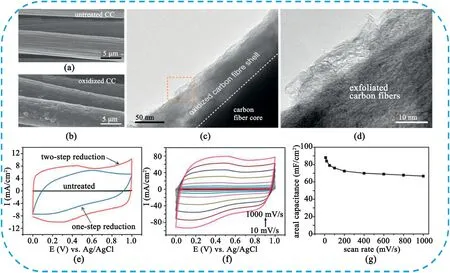
Fig.14.SEM images of(a)untreated and(b)oxidized CC substrates.(c)Low magnification TEM image collected at the edge of the oxidized carbon fiber.(d)Highresolution TEM image of the exfoliated carbon fibers.(e)CV curves of for untreated CC(black)and activated CC that is reduced by hydrazine(blue,one-step reduction process)and followed by annealing in ammonia(red,two-step reduction process)at100mV s-1.(f)CV curves of activated CC at different scan rates.(g)Calculated areal capacitance of activated CC at different scan rates.Copyright2014,WILEY-VCH Verlag GmbH & Co.KGaA.

Fig.15.(a,b)TEM of the FGN-300(inset:SAED of FGN-300).(c)Gravimetric capacitance of FGN-300,FGN-650,thermal exfoliated graphene sheet(TEGS)and hydrazine hydrate reduction graphene(RGO)electrodes at different current densities.Copyright2014,American Chemical Society.
Chemical activation of graphene is an effective way to prepare porous graphene materials from graphene sheets without the assistance of template.Ruoff et al.[150]firstly developed an activation process of microwave-exfoliated GO(MEGO)using KOH as activator.The obtained materials achieved a high specific surface area of3100m2g-1,a high electrical conductivity of~500S m-1,and a low oxygen and hydrogen content.The MEGO-based two-electrode symmetrical SCs delivered a specific capacitance of166F g-1at5.7A g-1in1-butyl-3-methyl-imidazolium tetrafluoroborate/acetonitrile(BMIM BF4)/AN.A high energy density of70Wh kg-1and a high power density of250kW kg-1were achieved in the voltage range of0–3.5V.Subsequently,the effect of activation conditions on the electrochemical properties of graphene was studied by Ruoff[151].The optimized samples possessed a specific surface area of3100m2g-1and a high specific capacitance of172F g-1in an organic electrolyte.Our groups[144]reported an easy synthesis of porous graphene nanosheets(PGNs)using the etching of graphene sheets by MnO2.The PGNs possesses a high specific surface area of1374m2g-1with massive mesopores.The PGNs electrode exhibited a specific capacitance of241F g-1at2mV s-1,superior rate performance(157F g-1at500mV s-1)in6M KOH.Duan et al.[152]developed a one-step process with simultaneous low temperature etching of nanopores in graphene and self-assembly of graphene into3D network structures(Fig.17).During the hydrothermal process,GO sheets were reduced and self-assembled into hydrogels with an interconnected3D macroporous network with massive macropores.H2O2molecules can partially oxidize and etch the carbon atoms around more active defect sites of GO,leaving behind carbon vacancies,which gradually extend into nanopores.Due to the large ion-accessible surface area,efficient electron and ion transport pathways as well as a high packing density,the holey graphene framework(HGF)electrode displayed a gravimetric capacitance of298F g-1and volumetric capacitance of212F cm-3in organic electrolyte.A fully packaged device showed gravimetric and volumetric energy densities of35Wh kg-1and49Wh L-1,respectively.Li et al.[153]synthesize a3D reduced graphene oxide hydrogel(RGH)prepared by hydrothermal reaction using NaI as activation agent followed with thermal treatment.Such material has interlayer pores between reduced graphene oxide nanosheets and in-plane pores and possessed a specific surface area up to835m2g-1and a high powder conductivity up to400S m-1.As binder-free SCs electrode,it shows a specific capacitance of169F g-1(21μF cm-2)and a power density of 7.5kW kg-1in6M KOH aqueous electrolyte.
More recently,Guo et al.[154]designed a freestanding graphene laminate film electrode with highly efficient pore utilization for compact capacitive energy storage(Fig.18).The interlayer spacing of the film can be precisely adjusted,which enables a tunable porosity.By systematically tailoring the pore size for the electrolyte ions,pores were utilized optimally and thereby the volumetric capacitance is maximized.Consequently,the fabricated SCs delivered a stack volumetric energy density of 88.1Wh L-1in an ionic liquid electrolyte,representing a critical breakthrough for optimizing the porosity towards compact energy storage.
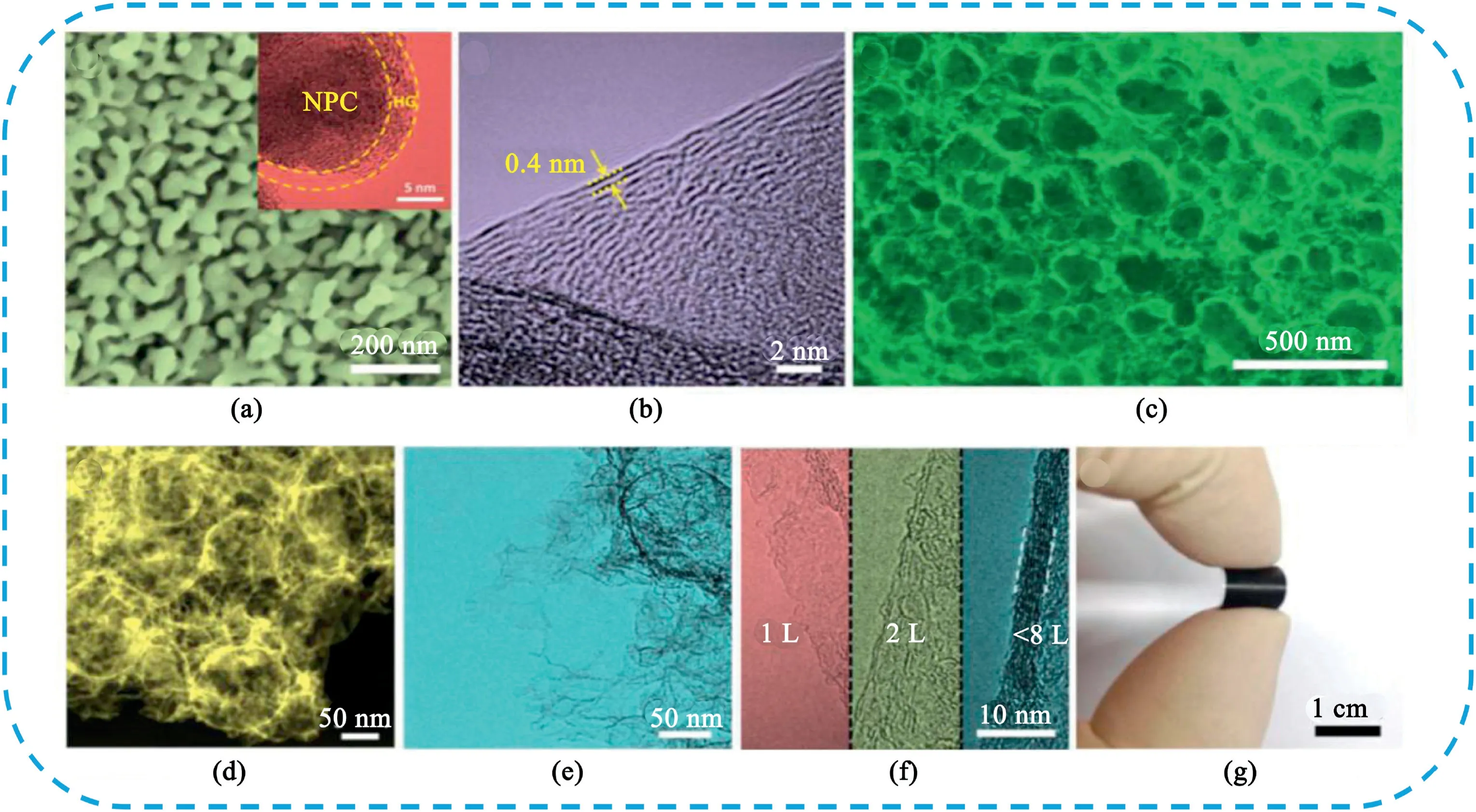
Fig.16.(a)SEM image of the NPC coated with HG,the insert is TEM image.(b)HRTEM image of the HG after remove the NPC.(c),(d)SEM and HAADF-STEM images of the HPG after treated by HNO3.(e)High-magnification TEM image of ultra-thin graphite nanosheets located onto the surface of HPG.(f)High-magnification TEM images of graphene sheets with different number of layers in HPG.(g)Photograph of HPG film,demonstrating its integrity and flexibility.
Self-assembly of2D graphene nanosheets into3D framework structures can largely retain the intrinsic characteristics of individual graphene nanosheets and have drawn massive attention in recent years[155].3D graphene frameworks have high specific surface areas,fast electron and ion transport,excellent conductivity,superior thermal stability,and good mechanical strength,which make them as the promising candidate for SCs.Graphene with3D framework structures mainly including graphene hydrogel(GH),graphene nanomesh(GH)and crumpled graphene(CG).
GH was firstly reported by Shi et al.[156]using a facile one-step hydrothermal method.The obtained GH is electrically conductive,mechanically strong,and thermally stable and exhibits a high specific capacitance.Afterwards,Duan et al.[155]reported a modified hydrothermal reduction method using ascorbic acid as reductant.The prepared GH possessed a highly interconnected3D network structure with excellent conductivity and mechanical robustness(Fig.19).The obtained flexible SCs showed a high gravimetric specific capacitance of186F g-1,a high areal specific capacitance of372mF cm-2,low leakage current(10.6μA),good cycling stability,and extraordinary mechanical flexibility.Other reductants such as,hydrazine,hydroxylamine was also used to prepare GH with the superior electrochemical performance[157,158].Moreover,Heteroatom doped GHs,such as N doped GH,S doped GH,F doped GH,S,P co-doped GH,N,S co-doped GH,N,F co-doped GH were synthesized by hydrothermal method with these heteroatoms precursors assist and show higher specific capacitance than pure GH[159–168].
GNM as a novel type of graphene structures with a high density of the nanoscale pores on the conjugated carbon surface,open porous structure,high surface area,as well as the combination with the inherent properties of graphene have great potential applications in the fields of energy storage[169].Duan et al.[170]firstly prepared GNM with variable periodicities and neck widths down to5nm.They used the poly(-styrene-block-methyl methacrylate)(P(S-b-MMA))block copolymer thin film as the etching template,and CHF3plasma-based reactive ion etch process followed by using oxygen plasma etch to punch holes into the graphene layer.Ning and Our group[171]developed a template CVD method to produce GNM on porous MgO layers(Fig.20).The nanomesh graphene samples had only one to two graphene layers and a specific surface area of1654m2g-1.Benefiting from the unique porous structure,the eletrodes delivered excellent electrochemical capacitance(up to 255F g-1),good cycle stability and rate performance.Later,Ning et al.synthesized sulfur-decorated GNM(S@G)by155°C heat treatment of the mixture of nanomesh graphene and sulfur.The S@G eletrode showed a specific capacitance of257F g-1at0.25A g-1,which is23.6%higher than that of the pure graphene.Zheng et al.[172]prepared nitrogen-doped graphene aerogel nanomesh(N-GANM)by hydrothermal way from graphene oxide and ammonium hydroxide using iron nitrate as the etching agent.The N-GANM symmetric device displayed an energy density of30.9Wh kg-1at the power density of450.3W kg-1and excellent cycling stability retention(98%)after10,000cycles.
CG is a kind of the reconstruction for graphene from the bending and folding of a sheet into a crumpled configuration,which can prevent aggregation and possess high surface area and high pore volume[173,174].Chen et al.[175]developed a facile method to prepare highly crumpled nitrogen-doped graphene nanosheets(C-NGNSs)with a pore volume as high as3.42cm3g-1.The C-NGNSs showed significant improvement of different performance parameters of SCs owing to the abundant wrinkled structures,high pore volume,nitrogen doping,and improved electrical conductivity.Our group[176]reported a facile method to prepare highly corrugated graphene sheets(HCGS)by the thermal reduction of graphite oxide at900°C followed by rapid cooling using liquid nitrogen.The wrinkling of the graphene sheets can significantly prevent them from agglomerating and restacking with one another face to face and thus increase the electrolyte-accessible surface area(Fig.21).The HCGS electrode showed a specific capacitance of349F g-1at2mV s-1and excellent electrochemical stability.Cao et al.[177]reported a novel surface-crumpled reduced graphene oxide hydrogel with hierarchical pores by adjusting the temperature and time of hydrothermal reaction with the addition of2-butanol molecules.The pressed hydrogel electrode provided an outstanding specific capacitance of549F cm-3at 1.14A cm-3and high rate capability of62.2% from1.14A cm-3to 114A cm-3.
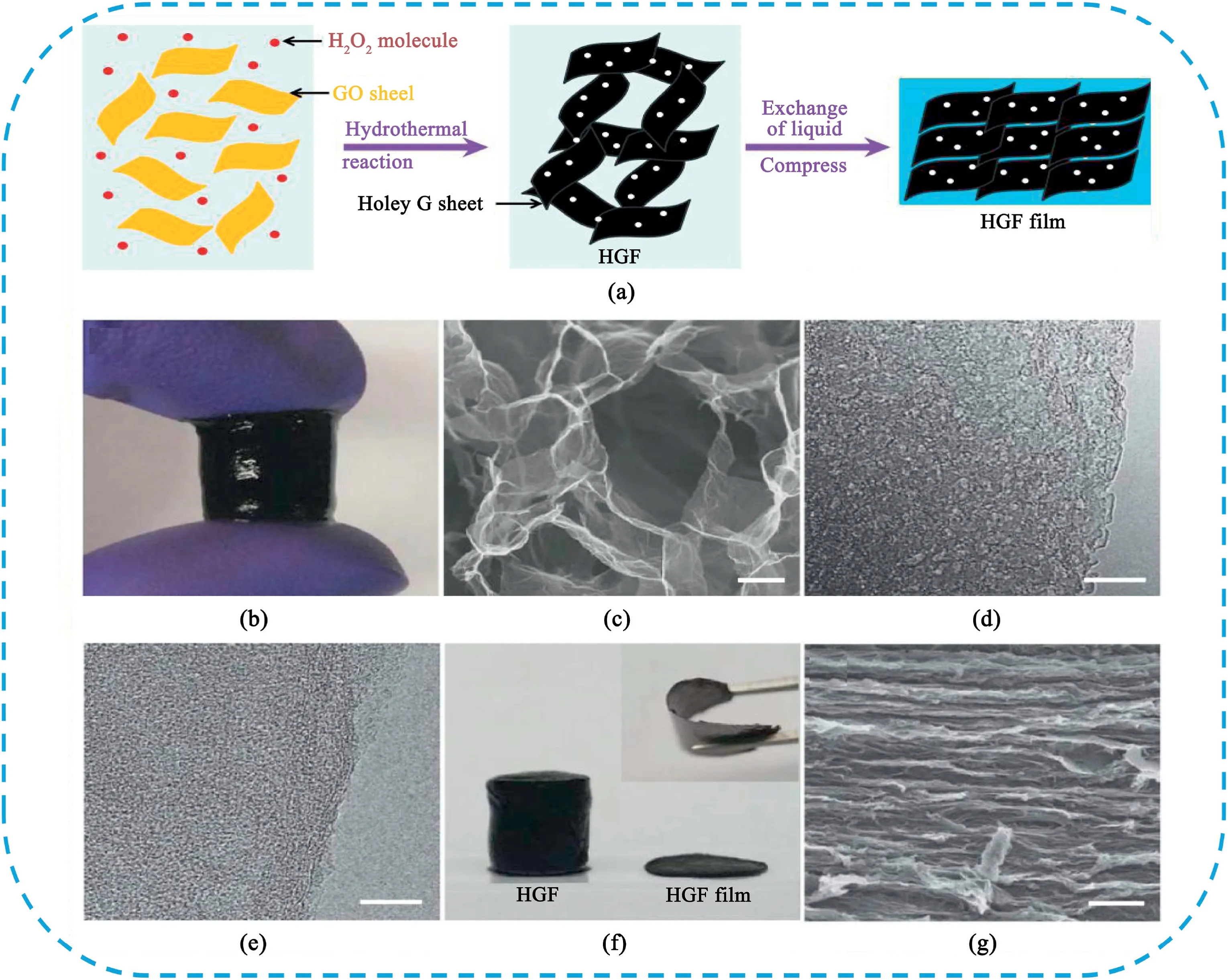
Fig.17.(a)Schematic illustration of the preparation process of HGF and HGF films.(b)Photograph showing a free-standing HGF.(c)Scanning electron microscope image of interior microstructures of HGFs.(d)Transmission electron microscopy(TEM)image of holey graphene sheets in HGF.(e)TEM image of non-holey graphene sheets in GF for comparison.(f)A photograph showing HGF before and after mechanical compression with the flexibility of the compressed HGF film shown in the inset.(g)Cross-sectional SEM image of the compressed HGF film.Scale bar,1mm.Copyright2014Nature Publication Group.

Fig.18.(a)Schematic diagram illustrating the production of EGM-GO films from an aqueous dispersion with a tunable precursor ratio of GO to EG and photographs of a freestanding EGM-GO film after being peeled off from the filter membrane.(b)X-ray diffraction patterns of the as-prepared EGM-GO films.
Despite tremendous achievements have obtained,the researches in this field are confronted with serious challenges in practical applications.Firstly,how to synthesize high-quality graphene with controllable layer thickness on a large scale by a low cost and environmentally friendly way is the major obstacle for the application of graphene.Consequently,it is highly urgent to develop low-cost,scalable,effective,and environmentally approaches to prepare high-quality graphene.Secondly,graphene materials easily agglomerate and restack with each other during the synthesis or electrode preparation process,resulting in a low SSA and poor electrochemical performance.At last,a better understanding of the correlation between the electrochemical performance and graphene structures,physical properties,and interactions within hybrids is essential.
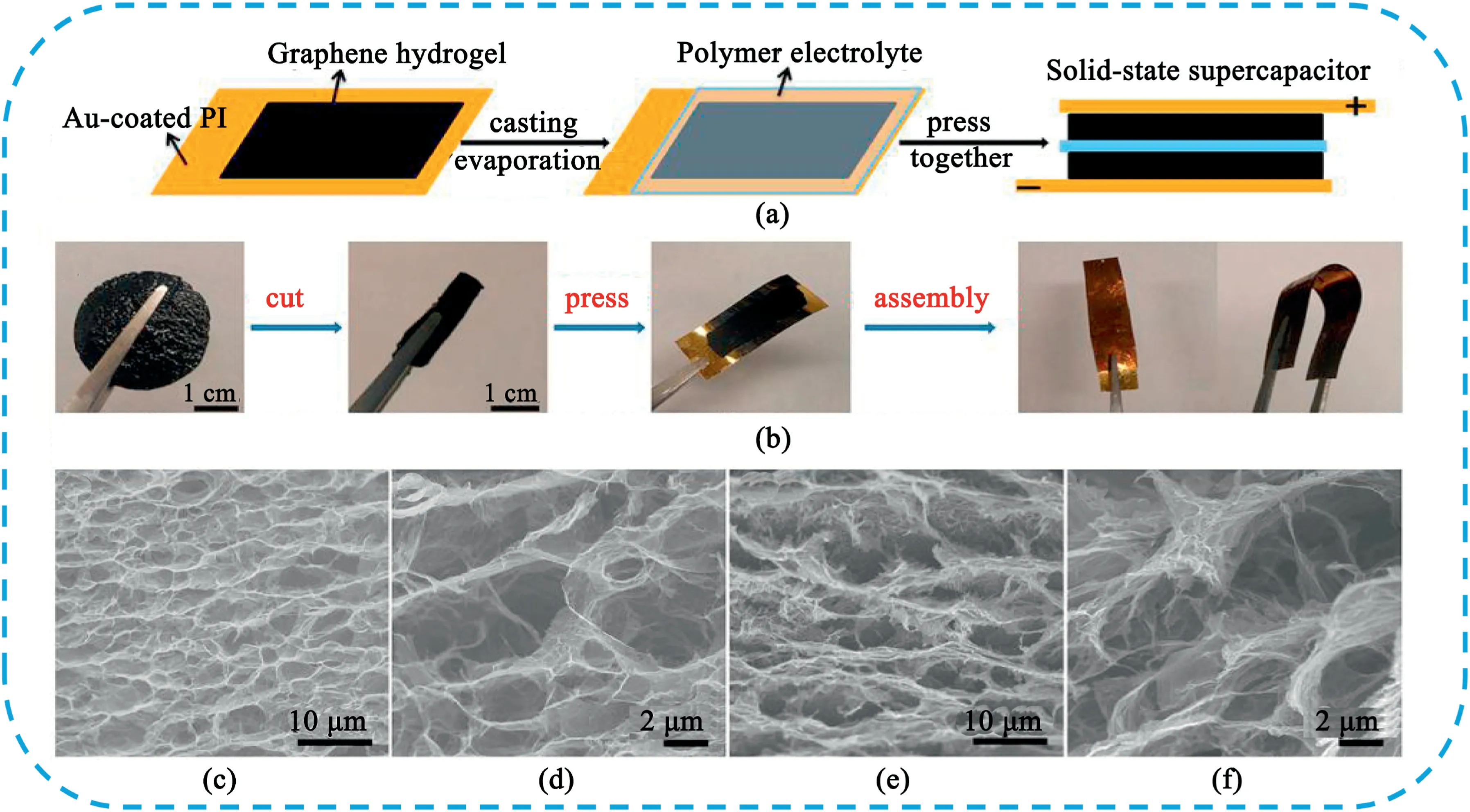
Fig.19.(A)Schematic diagram and(B)photographs of the fabrication process of flexible solid-state SCs based on GH films.(C)Low and(D)high-magnification SEM images of the interior microstructure of the GH before pressing.(E)Low and(F)high-magnification SEM images of the interior microstructure of the GH film after pressing.Copyright2013,American Chemical Society.
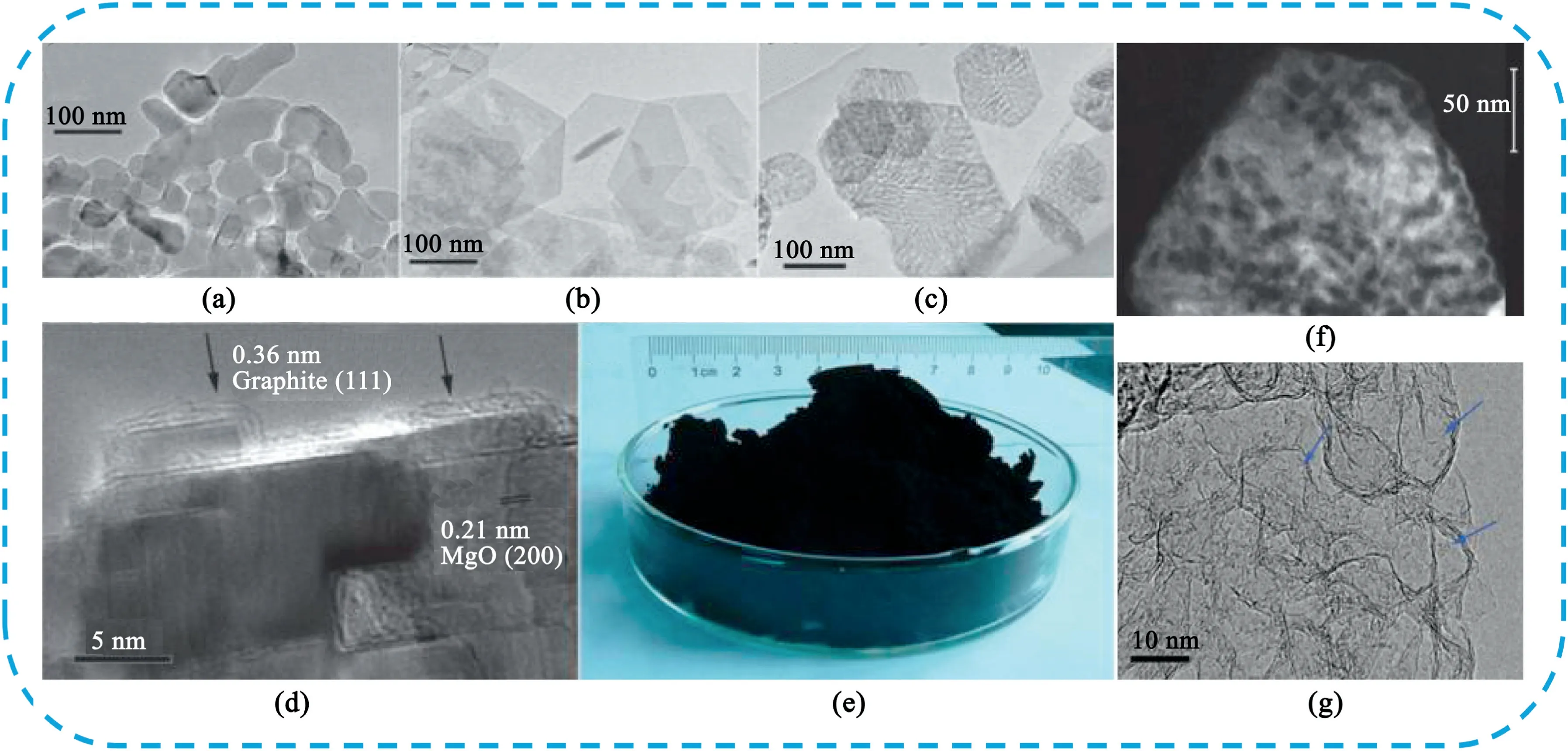
Fig.20.TEM images showing the transformation of MgO from(a)the original particles into(b)polygonal Mg(OH)2layers and then(c)porous MgO layers.(d)TEM photos of the MgO layers after carbon deposition.(e)Example of the bulk quantity of graphene product.The image consists of approximately10g of sample.(f)TEM image of the GNM.Copyright2011,Royal Society of Chemistry.
4.2.2D porous carbon nanosheets
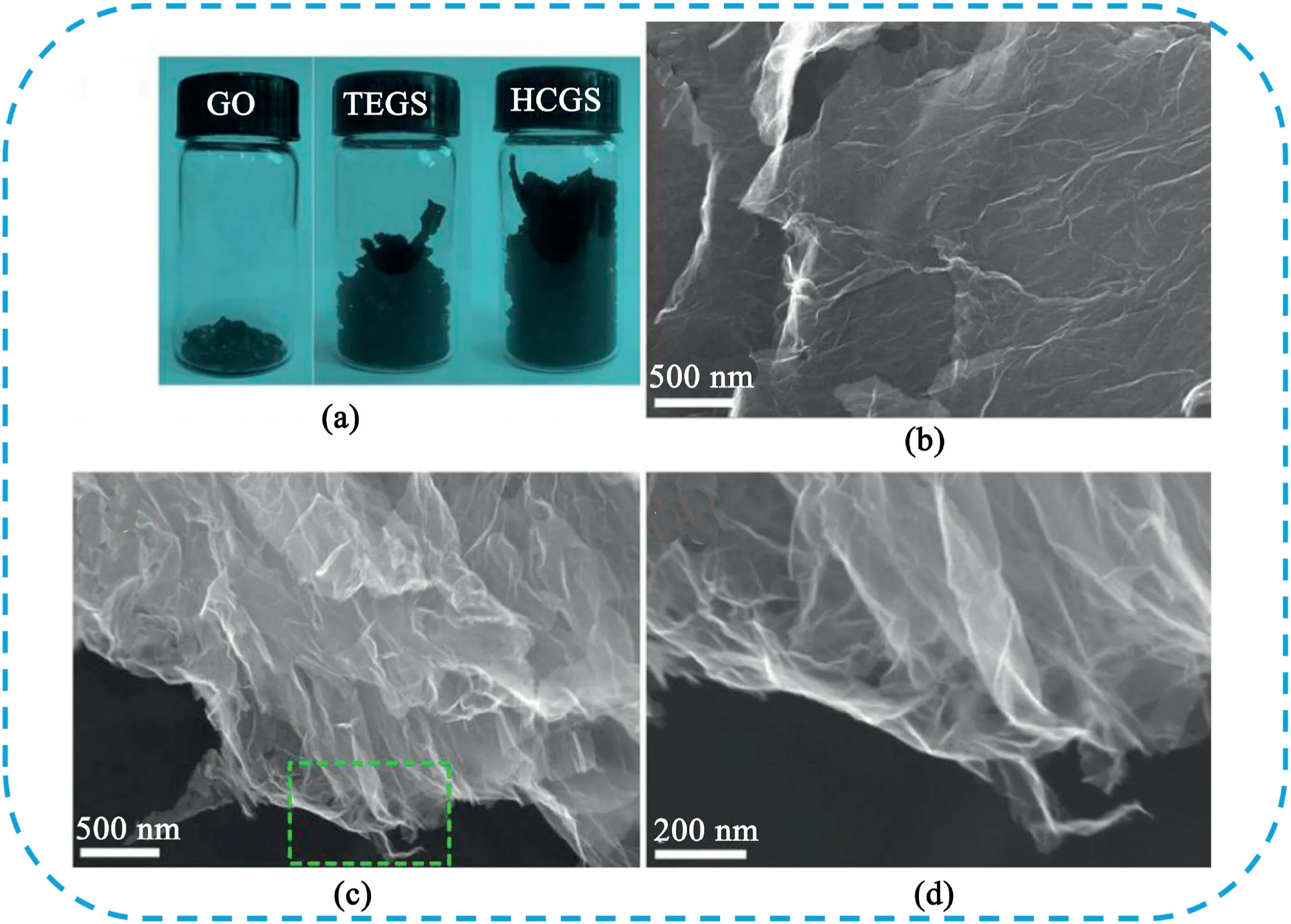
Fig.21.(a)Photographs of pristine GO and as-prepared thermally expanded graphene sheets(TEGS)and HCGS samples.Representative SEM images of(b)TEGS and(c and d)HCGS samples,(d)is the enlargement of the dashed frame region in(c).Copyright2012,Elsevier.
2D PCNs are good candidates for SCs due to their advantages in high aspect ratio for electrode packing and electron transport,hierarchical pore structures for ion transport,and shorten ion transport distance[117,178].Compared with graphene,the PCNs possess additional structural advantages.Firstly,the porous characteristics of PCNs ensure fast ions transport and efficient electrolyte infiltration to the electrode surfaces,thus providing more electrode-electrolyte interfaces.Secondly,PCNs can provide high specific surface areas and rich electrochemically active sites for electrochemical reactions.Thirdly,porous structure of PCNs can effectively prevent the irreversible restacking and overlapping that reduce the available active surfaces.Finally,the internal free spaces in the PCNs can provide sufficient voids for accommodating volume changes during charge/discharge cycles,thus achieving long cycling stability.Currently,the synthesis of PCNs mainly includes template method and template-free method[179].
Carbonization of natural or synthetic organic precursors confined within layered nanostructured inorganic frameworks,such as MgO[119,180,181],montmorillonite[182],MgAl-layered double oxides[183],GO[184,185],CaO[186],zinc acetate[187]and NaCl[188]to obtain 2D PCNs.Our group[119]synthesized3D pillared-porous carbon nanosheets with supporting carbon pillars in between the carbon layers by carbonization of pitch on porous MgO templates.The unique structure endows the high-rate transportation of electrolyte ions and electrons throughout the electrode matrix,resulting in excellent electrochemical performance.Qiu et al.[183]developed a facile and effective CVD method to synthesize carbon nanomesh(CNM)using MgAl-layered double oxides(LDO)as sacrificial template and ferrocene as carbon precursor(Fig.22).The as-made hexagonal thin-sheet CNM features a hierarchical pore system consisting of micropores and small mesopores with a size range of1–6nm,and many large mesopores with their pore sizes of10–50nm.As an electrode for SCs,the CNM exhibited the enhanced capacitance,high rate capability,and outstanding cycling performance with a much-shortened time constant.Feng et al.[184]presented a graphene-directed synthesis for the large-scale production of Schiff-base-type2D porous polymer(TPP)nanosheets.The resulting TPPs can act as robust precursors for the creation of nitrogen-enriched2D porous carbon(TPC)nanosheets.The as-prepared TPCs based on10wt%aminated graphene oxide(AGO)and1,3-phthalaldehyde(TPC-1)showed nitrogen-doping contents of12.3%,8.8%,and5.6% after pyrolysis at700,800,and900°C,respectively.Remarkably,the TPC-1 obtained by thermal treatment at800°C,exhibited ultrahigh specific capacitance up to424F g-1at0.1A g-1in6M KOH,superior to the pyrolyzed porous carbons obtained from Schiff-base porous polymers without graphene.Duan et al.[189]recently reported a self-templated strategy for the synthesis of highly porous and ultrathin2D carbon nanosheets by direct pyrolysis of layered zinc acetate.The obtained PCNs possessed a high specific surface area up to1780cm3g-1and pore volume to2.7cm3g-1.The symmetric SCs device showed excellent cycling durability(98.3%)with an energy density of18.39Wh kg-1.
Template-free method is another optional strategy to prepare2D carbon nanosheets,which can avoid the complex process of template preparation and removal[179].The precursor materials can spontaneously form2D carbon nanosheets by pre-treatment of carbon precursor and subsequent direct pyrolysis or further chemical activation.Carbon precursors mainly include biomass materials[190–195],polymers[196,197],carbon-containing metal salts[198,199]and metal-organic frameworks(MOF)[200].Our group[201]reported a facile one-step pyrolysis and activation synthesis strategy to convert common biomass of willow catkin into interconnected porous carbon nanosheets and then followed by effective nitrogen and sulfur co-doping(Fig.23).Due to the unique hollow and multilayered structure of willow catkin fiber,the pore structure of obtained carbons can be controlled by adjusting the mass ratio of raw material to alkali.The nitrogen and sulfur co-doped PCNs demonstrated a high specific capacitance of298F g-1at0.5A g-1and 233F g-1at50A g-1,revealing excellent rate performance.Liu et al.[195]developed a convenient strategy to transform bark into a highly porous carbon nanosheet by CuBr2,and the structure of the obtained carbon materials can be adjusted easily by temperature control.The prepared bark-based carbon showed a3D porous nanosheet structure and the ultrahigh specific surface areas from1955to2396m2g-1and delivered a high specific capacitance of345F g-1and outstanding capacitance retention.Sevilla et al.[199]prepared PCNs based on the carbonization of an organic salt(potassium citrate)at a temperature in the750–900°C range.The PCNs possessed a thickness of<80nm,BET surface areas in the1400–2200m2g-1range and electronic conductivities of1.7–7.4S cm-1.The obtained materials exhibited an excellent power handling ability and superb robustness over long-term cycling in organic electrolyte.Tang et al.[197]reported an easily operated way to efficiently convert waste poly(ethylene terephthalate)beverage bottles into PCNs by the combined processes of catalytic carbonization and KOH activation.PCNs had an ultrahigh specific surface area(2236m2g-1),hierarchically porous structure,and large pore volume(3.0cm3g-1).As an electrode material,it delivered a high specific capacitance of 169F g-1(6M KOH)and135F g-1(1M Na2SO4).Li et al.[200]developed a novel way to synthesize PCNs derived from Cu-MOF with an ultrahigh specific surface area of2491m2g-1and pore volume of 1.50cm3g-1.The PCNs showed a high specific capacitance of 260.5F g-1at0.5A g-1with a high load mass of8mg cm-2.
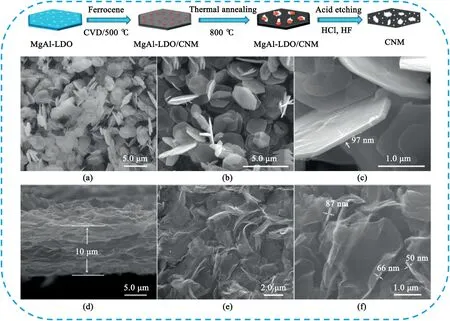
Fig.22.SEM images of(a)LDO,(b,c)LDO/CNM60-90,(d)a film made up of CNM60-90,and(e,f)the CNM60-90with different magnifications.Copyright2015,WILEY-VCH Verlag GmbH & Co.KGaA.
2D porous carbon nanosheets can alleviate the problem of stacking 2D nanomaterials,and improve ion or electron transport rate in the vertical direction of2D lamellar.Therefore,the ions or electrons can transporte to the inside of the electrode more quickly,which can greatly shorten the ion or electron transport distance.
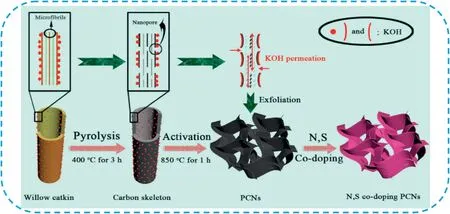
Fig.23.Schematic of the synthesis process for the N,S co-doping PCNs.Copyright2016,Elsevier.
5.3D carbons
As known,microstructure plays a key effect in the electrochemical performances of electrode materials for SCs.With the increasing dimensions,a larger proportion of the surface is exposed,which can reduced“dead surface”,improve the electron transport kinetics,provide hierarchical porous channels and short ionic diffusion distances[202].The3D microstructures with well-interconnected pores provide not only a continuous electron pathway for good electrical contact,but also effectively shorten ions diffusion distance[203].Consequently,3D microstructures carbon materials show new opportunities in the rational design and synthesis of new structures tailored to improve electrochemical performance.Currently,3D microstructures carbon materials mainly include3D hierarchical porous carbons(HPC),and self-assembly of3D graphene-carbon building blocks have demonstrated good performances in SCs.
5.1.Hierarchical porous carbons
HPC with different scales of pores(micropores,mesopores and macropores)interconnected together and assembled in3D carbon materials.Many studies have demonstrated that the presence of micropores is advantageous to provide a large surface area to enhance charge storage capability,mesopores can provide a high-speed channel to facilitate rapid diffusion of ions and macropores act as ion buffer pool in favor of shortening ions diffusion distance.At present,the preparation methods of HPC mainly include hard template method,soft template method and template-free method.
Hard template method is the most common and powerful method to prepare HPC[203].Hard templates with well-defined structures are incorporated into carbon precursors and carbonized at elevated temperatures in an inert atmosphere.After removing the template,the porous structure is formed,and the pore size depends on the type of template.Activation processes using physical or chemical activation agents,such as CO2[204],KOH[205],ZnCl2[206]and pyrolysis at high temperatures can further increase the amount of micropores and small mesopores.Common templates mainly including silica-based template,metal oxides and inorganic salts.
Silica-based template mainly includes silica spheres[207–210]and mesoporous silica templates[211–213].For example,Li et al.[209]prepared porous carbon foam with multiscale pore network(CF-MSP)derived from chitosan using silica spheres as template with further KOH activation(Fig.24).The porous carbon foam delivered an ultrahigh gravimetric capacitance of374.7±7.7F g-1at a current density of 1A g-1.More importantly,it retained235.9±7.5F g-1at an ultrahigh current density of500A g-1.Significantly,the electrochemical analysis showed that the hierarchical porous structure containing macropores,mesopores,and micropores allows efficient ion diffusion and charge transfer,resulting in excellent rate capability.Yang et al.[210]developed a spheres-in-tube nanostructure with hierarchical porosity by encapsulating heteroatom-doping hollow carbon spheres into carbonaceous nanotube(HCSs@CT)using anodic SiO2@aluminum oxide for subsequent polypyrrole coating,followed by carbonization and template removal(Fig.25).The unique spheres-in-tube architecture possessed high specific surface area,high heteroatom content and hierarchical porous structure.As a result,the obtained samples exhibited a specific capacitance of235F g-1at0.2A g-1and156F g-1at20A g-1in6M KOH solution.Tong et al.[211]synthesized nitrogen-rich carbon materials with hierarchical porosity by pyrolyzing melamine-ureaformaldehyde(MUF)resins using mesoporous silica as template,followed by KOH activation.The resulting HPC possessed a high surface area of2563.4m2g-1and nitrogen content varying from 14.25to11.68at.%.The samples carbonized at750°C possessed a high specific capacity of375F g-1at0.5A g-1,excellent rate capability,as well as excellent cycling retention.
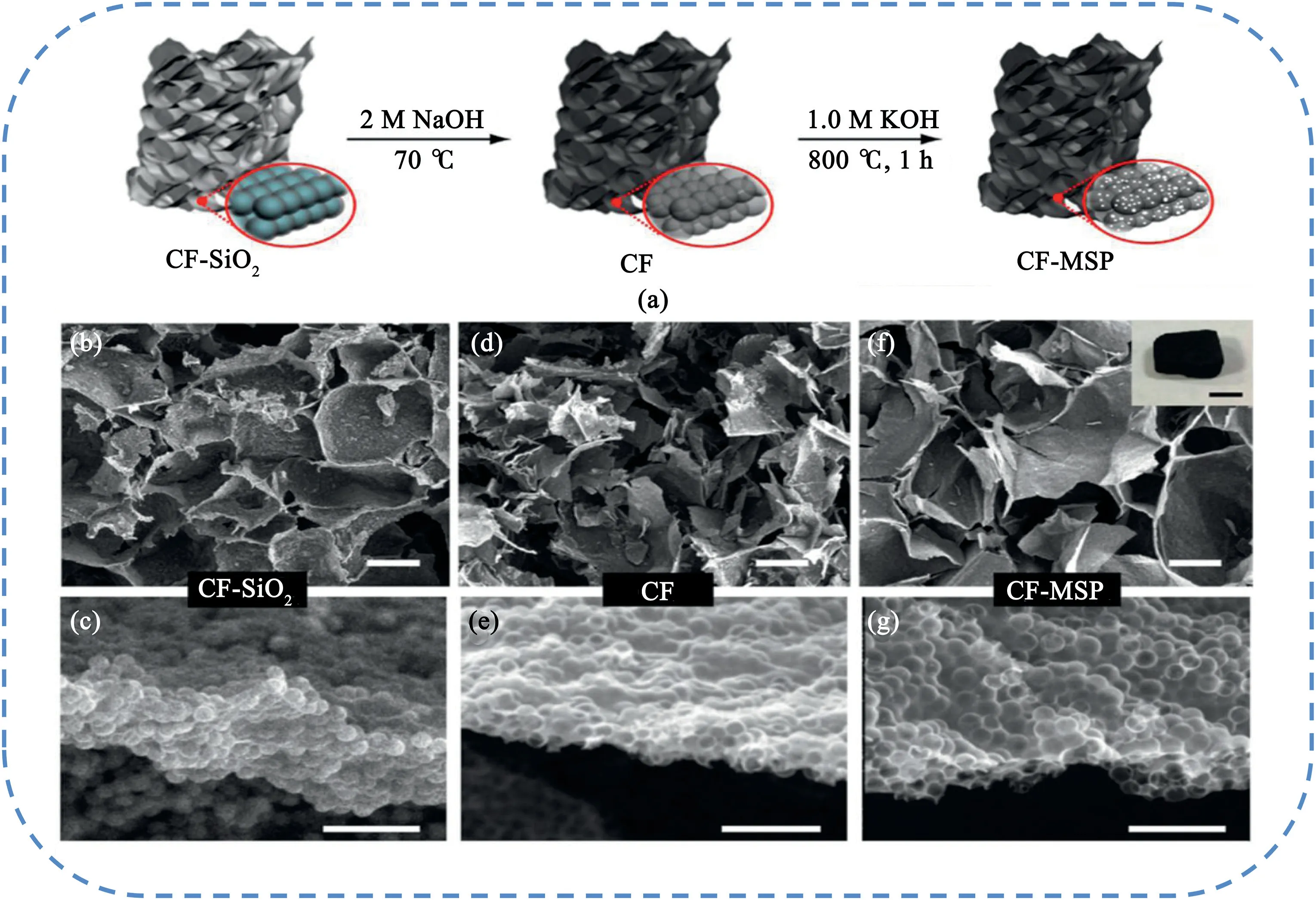
Fig.24.(a)Schematic illustration of the synthesis of CF-MSP.(b,d,f)SEM images of CF-SiO2,CF,and CF-MSP.Scale bars are25μm(c,e,g)SEM images collected from the edge of a carbon sheet in(b,d,f).Scale bars are1μm.Panel f inset shows a digital picture of a piece of self-standing CF-MSP foam;scale bar is1mm.Copyright2017,American Chemical Society.
Removal of silica templates requires acute toxic chemicals,for example,HF or highly corrosive concentrated and hot alkaline solutions.To overcome these obstacles,researchers have developed metal oxide templates,such as,MgO[214–217],ZnO[218,219]and Fe2O3[220],which can be easily removed in dilute hydrochloric acid.Guo et al.[215]used nanosized MgO as template quinone-amine polymer(PAQ)as precursor to synthesize nitrogen and oxygen co-doped carbon foam with hierarchical porous structure(Fig.26).The obtained carbon foam possessed a high content of heteroatoms(total12.26at%),hierarchical porous structure with a high specific surface area of1215m2g-1and a mediated pathway for electrolyte ion diffusion.The co-doped carbon foam delivered a high specific capacitance of321F g-1at1A g-1,a superior energy density of15.91W h kg-1at a power density of 0.4kW kg-1.Our group[218]synthesized3D flower-like HPC material by a simple and efficient carbonization way,and followed by chemical activation using flower-like ZnO as template and pitch as carbon precursor.The hierarchical porous structure was composed of numerous micropores and well-defined mesopores in the interconnected macroporous walls.The prepared electrode showed a high capacitance of 294F g-1at2mV s-1and excellent rate capability(71% retention at 500mV s-1)with superior cycle stability(only2% loss after5000 cycles).
Salt template is a convenient and efficient method to prepare HPC,which can avoid the tedious steps of hard template synthesis and removal.According to their pyrolysis process,salt templates can be classified into salt templates with extra carbon precursors and self-salt templates that directly produce carbon materials.
Salt templates:some common inorganic salts,such as,sodium chloride(NaCl)[221],potassium chloride(KCl)[222],sodium silicate(Na2SiO3)[221],sodium carbonate(Na2CO3)[221]and calcium carbonate(CaCO3)[223–226]are usually used as templates.These templates can be uniformly distributed in the carbon precursor,and play a role of template-directed in the process of high-temperature pyrolysis to obtain porous carbon.Zhang et al.[221]synthesized3D HPC by a facile way using the self-assembly of various water-soluble NaX salts(X:Cl-,CO32-,SiO32-)as structure-directing templates(Fig.27).The as-constructed3D HPC possessed high surface area,good conductivity,facile electrolyte penetration and robust structure.The HPC electrode delivered a high specific capacitance(320F g-1at0.5A g-1),outstanding high rate capacitance(126F g-1at200A g-1).Lee et al.[225]prepared HPC materials derived from CO2conversion by using NaBH4as the reducing agent and CaCO3as the template.The prepared HPC exhibited a high capacitance of270F g-1at1A g-1and retained 170F g-1at20A g-1in6M KOH aqueous electrolyte.Zhang et al.[222]developed a facile molten salt method to prepare hierarchical porous N-doped carbon submicrospheres(HPNCs)using diaminomaleonitrile as carbon precursor,FeCl3as catalyst,and ZnCl2–KCl as molten salt template.The optimized samples exhibited the highest specific surface area(3155m2g-1)and largest hierarchical pore volume(2.2cm3g-1).The resultant HPNCs showed a high specific capacitance of455F g-1,an ultrahigh energy density of93.6Wh kg-1and excellent capacitance retention of95%after5000cycles.
Self-salt templates:some organometallic salts,such as,sodium gluconate[227],potassium acid hthalate[228],sodium alginate[229],potassium alginate[230],potassium gluconate[231],metal organic frameworks(MOFs)[232–234]are commonly used as self-salt templates.Sevilla et al.[198]developed a one-pot approach for the synthesis of highly porous carbon nanosheets with micropore and mesopore structure.The porous carbon nanosheets had a large aspect ratio,a thickness within the range of40–200nm,high surface areas of1390m2g-1.Importantly,the textural properties can be substantially enhanced up to 1890m2g-1by an additional activation.The hierarchical porous nanosheets showed specific capacitances of140F g-1at150A g-1in 1M H2SO4and100F g-1at120A g-1in1M TEABF4/AN.Our group[231]report a facile method for the synthesis of porous carbons by a self-activation process using potassium gluconate as the precursor and then followed by effective nitrogen/sulfur dual-doping.The obtained carbon materials owned hierarchical porous structure with moderate specific surface area,numerous mesopores and rich heteroatoms doping.Benefitting from their synergistic effect,the nitrogen and sulfur dual-doped HPC electrode showed a high specific capacitance of 320F g-1at0.5A g-1,good rate characteristic and superior electrochemical stability.More interestingly,an asymmetric SCs using the nitrogen/sulfur dual-doped HPC as negative electrode and the HPC/MnO2composite as positive electrode exhibited a high energy density of42.5Wh kg-1and good electrochemical stabilization(90.3%capacitance retention after5000cycles).Pan et al.[232]reported a novel and facile way to prepare hierarchical porous rod-like carbon derived from aluminium-based metal organic frameworks.The optimized porous carbon materials possessed a large surface area(1886m2g-1),a large pore volume(1.29cm3g-1)and a uniform pore diameter(4.25nm).The carbon with the unique structure provided excellent electrochemical properties with high specific capacitance(345F g-1at1mV s-1and 332F g-1at1A g-1),and good cycling stability.
Soft templates are mainly organic molecules or block copolymers.In water,soft templates are usually self-assembled into micelles with the charged and hydrophilic tails facing outwards.These charged tails attract nearby carbon precursors through electrostatic interaction.These carbon precursors are covalently linked to the soft template via copolymerization to form rigid organic micelles.The carbon precursor micelle composites were carbonized in an inert atmosphere.This annealing process leads to the thermal decomposition or evaporation of soft template and the pyrolysis of carbon precursor.Due to the ordered assembly of organic molecules in the formation of micelles,the obtained HPC usually contain rich ordered mesopores[235].The main features of soft template method are as follows:(1)the shape of soft template is diverse,which is beneficial to obtain the desired structure;(2)soft templates are generally easy to construct without complicated equipment.Although the size and shape of the product can not always be strictly controlled by the soft template method,people have paid more attention in the template method due to its simple method,convenient operation and low cost[236–240].Wang et al.[235]reported a facile way to prepare nitrogen-doped HPC nanospheres(NHPC)through the carbonization of mesoporous polydopamine nanospheres fabricated by one-step self--polymerization of dopamine using Pluronic F127as a soft template.The NHPC sample had a high specific surface area of1725m2g-1with a pore volume of1.85cm3g-1and thus delivered a high specific capacitance of 433F g-1at0.5A g-1and excellent stability.More recently,Li et al.[241]synthesized a unique hierarchically porous N,O,S-enriched carbon foam(KNOSC)by a combination of a soft-template method,freeze-drying,and chemical etching(Fig.28).The carbon foam had a huge specific surface area of2685m2g-1and a macroporous structure containing a network of mesoporous channels filled with micropores.The KNOSC electrode delivered an excellent capacitance of402.5F g-1at 1A g-1and good rate capability of308.5F g-1at100A g-1.The assembled symmetric SCs device based on KNOSC as the electrodes achieved a specific energy density of15.2W h kg-1at a power density of 36kW kg-1.
Template free methods have been widely concerned in recent years because of the simple operation.In general,carbon precursors were carbonized to produce porous structure,then activated to further develop pore structure to form HPC.Sometimes,HPC can be prepared by one-step carbonization of the mixture of carbon precursor and chemical activating agents.Biomass and organic polymers with the intrinsic porous structure are two major carbon precursors.
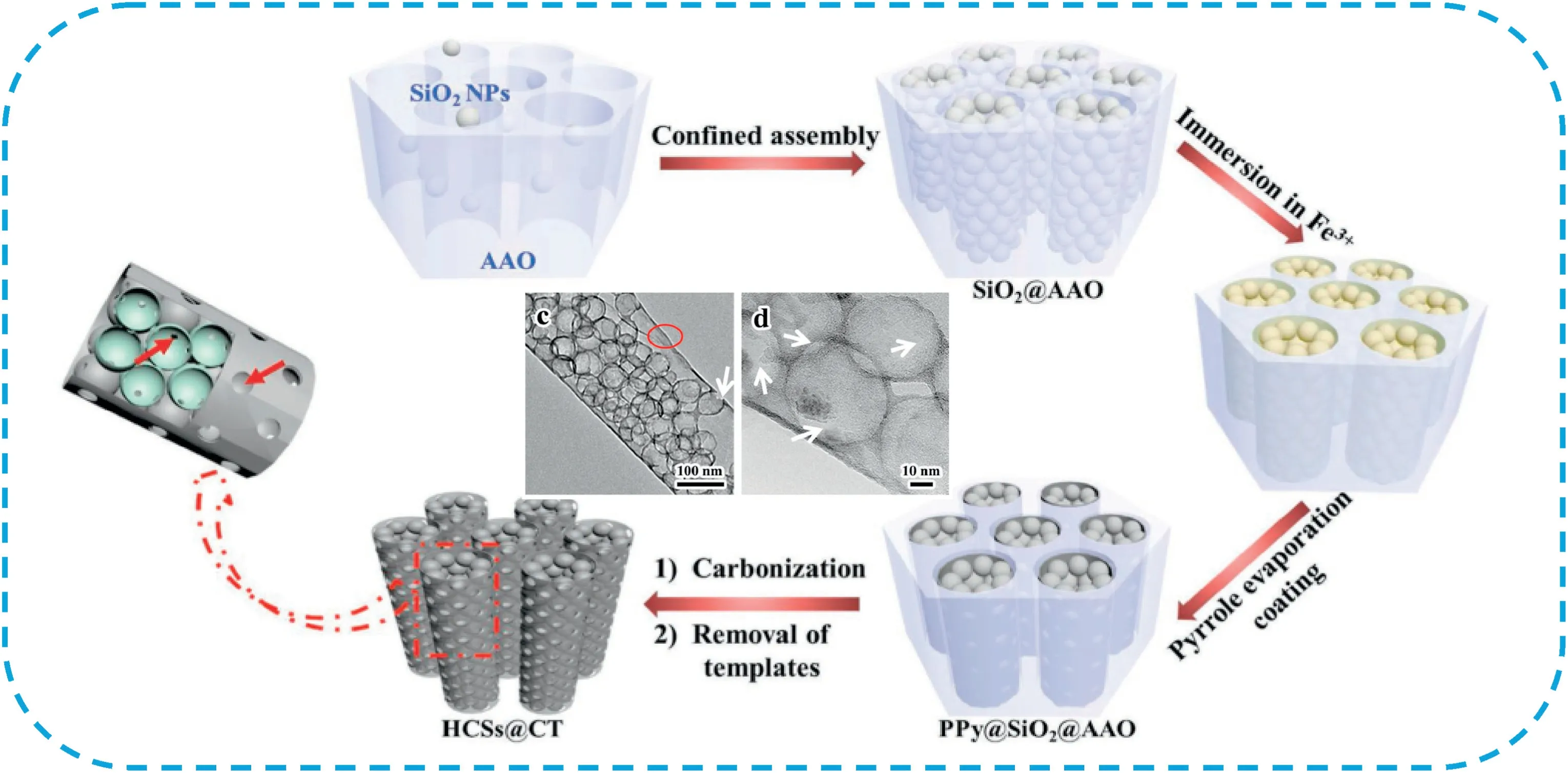
Fig.25.Schematic illustration of the production of HCSs@CT with the assistance of the dual template formed by confined assembly.In the enlarged view of HCSs@CT,meso/macropores exist between the interconnection of HCSs,and the connection of CT and HCSs(green spheres show the internal circumstances of HCSs).Copyright2018,WILEY-VCH Verlag GmbH & Co.KGaA.
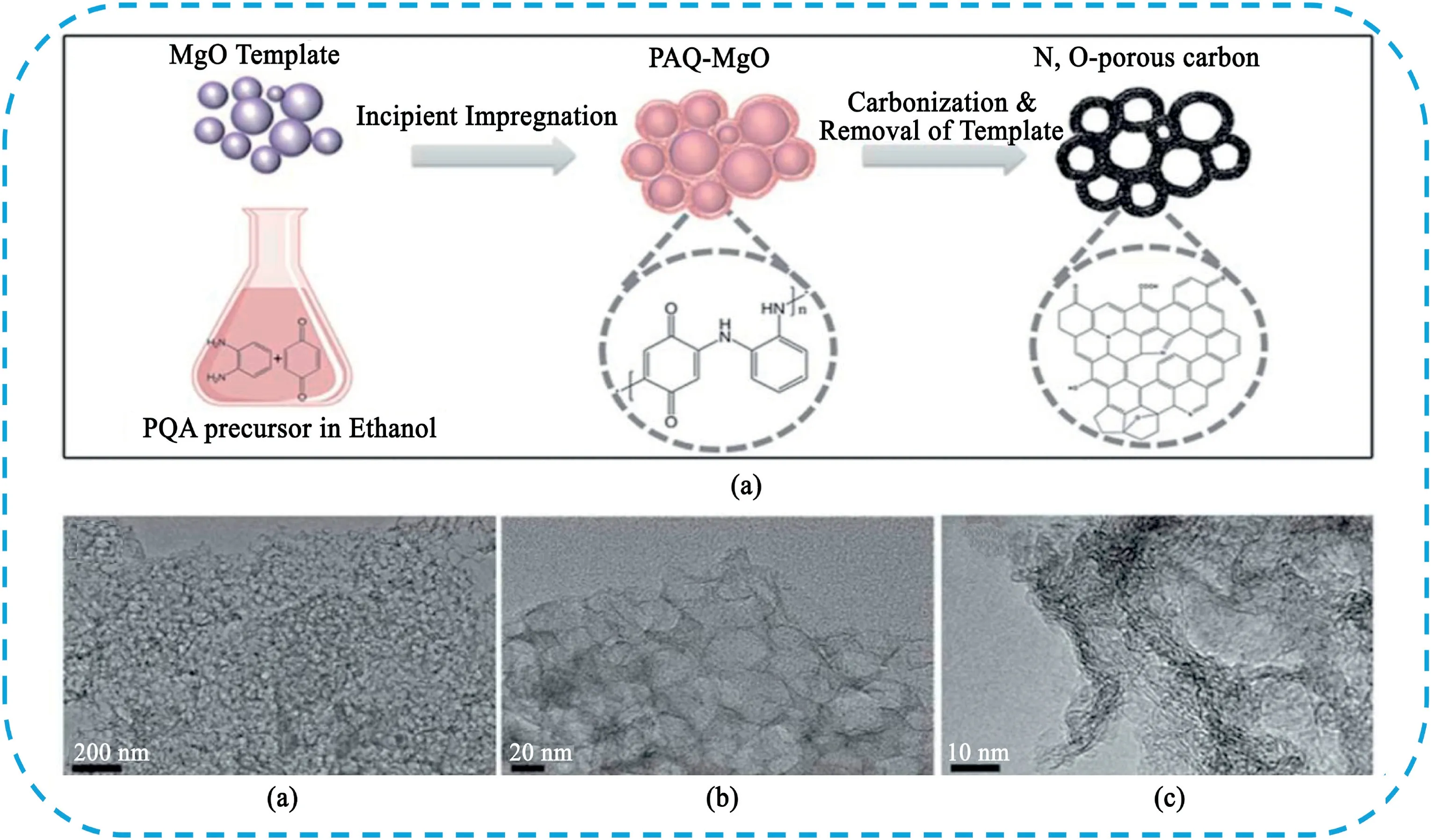
Fig.26.(a)Schematic illustration the synthesis of porous nitrogen and oxygen-doped carbon foam.(b–d)TEM images of carbon foam at different magnifications,(e)SEM image of carbon foam,Copyright2011,Royal Society of Chemistry.
In recent years,a large number of different types of biomass materials were used to prepare HPC and exhibited excellent electrochemical performance(Table1).Our groups[242]reported one-step carbonization of alkali-treated wheat flour for the synthesis of3D interconnected honeycomb-like porous carbon foam(HPC)with hierarchical porous structure(Fig.29).Owing to the interconnected hierarchical porous structure,large specific surface area,and rich heteroatom doping,the HPC electrode showed a high specific capacitance of473F g-1at 0.5A g-1and outstanding electrochemical stability.More interestingly,the asymmetric SCs using HPC as the negative electrode and MnO2/HPC as the positive electrode achieved a high energy density of63.5W h kg-1and excellent cycle stability with93.4% of initial capacitance retention after5000cycles.Tong et al.[243]reported a facile one-step method to synthesize N,S co-doped graphene-derived hierarchical porous carbon foam(N,S-GHPCF)by the carbonization of the mixture of graphene oxide,chitosan,K2CO3and thiourea.The N,S-GHPCF sample possessed high porosity,large surface area,high conductivity and high heteroatom content,it delivered a high specific capacitance of405F g-1at1A g-1,as well as superior rate performance(295F g-1at100A g-1)and cycling stability.Xu et al.[244]developed a green method of combining solvent-free ball milling to prepare HPC using biochar carbon source and KHCO3as the activating agent.The optimized sample possessed high specific surface area up to2147.9m2g-1and abundant hierarchical pores including micropores,mesopores,and macropores.The specific capacitance of the HPC material showed a high capacitance of302.7Fg-1at a current density of0.5A g-1.
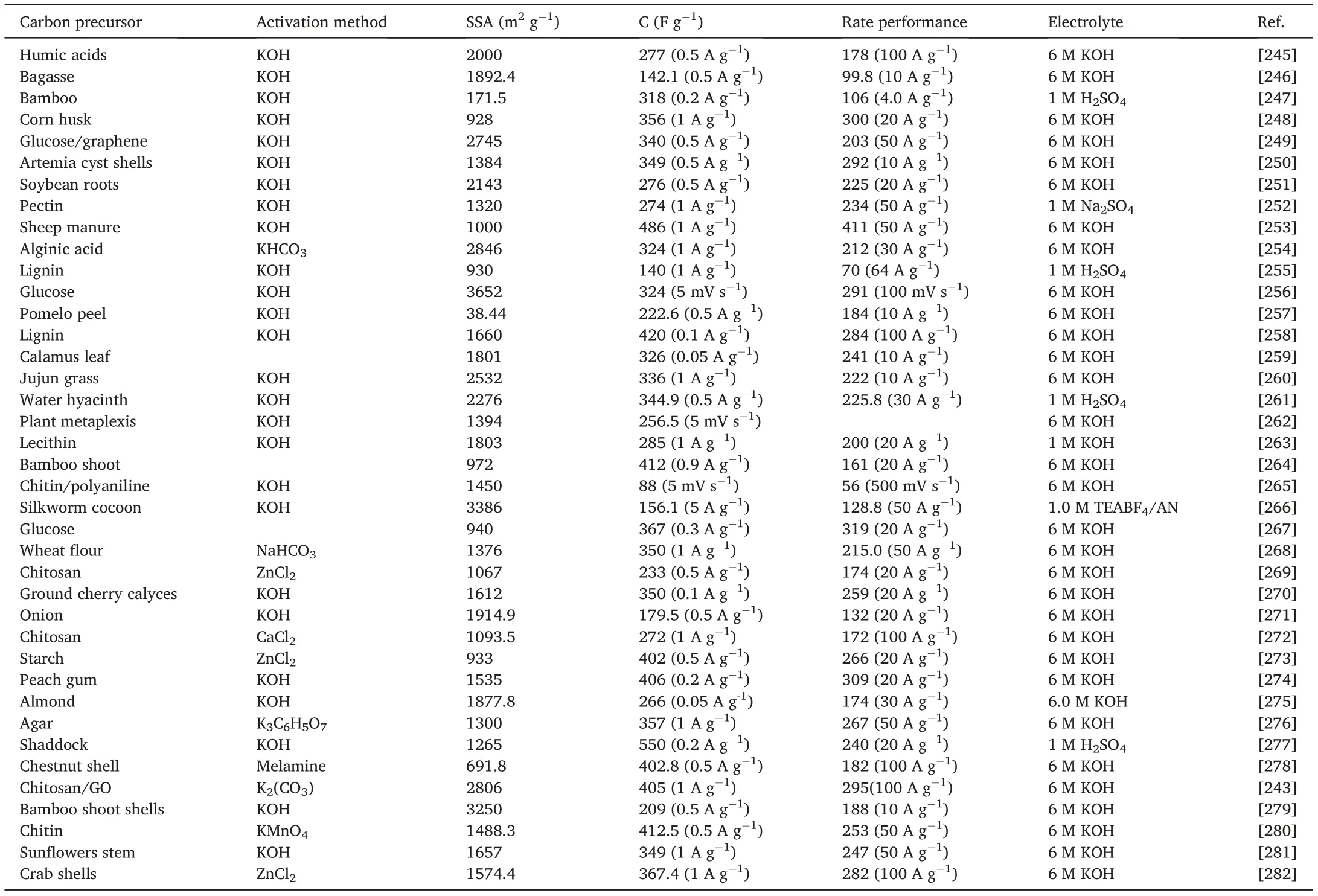
Table1 The electrochemical performance of the reported biomass carbon-based electrodes

Fig.27.Schematics demonstrating the fabrication process of3D HPC with the assistance of multi-scale soluble salt self-assembly.Copyright2015,Royal Society of Chemistry.
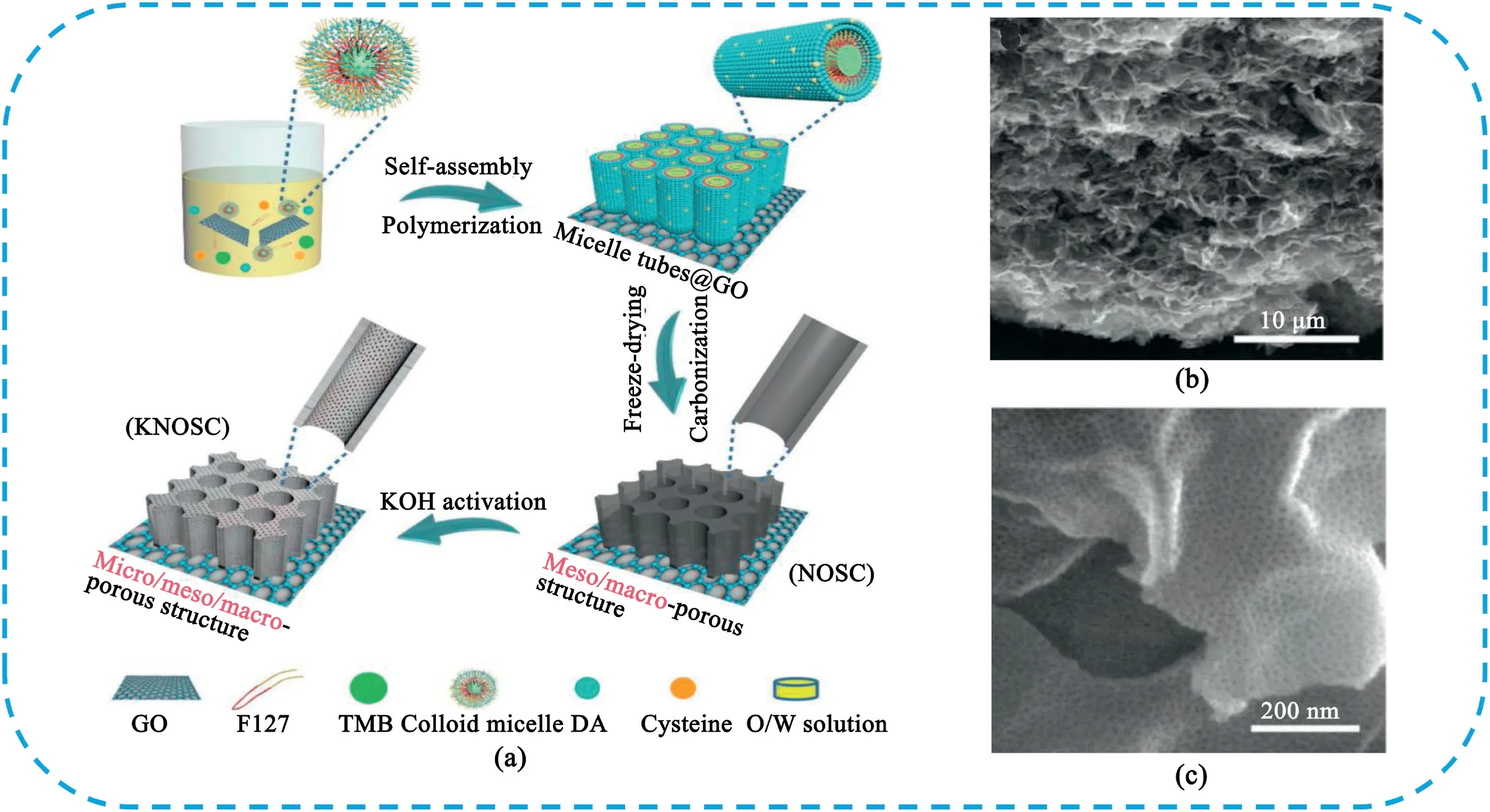
Fig.28.(a)Schematic illustration of the synthesis of KNOSC.(b).SEM image of KNOSC.(c).High-magnifcation SEM image of KNOSC.Copyright2018,WILEY-VCH Verlag GmbH & Co.KGaA.
Currently,many polymers are used as carbon precursor to prepare HPC,especially some precursors containing heteroatoms can enhance the electrochemical performance by self-dopped heteroatoms.The electrochemical comparison of HPC prepared from the polymer is listed in Table2.Huang et al.[283]reported a facile modified chemical activation strategy using polypyrrole microsheets as precursor and KOH as activating agent to prepare functionalized3D HPC(Fig.30).Benefiting from the high specific surface area(2870m2g-1),rich heteroatom doping(N:7.7wt%,O:12.4wt%),excellent electrical conductivity(5.6S cm-1),hierarchical porous structure,mesoporous walls and microporous textures.As an electrode material,it showed high capacitance,good rate performance and long-term stability in both aqueous and organic electrolytes.Li et al.[284]developed a self-assembly foaming strategy to synthesize honeycomb-like HPC derived from pitch based polymers.The HPC samples possessed rich mesopores specific in sizes of2–5nm,and abundant open-ended honeycomb-like holes endow a hierarchical pore-in-pore feature and an ultrahigh specific surface area of 3473m2g-1.The HPC electrode delivered a high specific capacitance of 339F g-1at1A g-1and retained85.6%of the capacity even at50A g-1in6M KOH,and the HPC//HPC supercapacitive cell delivered a high energy density of34.5Wh kg-1.
Table2 The electrochemical performance of the reported polymers carbon-based electrodes
HPC with unique connectivity of various scales of pores,have already displayed superior performance over traditional porous carbon materials.Consequently,HPC provides new opportunities in the rational design and synthesis of new structures tailored to improve electrochemical performance.Howover,most HPC materials possess rather low mass density,which are disadvantageous in volumetric capacitance.Therefore,it remains a significant challenge to design and develop HPC materials with high gravimetric and volumetric performances to satisfy the urgent requirement fo future practical applications.
5.2.Assembly of3D graphene-carbon building blocks(GC)
Assembly of graphene with other dimensional carbons to form3D building blocks structure can not only effectively prevent the agglomeration of graphene nanosheets,but also provide more ion diffusion channels,exhibiting high-performance electrochemical properties.Up to now,numerous types of carbon materials are used such as CNTs[303–307],carbon black[308],carbon sphere[309,310],activated carbon[311,312]and carbon nanofibers[313–315-].Our groups[304]reported that a facile way to prepare3D sandwich-like graphene/CNT composite with CNT pillars intercalated between graphene nanosheets via CVD process.The obtained sample displayed a high specific capacitance of385F g-1with120% of initial capacitance after2000cycles.Later,Lee et al.[307]developed the free-standing,flexible and highly conducting films consisting of stacked nanoporous graphene layers pillared with single-walled CNTs(Fig.31).Due to the unique3D porous structure,the electrodes achieved a remarkable electrochemical performance of a maximum energy density of117.2Wh L-1or110.6Wh Kg-1at a maximum power density of424kW L-1or400kW kg-1.More recently,Lee et al.[313]prepared a hierarchical ternary carbon aerogel structure consisting of graphene,carbon nanofibers,and carbon nanotubes(Fig.32).Benefiting from the unique3D structure of CNTs grown on graphene-CNFs aerogel,in which the CNF provides the support and the graphene flakes act as linkers between the CNFs to maintain a stable 3D structure with high porous characteristics,the obtained electrode material exhibited a high specific capacitance of521.5F g-1at 0.25A g-1.A hybrid SCs device based on the obtained electrode as the negative electrode and NiCo2S4grown on nickel foam as the positive electrode delivered an energy density of62.13Wh Kg-1at a power density of789.66W kg-1.In spite of numerous great achievements have obtained,a number of problems still need to be addressed such as high production cost,complex preparation process and poor volumetric performance.
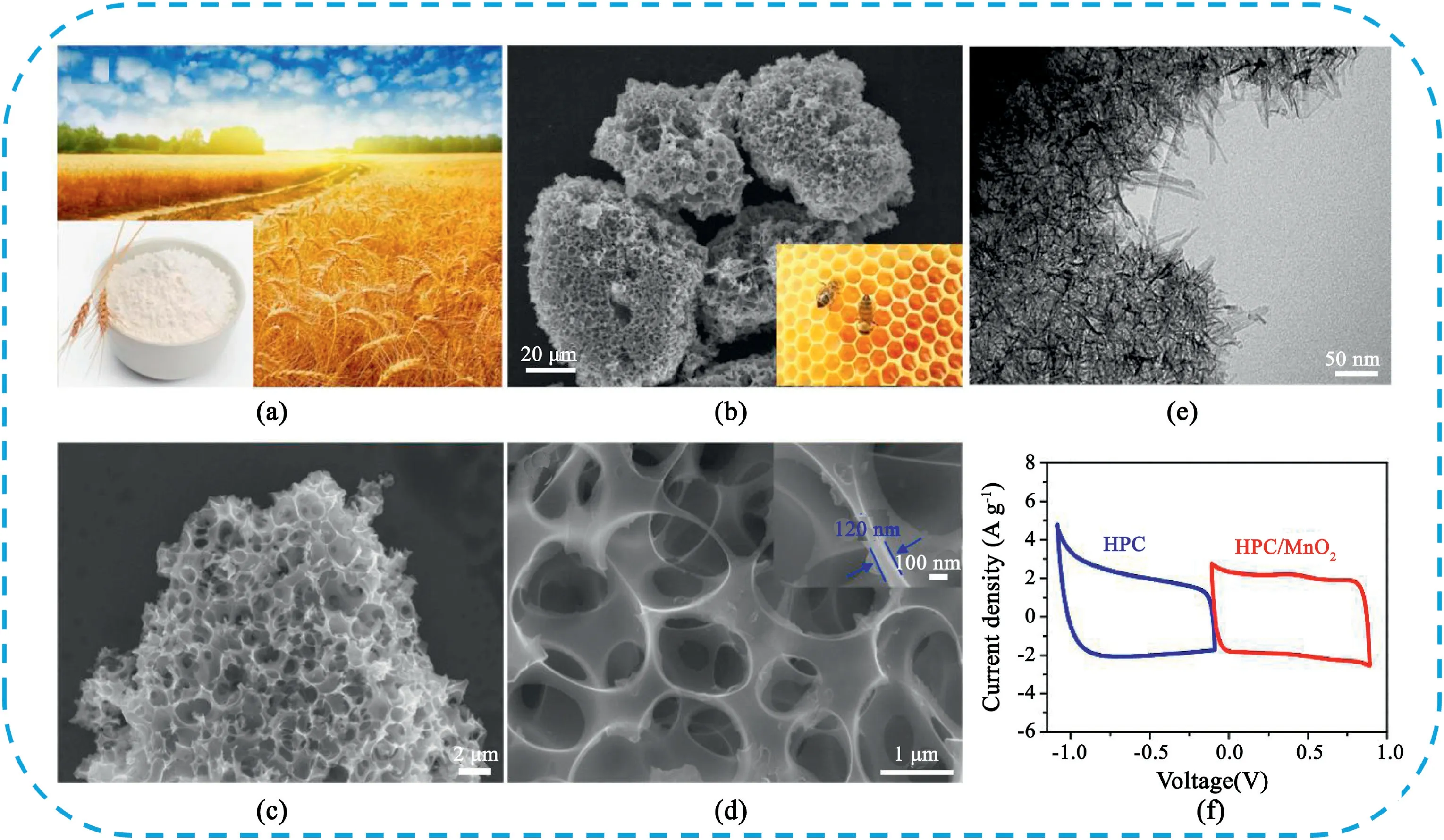
Fig.29.(a)Photograph of wheat field,the inset is wheat flour.(b)SEM image of HPC,the inset is the photograph of a honeycomb.(c,d)High resolution SEM images of HPC.(e)TEM image of HPC/MnO2.(f)Comparative CV curves of HPC and HPC/MnO2electrodes Copyright2015,Elsevier.
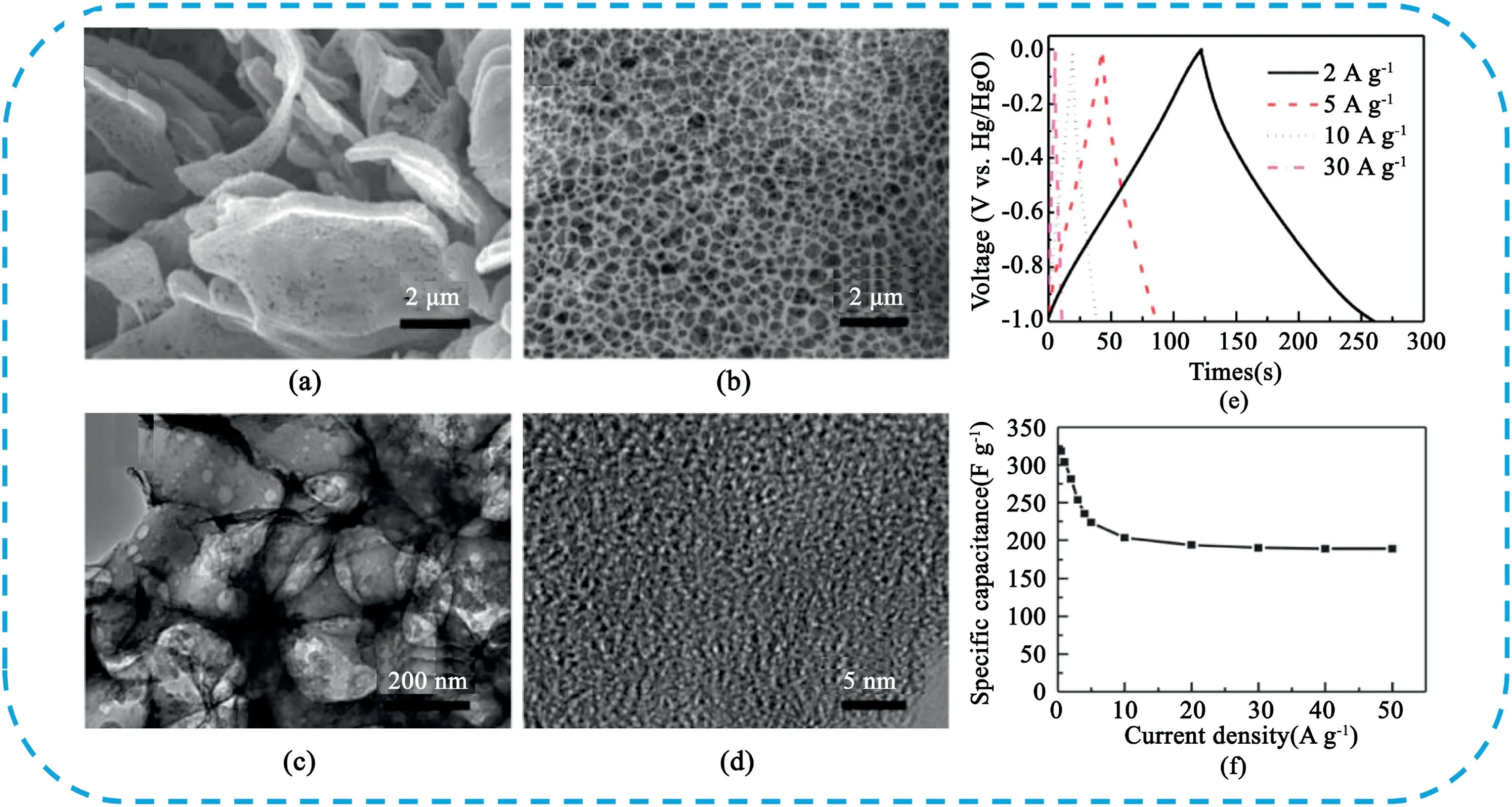
Fig.30.(a)SEM image of PPy microsheets;(b)SEM,(c)TEM and(d)HRTEM images of THPC.Copyright2013,Royal Society of Chemistry.
6.Conclusions and outlook
Carbon-based materials are considered as the most promising candidates of electrode materials for SCs due to their rich resources,high specific surface area,adjustable physical/chemical properties and environmental friendliness.In this review,we summarized recent great achievements of different spatial dimensions carbon-based electrode materials application in SCs.As mentioned in this review,1D carbon structure has short ion and electron transport pathways,which are in favor of achieing high rate capability.Thus the capacitance of SCs greatly depends on the specific surface area of the electrode materials.With the increase in dimensionality,2D carbon structure with more percentage of certain facets,are exposed in the electrolyte,which can significantly enlarge the available active surface and accordingly enhance the electrochemical performances of electrode materials.Moreover,the3D carbon structures with well-interconnected pores provide not only more availability surface area,but also continuous ion/electron pathways and thus effectively enhance the electrochemical performances.
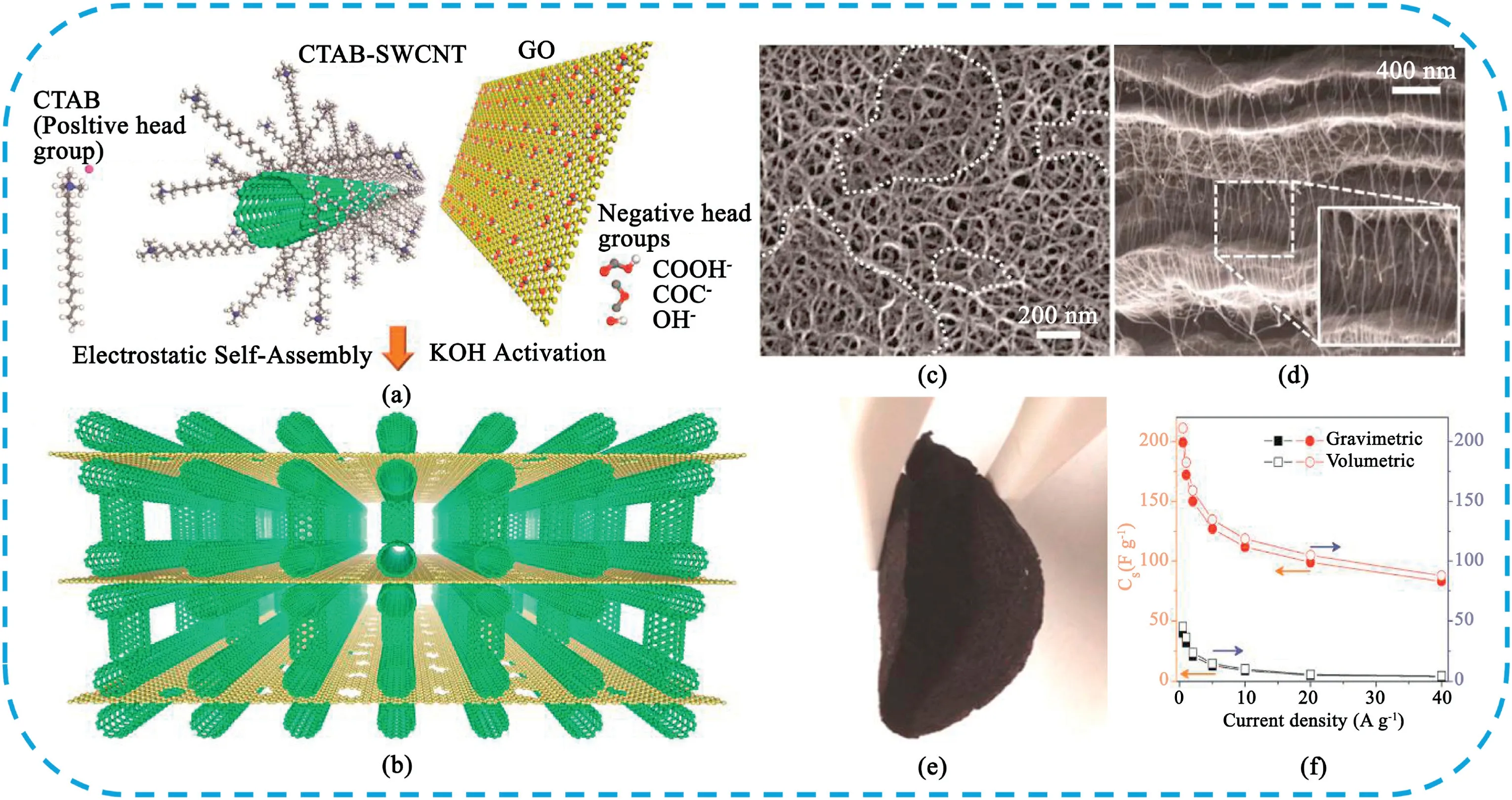
Fig.31.(a,b)Schematic for fabricating the graphene/CNTs hybrid nanostructure.(c)Top-view SEM image showing the porous graphene/CNTs composite.(c)Crosssectional SEM image containing dangling CNTs.(e)Optical micrograph showing the free-standing and flexible film.(f)Gravimetric and volumetric specific capacitances as a function of the current density.Copyright2015,American Chemical Society.
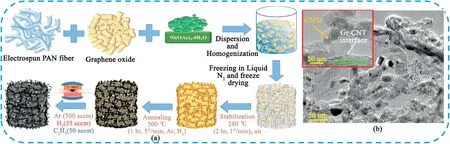
Fig.32.(a)Schematic representation of the fabrication of hierarchical CNTs@graphene-CNFs aerogel.(b)SEM image of the hierarchical CNTs@graphene-CNFs aerogel.
Although significant achievements in carbon-based materials with different spatial dimensions for high-performance SCs have been made in recent years,there are still some issues that need to be addressed in the future.(1)Most relevant studies in this field pay more attention for the enhance of electrochemical performance but ignore the underlying electrochemical mechanism.(2)Introduction of heteroatom into the carbons can increase the interlayer spacing and active sites,which can be beneficial for improving the electrochemical performance.However,some heteroatoms may decrease the electrical conductivity of carbon materials,such as oxygen and sulfur functional groups.(3)Controllable microstructures with the combination of interconnected pores would effectively improve the electrochemical properties of carbon electrodes.(4)Biomass-derived porous carbon with wide sources,low cost,and excellent electrochemical performance have been widely concerned,but its volume performance still needs to be greatly improved.
Declaration of competing interest
There is no conflict of business interests in this paper.
Acknowledgements
The work was supported by National Natural Science Foundation of China51702043(51702043,51672055,51972342,51902345,51872656),Fundamental Research Funds for the Central Universities(20C×06024A,19C×05001A),Taishan Scholar Project of Shandong Province(ts20190922),Key Basic Research Projects of Natural Science Foundation of Shandong Province(ZR2019ZD51).
杂志排行
Namo Materials Science的其它文章
- Low-cost fabrication of highly dispersed atomically-thin MoS2nanosheets with abundant active Mo-terminated edges
- Hierarchically electrospun nanofibers and their applications:A review
- RTV silicone rubber composites reinforced with carbon nanotubes,titanium-di-oxide and their hybrid:Mechanical and piezoelectric actuation performance
- Surface microstructure-controlled ZrO2for highly sensitive room-temperature NO2sensors
- Applications of carbon nanomaterials in perovskite solar cells for solar energy conversion
- Synthesis of hexagonal boron nitrides by chemical vapor deposition and their use as single photon emitters
