Chrysanthemum indicum ethanol extract attenuates hepatic stellate cell activation in vitro and thioacetamide-induced hepatofibrosis in rats
2021-11-15YunJinChaeSushrutaKoppulaMyongKiKimTonyYoonMinDongSong
Yun-Jin Chae, Sushruta Koppula, Myong-Ki Kim, Tony Yoon, MinDong Song
1Department of Applied Life Science, Graduate School of Konkuk University, 268 Chungwon-daero, Chungju-si, Chungbuk, 27478, Republic of Korea
2Department of Biotechnology, College of Biomedical and Health Sciences, Konkuk University, 268 Chungwon-daero, Chungju-si, Chungbuk, 27478,Republic of Korea
3Research Institute and College of Biomedical & Health Science, Konkuk University, 268 Chungwon-daero, Chungju-si, Chungbuk, 27478, Republic of Korea
4Scholar Foxtrot Co., LTD, 501, SinGwan-dong 5F, Jongam-ro 36-gil, Seongbuk-gu, Seoul, 02796, Republic of Korea
5Department of Food Science and Engineering, Seowon University, Cheongju, Chungbuk 28674, Republic of Korea
6Food One Corp, 127, Sincheoksandan 5-ro, Deoksan-eup, Jincheon-gun, Chungbuk 27850, Republic of Korea
ABSTRACT
KEYWORDS: Chrysanthemum indicum; Fibrosis; Hepatoprotective;Hydroxyproline; Thioacetamide
1. Introduction
Liver disease is an increasing incidence globally and represents a massive health care burden worldwide[1]. Several types of chronic liver damage can cause fibrosis and its end-stage, cirrhosis. This condition of hepatic fibrosis can be seen especially in patients with chronic hepatitis C virus infection, alcoholism, and non-alcoholic fatty liver disease[2]. Hepatic fibrosis is characterized by diffused nodule regeneration surrounded by high-density collagen deposition and liver structure collapse ultimately leading to fibrosis, cirrhosis,and hepatocellular carcinoma which involves severe morbidity and mortality[3].
It is well known that hepatic stellate cells (HSCs) are the key player cells and the primary effector cells in hepatofibrosis. HSCs are resident perisinusoidal cells largely present between hepatocytes and sinusoidal endothelial cells in a quiescent state. In the conditions of chronic liver damage provoked by viral hepatitis, toxin induction,autoimmune attacks, alcoholic and non-alcoholic steatohepatitis,HSCs get activated and transformed into proliferative, fibrogenic, and contractile myofibroblasts secreting excessive extracellular matrix(ECM) proteins and releasing cytokines and profibrogenic factors,especially transcription growth factor-beta (TGF-β)[4]. Overexpression of ECM proteins ultimately leads to hepatic dysfunction, fibrosis,cirrhosis, or cancer by HSCs activation[5,6].
The differentiation process of HSC into myofibroblasts involves the expression of alpha-smooth muscle actin and the reconstruction of the cytoskeleton by lipid loss[7]. Myofibroblasts increase gene expression of ECM proteins (such as collagen), proinflammatory cytokines, and profibrotic genes[2]. Earlier studies reported that blockade of fibrosis by inhibiting the activation of HSCs is a key step and has prominent potential in preventing or reversing liver fibrosis in experimental models[8-10]. Further, hepatotoxic agents including thioacetamide (TAA) have been well reported to induced liver fibrosis and cirrhosis and are regarded as a valid model in various in vivo experiments to study liver fibrosis protecting agents and mechanisms[11,12].
Although there is considerable progress in understanding hepatofibrosis pathogenesis, no effective therapeutic agent has been developed to control fibrotic process. Several studies have been reported using natural herbs with promising effects in controlling hepatofibrosis via inhibition of HSCs activation or propagation,and modulation of the molecular mechanisms leading to hepatic fibrosis[13,14].
Chrysanthemum indicum Linn. (C. indicum, family: Asteraceae)is a widely used traditional medicinal herb for treating various immune-related diseases such as hypertension and various other symptoms including pemphigus, swelling, pain, and scrofula in Asian countries[15]. In culinary practices, C. indicum is used as a mixed spicy food additive for taste and has been used in Korean tea and liquor since ancient times[16]. Further, C. indicum has been used in Oriental medicine for the treatment of pertussis, pneumonia,stomatitis, colitis, fever, vertigo, and sores[17]. Earlier reports indicated that C. indicum possesses a variety of pharmacological activities ranging from antioxidant, antinociceptive, antibacterial,antiviral, anticancer, immune regulation, hepatoprotective, and antiinflammatory effects[15,18].
Regarding its hepatoprotective effects, C. indicum attenuated the CCl-induced cytotoxicity hepatocellular cell lines and protected the liver injury in paracetamol-induced experimental rats[19,20]. However,no studies have been performed regarding the antihepatofibrosis effects of C. indicum in HSCs and TAA-induced experimental models of fibrosis. In light of such reports, the present study focused on the antihepatofibrosis effects of C. indicum using HSCs and TAAinduced liver fibrosis experimental models.
2. Materials and methods
2.1. Reagents and chemicals
TAA, 3-(4, 5-demethylthiazol-2yl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) and other reagents, including silymarin,hydroxyproline (HP), p-dimethylaminobenzaldehyde,1,1,3,3-tetraethoxypropane, chloramine-T, 5,5-dithiobis-2-nitrobenzoic acid, glutathione (GSH), and β-nicotinamide adenine dinucleotide phosphate, reduced form (β-NADPH) were purchased from Sigma(St. Louis, Missouri, USA). Dulbecco's modified Eagle's medium(DMEM) and fetal bovine serum (FBS) were acquired from Invitrogen (Carlsbad, CA, USA). Perchloric acid was obtained from GFS Chemical Co (Columbus, Ohio, USA). GOT-GPT assay kit was purchased from Asan Pharmaceutical (Hwaseong-si, Korea).
2.2. Plant material and extraction
The whole plant material of C. indicum was procured from Uiseonggun, Gyeongsangbuk-do, South Korea and was authenticated by a botanist at Konkuk University, South Korea. For extraction, shade dried whole herb of C. indicum L (100 g) was finely powdered and extracted with 1 L ethanol (70%) using Soxhlet’s apparatus for 24 h.The extract was filtered by Whatman filter paper, using a Buchner funnel and water suction. The extract was then concentrated in a vacuum under reduced pressure and lyophilized. The final yield of the lyophilized C. indicum ethanol extract (13.6% w/w) named CIEE hereafter was stored at 4 ℃. The lyophilized powder of CIEE was dissolved in 10% dimethyl sulfoxide (DMSO; Junsei Chemical Co., Ltd., Tokyo, Japan) with the final concentration of DMSO not exceeding 0.1% for experimental studies.
2.3. High performance liquid chromatography (HPLC)fingerprint analysis of CIEE
The constituents of CIEE were analyzed with a Prominance LC-20AD HPLC system (Shimadzu, Kyoto, Japan). HPLC analysis was conducted using a SunFire C18 (4.6 mm × 250 mm, 5 μm, Waters,Ireland). The flow rate of the mobile phase was 1.0 mL/min. The chromatogram was monitored at 330 nm. The injection volume was 10 μL. The column temperature was maintained at 30 ℃. Gradient flows(solvent A, 0.1% formic acid in water; solvent B, acetonitrile) were used as follows: 0 min, 95% A; 5 min, 95% A; 20 min, 85% A; 40 min, 80% A; 60 min, 65% A; 80 min, 10% A. And constituents were analyzed using SYNAPT G2 system (Waters, U.K.) with electrospray ionization (ESI). The mass range (m/z) was 100-1 000 amu.
2.4. Cell lines and culture
Immortalized rat HSCs (HSC-T6) received by Prof. Chang-Gue Son (Korean Hospital of Daejeon University, Korea) and Chang Liver cells purchased from American Type Culture Collection(Manassas, VA, USA) were cultured in DMEM (Invitrogen,Carlsbad, CA, USA) supplemented with 5% FBS (Invitrogen,Carlsbad, CA, USA) and 1% antibiotic-antimycotic in a humidified atmosphere of 5% COat 37 ℃. HSC-T6 cells were activated by serum starvation before treatments.
2.5. Primary HSCs isolation and culture
The protocol for HSCs isolation was performed according to earlier reports[21,22]. HSCs culturing was done in a low glucose DMEM(GIBCO, USA) containing 10% FBS (GIBCO, USA) and 1%antibiotic-antimycotic (GIBCO, USA) at a humidified atmosphere of 5% COat room temperature and these activated HSCs were used in the studies. Cells were replaced with a fresh medium daily for 7 d.
2.6. Cell viability and proliferation assay
Cell viability and proliferation assays were evaluated by MTT method. For cell viability, in a 96-well plate, HSC-T6 (6 × 10cells/well) and/or Chang liver cells were cultivated in DMEM medium as described in section 2.4. CIEE was evaluated at various concentrations (0.05, 0.1, 0.25, 0.5, and 1.0 mg/mL) for 24 h at 37 ℃in an atmosphere of 5% COand 95% humidity. For proliferation assay, following the same procedure as in cell viability assay,HSC-T6 cells were treated with various concentrations of CIEE (0.1,0.25, and 0.5 mg/mL) for 24 h and then exposed to TGF-β (5 ng/mL)for 30 min. The cells were then incubated with 0.5 mg/mL MTT(Sigma, USA) for 3 h, and the reaction was interrupted by addition of DMSO (JUNSEI, Japan). An ELISA reader was used to obtain the results at 540 nm. The viabilities of the control cells were used as the control values at 100%.
2.7. Animals and experimental setup
Thirty Sprague Dawly male rats were purchased from a commercial animal breeder (Orient Bio, Gyeonggi-do, Korea). Animals(six-weeks-old, 190-210 g) were acclimatized to the laboratory conditions of temperature [(23 ± 3) ℃], relative humidity [(50 ±20)%], and 12/12-h light/dark cycle. After 1 week of acclimation,the rats were divided randomly into five groups of six animals each and were housed in separate standard cages under controlled laboratory conditions as follows: Control group, TAA only group,CIEE 100 (TAA plus 100 mg/kg CIEE) group, CIEE 500 group(TAA plus 500 mg/kg CIEE), and positive control silymarin group(TAA plus 50 mg/kg silymarin). Earlier studies indicated that C.indicum extract possesses hepatoprotection in various experimental models of liver toxicity in the dose range of 50-600 mg/kg[19,20,23].The dose selection was based on our preliminary experiments and earlier reported studies. Liver fibrosis was induced by intraperitoneal(i.p.) injections of TAA (200 mg/kg) two times a week for 13 weeks to four groups except for the control group (normal saline, i.p.).CIEE (100 or 500 mg/kg), silymarin (50 mg/kg), or distilled water was given six times per week orally by gastric gavage starting from the 7th week to the 13th week. After the last treatment schedule followed by fasting for 18 h, blood was collected by cardiac puncture under the influence of COanesthesia and the serum was separated by centrifugation at 3 500 g for 15 min for biochemical analysis. The liver was excised for relative/absolute weight determinations and a small portion of liver tissue was stored separately(-80 ℃) for biochemical and protein expression determination. For histomorphological identifications, the liver tissue fixed in Bouin’s solution was used. For gene expression experiments, liver tissue fixed in RNA later solution stored at -80 ℃ was used.
2.8. Serum biochemical analysis
Serum levels of aspartate transaminase (AST) and alanine transaminase (ALT) were determined spectrophotometrically(Sunrise, Tecan, San Jose, CA, USA) using the commercially available assay kits, respectively following the manufacturer’s instructions (Asan Pharmaceutical, Hwaseong-si, South Korea).
2.9. Determination of total glutathione (GSH) contents in liver tissues
The level of total GSH was estimated in liver homogenates according to the method described by Ellman[24]. The absorbance was measured at 405 nm and the amount of GSH was expressed as mM of GSH per gram of tissue.
2.10. Determination of HP contents in liver tissues
The HP content was measured according to the previous report with slight modification[25]. The absorbance was measured at 558 nm using a spectrophotometer (Tecan, USA). HP content was measured using a standard curve obtained by dilutions of 0.5 mg/mL HP solutions.
2.11. Histopathology of liver tissue
For histomorphological examinations, the liver tissues fixed in Bouin’s solution and embedded in paraffin were cut into sections of 5 μM thickness. Each section slides were dried followed by staining with hematoxylin and eosin (H & E).
2.12. Real-time polymerase chain reaction (RT-PCR)
Total RNA was extracted from HSC-T6 cells and liver tissue using TRIzol reagent (QIAGEN, Valencia, CA, USA). cDNA was synthesized from total RNA (2 μg) in a 20 μL reaction using a highcapacity cDNA reverse transcription kit according to manufacturer’s instructions (Applied Biosystems, Foster, CA, USA). The primers for TGF-β
andβ
-actin were as follows (forward and reverse,respectively): TGF-β
, 5'-AGGAGACGGAATACAGGGCTTT-3'(Forward) and 5'-AGCAGGAAGGGTCGGTTCAT-3' (reverse);β
-actin, 5'-CTAAGGCCAACCGTGAAAAGAT-3' (forward) and 5'-GACCAGAGGCATACAGGGACAA-3' (reverse). The gene expression levels were compared withβ
-actin as an internal standard.2.13. Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM)(n=6). Statistically significant differences between groups were analyzed using one-way analysis of variance (ANOVA) followed by student’s t-test using Graph Pad Prism software version 4.00 (Graph Pad Software Inc., San Diego, CA). The values of P<0.05 were considered statistically significant.
2.14. Ethical statement
The experimental treatments were performed with prior approval by the Committee of Laboratory Animals according to institutional guidelines of Konkuk University, Republic of Korea (IACUC No.KU19023).
3. Results
3.1. Effect of CIEE on HSC-T6 cell viability, proliferation and primary HSCs morphology
CIEE treated at various indicated concentrations (0.05, 0.1, 0.25,and 0.5 mg/mL) and DMSO did not influence the overall Chang liver and HSC-T6 cell viability analyzed by MTT assay. However,CIEE treated at 1 mg/mL concentration exhibited cytotoxicity in both Chang cells and HSC-T6 cells (Figure 1A). Therefore, the nontoxic concentrations of CIEE (0.1, 0.25, and 0.5 mg/mL) were used to evaluate the cell proliferation in HSC-T6 cells. Data showed that TGF-β induced significant HSC-T6 cell proliferation when compared with control cells (199.87% increase; P<0.001). Treatment with CIEE dose-dependently and significantly (P<0.05 at 0.1 and 0.25 mg/mL and P<0.01 at 0.5 mg/mL) inhibited the TGF-β-induced HSC-T6 cell proliferation (Figure 1B).
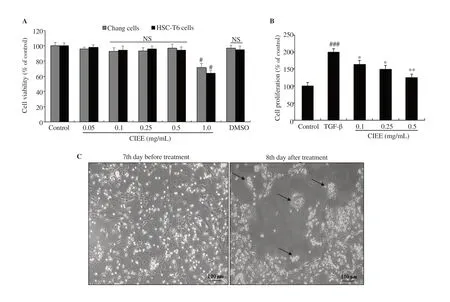
Figure 1. Effect of CIEE on cell viabilities and morphological changes. A: HSC-T6 and Chang liver cells were incubated with CIEE at indicated concentrations for 24 h and cell viability was determined by MTT assay. B: TGF-β (5 ng/mL)-induced HSC-T6 cells were incubated with CIEE at indicated concentrations(0.1, 0.25, and 0.5 mg/mL) and cell proliferation was determined by MTT assay. C: Primary HSCs were cultured for 1 week and exposed to the CIEE (0.5 mg/mL) for 24 h. Pictures were taken before and after 24 h treatment with CIEE. Black arrows represent the corresponding morphological changes (magnification was 100×). NS, not significant, #P<0.05, ###P<0.001 compared with control, *P<0.05 and **P<0.01, compared with TGF-β-induced HSC-T6 cell group. DMSO:Dimethyl sulfoxide; TGF-β: Transforming growth factor-beta; CIEE: Chrysanthemum indicum ethanol extract; HSC-T6: hepatic stellate cells-T6.
Further, untreated primary activated HSCs showed typical myofibroblast morphology (7th day). However, primary HSCs treated with CIEE (0.5 mg/mL) for 24 h showed shrinking collagen fiber, cell degradation and preserved the features of quiescent HSCs with astral-like morphology (Figure 1C). The observations revealed a decrease in the viable cell number and stretched fibers in the activated cultured primary HSCs morphology treated with CIEE compared with the non-treated activated HSCs.
3.2. CIEE attenuated TAA-mediated body and liver weight changes
The average bodyweight of the TAA-induced group was decreased significantly (P<0.05) when compared with the control group (Figure 2A). No significance was observed in CIEE (100 and 500 mg/kg) or silymarin treated groups in the bodyweight gains when compared with TAA alone treated group. TAA (200 mg/kg) injected (i.p.)two times a week for 13 weeks resulted in morphological changes in livers of rats showing decreased glossiness and smoothness on the external surface, reduced bright red color, and enlargement when compared with the control group. However, treatment with CIEE (100 and 500 mg/kg) or silymarin (50 mg/kg) improved morphological changes in the rat liver. Further, a significant increase in the liver weight ratios in the TAA-induced group was observed when compared with the control group. However, CIEE (500 mg/kg)or silymarin (50 mg/kg) treatment in TAA-induced rats protected the increased liver weight ratios significantly (P<0.05) when compared with TAA alone treated group (Figure 2B).
3.3. Serum biochemical analysis
Serum levels of AST and ALT (Figure 3A and 3B) were increased significantly (P<0.001) in TAA-induced groups [(127.24 ± 9.75) IU/L and (38.78 ± 2.15) IU/L, respectively] and CIEE (100 and 500 mg/kg) could ameliorate the altered ALT and AST in TAA-induced group (P<0.05 and P<0.001 at 100 and 500 mg/kg, respectively).Especially, TAA-induced CIEE (500 mg/kg) and silymarin treated group reduced the increased ALT and AST values to more than half compared to the TAA group.
3.4. Determination of the total GSH and HP levels in TAAinduced liver tissues
TAA treatment group significantly (P<0.001) decreased the total GSH levels when compared with the normal group. CIEE treated groups at both doses (100 and 500 mg/kg) significantly (P<0.001)restored the total GSH contents in TAA-induced rats, which was similar to the positive control silymarin group (Figure 4A).Regarding HP content, TAA treatment significantly increased when compared to the control group in liver tissues (P<0.001). CIEE treatment groups (100 and 500 mg/kg) attenuated this increase significantly (P<0.05 and P<0.001) compared with TAA group. In particular, CIEE 500 mg/kg treatment group decreased HP level to the same level as standard silymarin (Figure 4B).
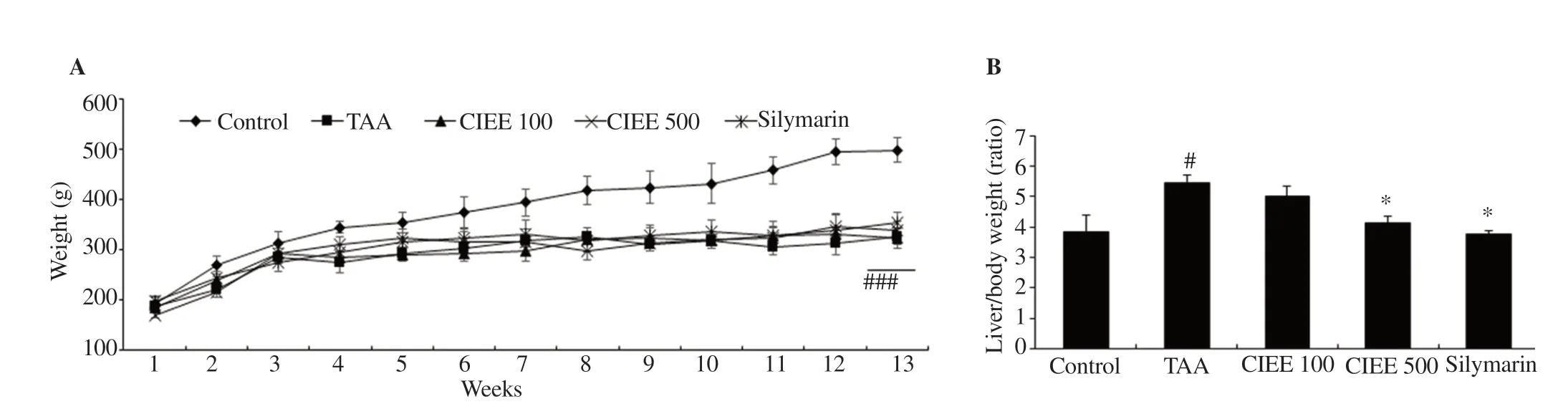
Figure 2. Effect of CIEE on body weights and liver/body weight ratio. A: Effect of CIEE or silymarin (50 mg/kg) on body weight changes. B: Relative liver/body weight ratio. #P<0.05 and ###P<0.001 compared with control, *P<0.05 compared with TAA-induced HSC-T6 cells. TAA: Thioacetamide (200 mg/kg);CIEE 100: Chrysanthemum indicum ethanol extract 100 mg/kg plus TAA; CIEE 500: Chrysanthemum indicum ethanol extract 500 mg/kg plus TAA.
3.5. Histological examination of TAA-induced liver tissues
The normal state of liver morphology in control rats was observed in H&E staining analysis (Figure 5A). Marked histological abnormalities showing hepatocellular degeneration, vacuolated cells with the altered liver morphological pattern were observed in TAAinduced groups. However, treatment with CIEE (100 and 500 mg/kg)showed improved morphology. Silymarin treated at 50 mg/kg dose also exhibited reversed and improved morphological pattern in the liver sections when compared with the TAA-induced group.
3.6. Liver fibrosis-related gene analysis in TAA-induced liver
To investigate the possible underlying mechanism of CIEE in exhibiting anti-fibrotic effects, the major liver fibrosis-related gene TGF-β
expression was analyzed by RT-PCR. TAA-induced group showed up-regulated TGF-β
expression. However, CIEE (100 and 500 mg/kg) treated groups showed a significant down-regulation of TGF-β
expression in TAA-induced liver tissues (Figure 5B).3.7. HPLC fingerprint analysis of CIEE
In the present study, HPLC analysis showed several constituents with distinctive peaks. The components in CIEE observed in the HPLC chromatogram are chlorogenic acid (R= 25.52), luteolin-7-O-glucoside (R= 42.94), luteolin-7-O-glucoronide (R= 49.89),apigenin-7-O-glucoside (R= 50.52), 1,5-didcaffeoylquinic acid (R= 51.82), 4,5-didcaffeoylquinic acid (R= 54.68), and acacetin-7-O-glucoside (R= 62.12), respectively, corresponding to the seven major peaks in the HPLC chromatogram shown in Figure 6.

Figure 3. Effect of CIEE on liver serum and oxidative stress biomarkers. Levels of serum biomarkers AST (A) and ALT (B) in TAA-induced serum of rats measured by commercially available GOT and GPT kits. ###P<0.001 compared with the control group, *P<0.05, and ***P<0.001 compared with TAA-induced group.

Figure 4. Effect of CIEE on total glutathione (GSH) and hydroxyproline (HP) levels. Total GSH (A) and HP content (B) were measured using spectrophotometry. ###P<0.001 as compared with the control group, *P<0.05, and ***P<0.001 compared with the TAA group.

Figure 5. Effect of CIEE on morphology and TGF-β expression. Hepatic sections were stained with hematoxylin and eosin (H&E) staining (×100) and observed under light microscopy (A). Black arrows represent the corresponding pathological changes (Scale bar=100 μm). Fibrosis-related gene expressions of TGF-β (B) in liver tissue were determined by real-time PCR. ###P<0.001 compared with control and *P<0.05 as compared to TAA-induced group.
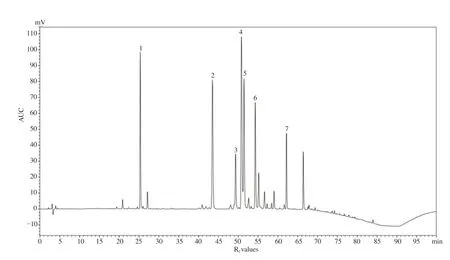
Figure 6. High performance liquid chromatography (HPLC) fingerprinting analysis of CIEE. HPLC chromatogram of CIEE used in the study showing major peaks of phytoconstituents. The seven major peaks were identified as, chlorogenic acid (1, Rf = 25.52), luteolin-7-O-glucoside (2, Rf = 42.94), luteolin-7-Oglucoronide (3, Rf = 49.89), apigenin-7-O-glucoside (4, Rf = 50.52), 1,5-didcaffeoylquinic acid (5, Rf = 51.82), 4,5-didcaffeoylquinic acid (6, Rf = 54.68), and acacetin-7-O-glucoside (7, Rf = 62.12), respectively. AU: Area under curve; Rf: Retention factor.
4. Discussion
In this study, CIEE was confirmed to possess hepatoprotection by significantly attenuating TAA-induced hepatofibrosis in both in vitro and in vivo experimental evaluation in various aspects. HSC activation is considered a crucial event that promotes increased production of ECM and contributes to liver fibrosis[26]. Activated HSCs are characterized by densification and proliferation of surrounding collagen scar cells and increased contractility of the ECM product. Thus, morphological changes of lipids reduce the elongation of collagen fibers and inhibit liver fibrosis.Treatment with CIEE at 500 μg/mL in activated primary HSCs culture showed cell degradation and collagen fiber loss by reducing the number of visible primary HSCs and collagen fibers elongated in 24 h after CIEE treatment compared to untreated activated primary HSCs. Earlier studies indicated that C. indicum extract possesses hepatoprotection in various experimental models of liver toxicity in the dose range of 50-600 mg/kg[19,20,23]. The dose selection was based on our preliminary experiments and earlier reported studies. Therefore, in the present study, we performed in vivo experiments using 100 and 500 mg/kg for optimum observation and statistical significance.
It is well known that TAA is toxic to liver inducing liver fibrosis with multiple metabolic disorders[27] and is regularly used as a classical model to develop hepatofibrosis in experimental animals. Earlier reports indicated that hepatotoxins including TAA show a negative effect on the overall body weight and an increase in liver/body weight ratio in experimental rats[28,29]. In agreement with the reports in the present study, decreased body weight and increased liver weight were observed.Although CIEE and silymarin treated groups in TAA-induced rats did not influence the overall average body weights, the increased liver/body weight ratio in TAA-induced rats was ameliorated significantly (P<0.05).However, reports also indicated that the alteration in body weights and liver weight ratios in toxin-induced experimental models may not be considered as a direct indication in the pathology of chronic liver damage[30,31].
Elevation of ALT and AST is an indicator of liver damage and routinely used as markers in liver function tests. These aminotransferase enzyme levels are an indication to understand the disease state (acute or chronic)and also indicate its site (intracellular or extra-liver) of damage[32]. In the present study, CIEE (100 and 500 mg/kg) reduced the TAA-induced increase in AST and ALT levels. Further, H&E staining revealed that the morphological abnormalities observed in TAA-induced rat liver tissues were reversed in the CIEE treated groups.
Oxidative stress and the production of large amounts of reactive oxygen species plays a major role in hepatofibrosis[33]. TAA is a carcinogen well known to induce hepatotoxicity in experimental animals and produce excessive reactive oxygen species. GSH is the major antioxidant enzyme that helps in protecting the cells from oxidative insults and serves as an indicator of oxidative stress in liver fibrosis[34]. Therefore, measuring total GSH contents and whether CIEE could restore the decreased GSH enzyme in TAA-induced liver tissues might support the beneficial role of CIEE as a natural antioxidant defense agent in protecting liver fibrosis.CIEE at both doses (100 and 500 mg/kg) significantly attenuated the decreased levels of GSH and the effect was comparable with positive control silymarin.
HP is a major protein collagen component and an importnat factor to make collagen stabilization and ECM accumulation[35]. ECM accumulation and abnormal activity may cause HSC proliferation and liver fibrosis[36]. In this study, CIEE administration significantly decreased the TAA-induced increased content of HP indicating a possible beneficial effect as an antifibrotic agent.
TAA exposure induces tissue deposition, necrosis, abnormally patterned,solidified, and shrunken cells with fibrotic scar tissues, which are due to the regeneration of hepatic centrilobular in the liver. H &E staining is the most used technique for observation of general tissue structure and diagnosis of disease conditions in tissues and cells[37]. In the present study, H & E staining of liver tissues confirmed that CIEE (100 and 500 mg/kg) treatment could recover the TAA-induced hepatic fibrosis (darkly stained areas).
In liver fibrosis, TGF-β is the main pro-fibrogenic related gene. During HSCs activation TGF-β is produced in the damaged liver and stimulates cytokines signal transduction pathways. TGF-β is produced by Kuffer cells and HSCs and up-regulates the transcription of the collagen 1 (Ⅰ)and 2 (Ⅱ) genes, which are expressed in HSCs and observed in the cirrhotic liver[37]. Therefore, suppressing liver fibrosis-related genes such as TGF-β
is important in reducing liver damage. In this study, the TGF-β
expression evaluated using RT-PCR demonstrated a significant decrease in CIEE treated group (500 mg/kg, P<0.05) when compared with the TAA-induced group. The positive control silymarin-treated group also showed a potent effect in ameliorating TGF-β
expression (P<0.05).Earlier reports revealed that CIEE contains several valuable phytoconstituents including essential oils, terpenoids, flavonoids, and phenolic acids. The nonvolatile active components include luteolin and acacetin glucosides, phenolic acids such as chlorogenic acid,caffeoylquinic acids, and apigenin[38,39]. These compounds were well reported to possess various biological activities including antioxidant,anti-inflammatory, and hepatoprotective properties[40-42]. In the present study, HPLC fingerprinting analysis of CIEE showed several distinctive peaks out of which seven corresponding active constituents were present in identifiable concentrations at different retention times. The active constituents present in CIEE might act individually or in a synergistic manner in exhibiting a potent effect against hepatofibrosis. However,further works on the isolation of individual active constituents and exploring the underlying mechanisms of CIEE in other experimental models of hepatofibrosis is quite necessary.
In conclusion, CIEE inhibited the TGF-β-induced HSC-T6 cell proliferation and decreased the collagen and ECM accumulation in activated primary HSCs. CIEE also attenuated the TAA-induced changes in liver functional parameters (AST and ALT) and restored the altered GSH and HP content. Further, CIEE ameliorated the degree of liver fibrosis on histological examinations and down-regulated liver fibrosisrelated genes such as TGF-β
in TAA-induced fibrosis rats. Our study strongly suggests that CIEE has potential hepatoprotective effects and can be used as a therapeutic against hepatofibrosis in chronic liver diseases.Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgments
This paper was supported by Konkuk University in 2020.
Authors’ contributions
YJC, SK, and MDS conceived and designed research. YJC, MKK,and TY conducted experiments and contributed new reagents.MDS, SK, and YJC analyzed data. YJC, SK, and MDS wrote the manuscript. All authors read and approved the manuscript.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Phytochemicals, pharmacological and ethnomedicinal studies of Artocarpus: A scoping review
- Crotalaria ferruginea extract attenuates lipopolysaccharide-induced acute lung injury in mice by inhibiting MAPK/NF-κB signaling pathways
- Anti-inflammatory and antipyretic potential of Arbutus andrachne L. methanolic leaf extract in rats
- Nanoemulsion containing a synergistic combination of curcumin and quercetin for nose-to-brain delivery: In vitro and in vivo studies
