Association of peripheral anterior synechia, intraocular pressure, and glaucomatous optic neuropathy in primary angle-closure diseases
2021-11-08MingZhangGuangYunMaoCongYeSuJieFanYuanBoLiangNingLiWang
Ming Zhang, Guang-Yun Mao, Cong Ye, Su-Jie Fan, Yuan-Bo Liang, Ning-Li Wang
1Department of Ophthalmology, ZhongDa Hospital, Southeast University, Nanjing 210009, Jiangsu Province, China
2Glaucoma Institute, Eye Hospital, School of Optometry and Ophthalmology, Wenzhou Medical University, Wenzhou 325027, Zhejiang Province, China
3Center of Evidence-Based Medicine & Clinical Epidemiological Research, School of Public Health, Wenzhou Medical University, Wenzhou 325035, Zhejiang Province, China
4Handan Eye Hospital, Handan 056001, Hebei Province, China
5Beijing Tongren Hospital, Beijing Institute of Ophthalmology,Capital Medical University, Beijing 100730, China
Abstract
● KEYWORDS: primary angle-closure glaucoma;peripheral anterior synechiae; intraocular pressure
INTRODUCTION
Glaucoma is the leading cause of irreversible blindness worldwide[1]. Primary angle-closure glaucoma (PACG)causes more blindness than primary open angle glaucoma(POAG)[2]. It is estimated that by 2020 there will be 23.4 million cases of PACG worldwide with 17.96 million in Asia[3].In China, PACG was estimated to be the predominant subtype of glaucoma, and the prevalence was about at the level of 1.4%, expected to increase to 2.01% until 2050[4].
Primary angle-closure diseases (PACD) comprises primary angle closure (PAC) and PACG[5]. The increase in intraocular pressure (IOP) in PACD is secondary to closure of the angle of the anterior chamber; this closure can be appositional or synechial[6]. A higher IOP in PACG is associated with a narrower width of the angle as well as the presence of peripheral anterior synechiae (PAS)[7-8]. While it is intuitive that higher IOP would be associated with extent of PAS and consequently more serious glaucomatous optic neuropathy(GON), this relationship is not well defined.
The aim of our study was to determine the association of PAS with raised IOP and the prevalence of GON in PACD.
SUBJECTS AND METHODS
Ethical Approval This was a retrospective analysis of baseline data obtained from a randomized controlled trial(RCT) conducted at the Handan Eye Hospital between October 1, 2005 and October 31, 2006 (registration number: ChiCTRTRC-00000034, www.chictr.org). The study was conducted in accordance with the principles of the Declaration of Helsinki.The informed consents were obtained from the patients.
The purpose of this RCT was to investigate the role of laser peripheral iridotomy (LPI) with or without argon laser peripheral iridoplasty (ALPI) in patients with PACD; the methodology has been described in detail elsewhere[9]. As part of the RCT, all eligible subjects underwent a comprehensive ophthalmic examination including visual acuity, refraction, IOP(Goldmann applanation tonometry), slit-lamp examination,static and dynamic gonioscopy, fundus examination, and visual field (VF) testing (Humphrey Field Analyzer 750i, SITA fast strategy, 24-2 threshold test; Humphrey Instrument, San Leandro, California, USA).
Inclusion criteria were: 1) Eyes defined as PAC or PACG based on the International Society of Geographical and Epidemiological Ophthalnology (ISGEO) classification[10].PAC was defined as non-visibility of the trabecular meshwork for ≥180° on gonioscopy with PAS and/or an IOP ≥21 mm Hg in the absence of GON. PACG was defined as PAC with evidence of GON. 2) Ability to undergo an ophthalmic examination.Exclusion criteria were: 1) unwillingness or inability to provide consent; 2) prior intraocular surgical treatment; 3)history or signs of trauma to the eye; 4) any ocular disorders such as uveitis that could impact the structure or function of the drainage angle.
PAS was defined as adhesion of the iris stroma to the trabecular meshwork or higher that was at least half a clock hour in width.The presence and extent of PAS as determined by dynamic indentation gonioscopy was recorded. The examination of PAS was carried out at the lowest level of ambient illumination using a Goldmann-type two-mirror lens (Model OG3MS,Ocular instruments. Inc., USA). A 1-mm beam of light was reduced to a very narrow slit and was offset horizontally for assessing superior and inferior angles and vertically for nasal and temporal angles.
Gonioscopic angle width was graded in five categories(0=closed to 4=wide open) based on Shaffer’s classification.The average Shaffer score width was determined by dividing the sum of all four quadrants by 4[11]. The ophthalmologist(Fan SJ) had a good agreement with (Liang YB) to determine the extent of PAS: 83.3% of 30 subjects with PAC or PACG were assessed between the 2 investigators as having less than 1 clock hour of PAS.
对31个省级行政区域同年的人均教育支出均等化指数、人均医疗卫生支出均等化指数和人均社会保障与就业支出均等化指数取均值,可以得到如下2005—2016年基本公共服务供给均等化指数变化趋势图(见图1)。
PAS was confirmed by good clinical ophthalmologist agreement as well as GON although with no anterior segment imaging device. GON was diagnosed on the basis of abnormality of the vertical cup-disc ratio (vCDR) of 0.7 or more and VF abnormalities present in the same eye. An abnormal VF, was defined as VF damage consistent with retinal nerve fiber layer (RNFL) damage or VF defects consistent with glaucomatous optic nerve damage[12-13].
Statistical Analysis The predictor variable PAS was evaluated both as a continuous variable as well as a categorical variable in quartiles (Qs). If a subject’s visual acuity and function precluded reliable VF testing, the diagnosis was made on the basis of an abnormal vCDR[10]. The association of PAS with IOP and prevalence of GON was first examined with smoothing plots (Proc Loess). As both eyes of each participant were eligible for inclusion, the independent effect of extent of PAS category on IOP and the probability of GON was determined using multivariable generalized estimating equation (GEE) models.
Covariates were selected based on the published literature.Adjustment for IOP assessed the independent effect of PAS on the prevalence of GON. Adjustments were performed for age,gender, spherical equivalent, average Schaffer score and the number of medications to determine the combined effects of PAS and IOP on GON. All statistical analyses were performed using SAS version 9.4 for Windows (SAS Institute, Cary,North Carolina, USA).P<0.05 was defined as the significant level.
RESULTS
A total of 355 eyes (238 PAC and 117 PACG) of 181 patients fulfilled the inclusion and exclusion criteria. The mean age was 63.4±8.2y and 67.6% were female.
The baseline characteristics in PAC patients and PACG patients are summarized in Table 1. Extent of PAS, IOP, mean defect (MD), spherical equivalent, average Shaffer score, and number of medications were significantly different between PAC and PACG. PAS was more extensive (8vs1 clock hour,P<0.001) and IOP higher (28.01vs18.00 mm Hg,P<0.001) in PACG compared to PAC.
The results of the multivariable GEE models are shown in Table 2. The prevalence of GON among the PAS quartiles were 10.2% (PAS<0.5 clock hours), 16.9% (PAS≥0.5 and PAS<3 clock hours), 29.6% (PAS≥3 and PAS<7 clock hours)and 74.4% (PAS≥7 clock hours), respectively. Compared toPAS<0.5 clock hours, the crude prevalence of GON was 3.7(95%CI: 1.6-8.4) in eyes with PAS≥3 clock hours and 25.7(95%CI: 11.0-60.0) with PAS≥7 clock hours. After adjusting for age, gender, eyes, spherical equivalent, average Shaffer score and number of medications, the prevalence was 5.4(95%CI: 1.9-15.7) in eyes with PAS ≥3 clock hours and 22.7(95%CI: 7.4-69.1) with PAS≥7 clock hours. After adjusting for IOP as well as the characteristics mentioned above the probability of GON remained significantly increased: OR=4.4(95%CI: 1.5-13.0;P=0.007) for PAS≥3 clock hours and OR=13.8 (95%CI: 4.3-43.6;P<0.001) for PAS≥7 clock hours.The association between PAS and IOP with GON is shown in Table 3. Adjusting for age, gender, eyes, spherical equivalent,average Shaffer score and number of medications, the rate was 18.0 times higher (95%CI: 7.5-43.4;P<0.001) in eyes with both PAS≥6 clock hours and IOP≥21 mm Hg compared to eyes with PAS<6 clock hours and IOP<21 mm Hg. The OR was 8.2 for PAS≥6 clock hours compared to PAS<6 clock hours (95%CI: 4.4-15.3;P<0.001) and 5.2 for IOP≥21 mm Hg compared to IOP<21 mm Hg (95%CI: 2.6-10.4;P<0.001).
After adjusting for age, gender, spherical equivalent, average Shaffer score, number of medications and IOP, the prevalence of GON increased linearly with increase in extent of PAS; this was true for both right and the left eyes (Figure 1).
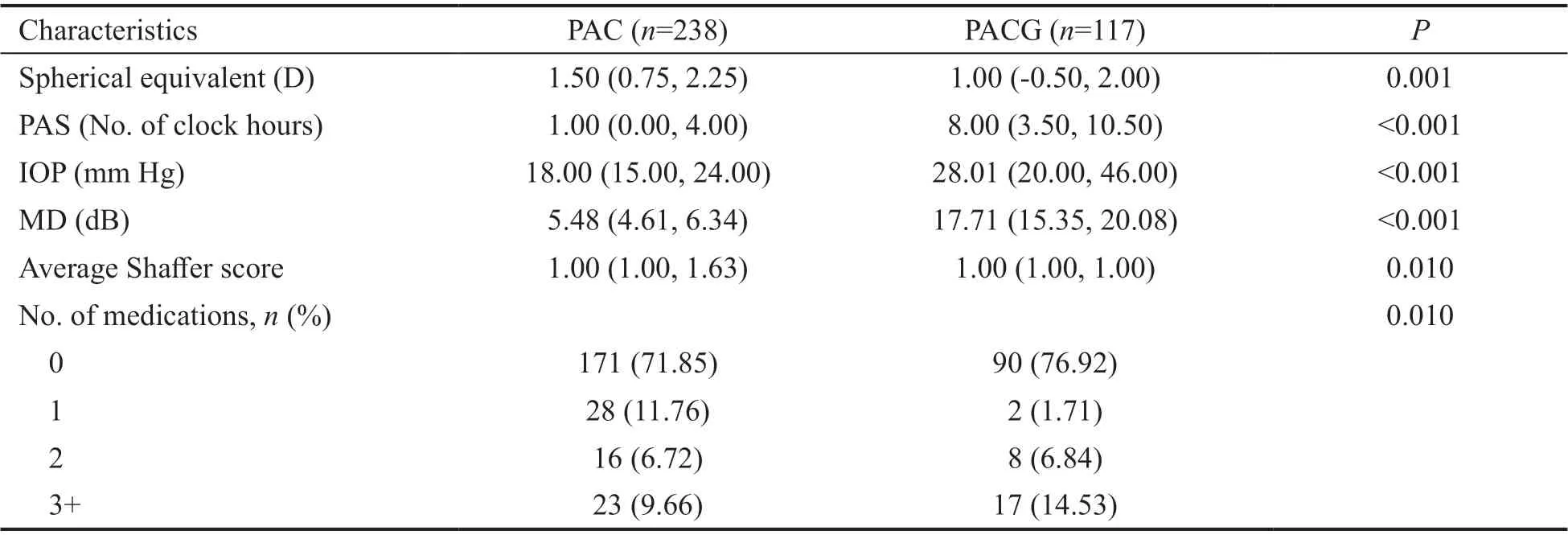
Table 1 Characteristics of study eyes mean (95%CI)

Table 2 Effect of peripheral anterior synechia (quartile) on GON
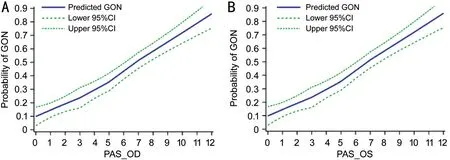
Figure 1 Association between PAS and the prevalence of GON in PACD A: The graph displays the crude association between PAS and the prevalence of GON in right eyes; B: The graph displays the crude association between PAS and the prevalence of GON in left eyes. The curves(95%CI indicated by dotted lines) were derived from smoothing plots (Proc Loess).
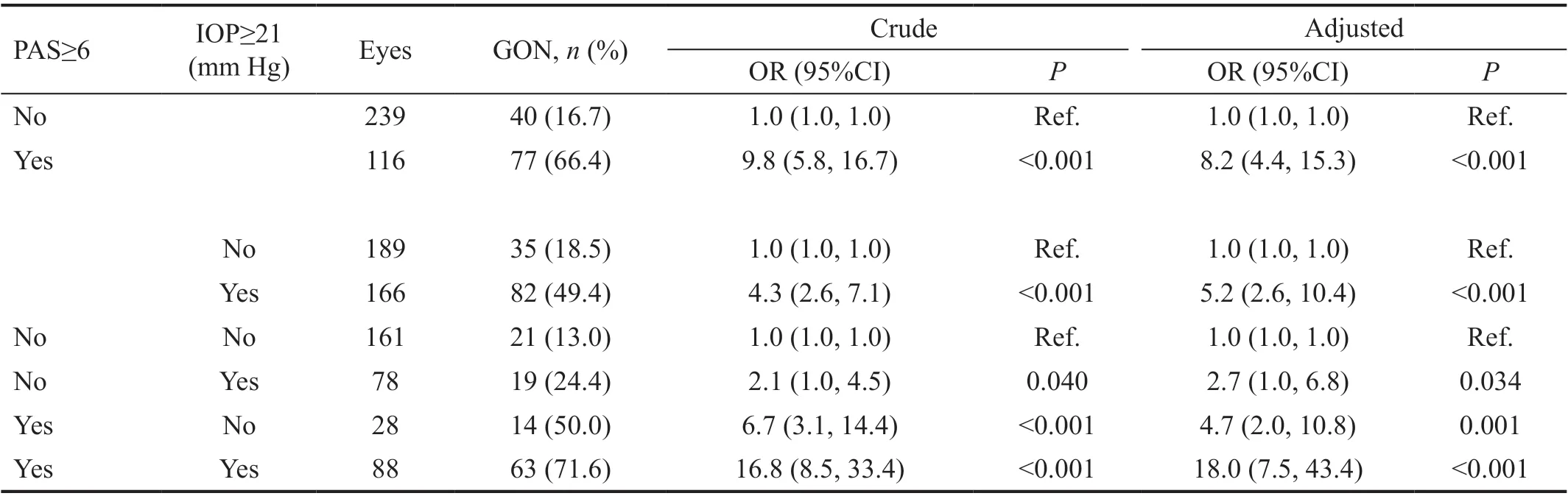
Table 3 Effect of peripheral anterior synechia, intraocular pressure, and their combined effect on the probability of GON
Figure 2 demonstrates that extent of PAS is related to higher IOP. The presence of 5 clock hours to 6 clock hours of PAS seems to be a turning point of IOP.
DISCUSSION
Our research demonstrated PAS is significantly more extensive in PACG patients than in PAC patients and the probability of GON is linearly associated with PAS. It is of great clinical significance that PAS could be a surrogate of PACG prevention or a predicator of GON development. There were a few surveys demonstrating that PAS were significantly associated with IOP and GON in PACG[6-7]. In a population based survey of Mongolian people, 16.7% with PAS had GON compared with 0.9% without PAS[14]. There was a 0.39 mm Hg increase in untreated IOP for each unit increase in clock hours of PAS.Odds of GON increased 1.2 times per 1-mm Hg increase in screening IOP. Choi and Kim[15]suggested that the extent of PAS may be regarded as a reliable indicator of the severity of GON in PACG, and especially in chronic PACG. Spearman’s correlation coefficient between the severity of VF defects and the extent of PAS was 0.348[15].

Figure 2 Distribution of IOP with clock hours of PAS PAS=6 seems the cut-off point for increase in pressure.
It is noteworthy that PAS was found to be independently associated with GON in our study, although with IOP as well as other characteristics such as age, gender, eyes, spherical equivalent, average Shaffer score, and the number of medications had been adjusted, which confirms that PAS is an important risk factor for the development of GON independent of IOP. Comparing with PAS<0.5 clock hours, probability of GON significantly increased while PAS≥3 clock hours(adjusted OR=4.4; 95%CI, 1.5-13.0), especially while PAS≥7 clock hours (adjusted OR=13.8; 95% CI, 4.3-43.6). The finding implied there may be some other mechanisms which have not been discovered previously where PAS can lead to GON solely and not because of higher IOP, emphasizing the need for careful examination of PAS in PAC diseases. It may be that the extent of PAS indicates the importance of the duration, in relation to GON, since the duration of PACG was not available.The more extensive the PAS was, the more chronic the disease process in PACG patients might have been and higher the probability of developing GON[15]. Inflammation factors such as tumor necrosis factor-α (TNF-α), interleukins (ILs), nuclear factor-kappa B (NF-κB) and various cytokines which may increase the susceptibility of optic nerve by leading to the death of the retinal ganglion cells (RGCs) are also considered to be associated with the development of PAS[16-17].
There seems to be no doubt that PAS is a risk factor for increased IOP and GON in PACG. To the best of our knowledge, PAS leads to permanent blockage of the access to the trabecular meshwork, while intermittent blockage happens in appositional angle closure. If untreated, PAS will extend and become confluent. In addition, the decrease of PAS signifies long-term well controlled IOP and GON. It was recorded that in eyes with synechial PAC or PACG, both LPI alone or combined with ALPI provided a significant and equivalent reduction in IOP, but combined laser technique released more of the PAS rather than LPI alone[18]. Goniosynechialysis(GSL) was demonstrated effective in eyes with broad PAS as a surgical technique to strip the PAS from the trabecular surface in the angle so that aqueous can regain access to the meshwork[19-20]. In PACG patients with concomitant cataract,phacoemulsification plus GSL or phacoemulsification alone may suffice to achieve medically well-controlled eyes with minimal PAS, and if not then trabeculectomy should be considered.
The approach to management of PACG varies greatly in different parts of the world. In most parts of the world, LPI is the preferred standard first-line treatment for PACG[21-23].In China, trabeculectomy is a mainstay of initial treatment for PACG and that decision was mainly based on the degree of PAS. LPI is only reserved for PAC with PAS<180°[24].Nevertheless, this threshold of PAS was based on the experiences of clinicians. Figure 2 of distribution of IOP with PAS showed that 5 to 6 clock hours of PAS seemed to be an obvious turning point for higher IOP. Furthermore, eyes with both PAS≥6 clock hours and IOP≥21 mm Hg had the highest risk of GON compared to eyes both with PAS<6 clock hours and IOP<21 mm Hg (OR=18.0, 95%CI: 7.5-43.4;P<0.001).This result was consistent with the viewpoint of Laiet al[25]that the main objectives of a surgical treatment in PACG were the reduction of the IOP, the reopening of the closed angle, and the prevention of a progressive angle closure or reclosure. It is worth mentioning that this study may offered some evidence for threshold of 180° of PAS, which seemed to be a guide to the likely success or failure of treatment in PAC diseases that IOP was more likely to be successful after LPI, or phacoemulsification alone if the PAS<180°[23,26-28].
Our study has some limitations. PAS was documented by gonioscopy and the total clock hours of PAS were recorded.We didn’t record the adhesiveness of the PAS to the trabeculum and the trabecular function, which may also affect IOP. The duration, which was usually difficult to retrospect accurately for PAC patients, especially for chronic PACG patients, can’t be adjusted in these models. But still,this study demonstrated that PAS was obviously associated with GON independent of IOP, which was considered as the medium of PAS and GON previously.
The presence of 5 to 6 clock hours of PAS seemed to be a turning point of IOP. PAS can be considered as an important predictor of high IOP for PAC diseases, and the turning point seemed to be 5 to 6 clock hours.
In conclusion, PAS was significantly associated with IOP and GON in PAC diseases. There may be some other mechanisms have not been discovered previously that PAS can lead to GON independent of IOP.
ACKNOWLEDGEMENTS
Foundations:Supported by Health Innovation Talents in Zhejiang Province (No.2016025); Wenzhou Medical University R&D Fund (No.QTJ13009).
Conflicts of Interest:Zhang M, None; Mao GY, None; Ye C,None; Fan SJ, None; Liang YB, None; Wang NL, None.
猜你喜欢
杂志排行
International Journal of Ophthalmology的其它文章
- lmpact of intraocular pressure fluctuations on progression of normal tension glaucoma
- Effective treatment for secondary angle-closure glaucoma caused by traumatic lens subluxation:phacoemulsification with capsular-tension-ring implantation combined with ophthalmic endoscopecontrolled goniosynechialysis
- Efficacy and safety of newly developed preservativefree latanoprost 0.005% eye drops versus preserved latanoprost 0.005% in open angle glaucoma and ocular hypertension: 12-week results of a randomized,multicenter, controlled phase III trial
- Progressive restrictive strabismus in an infant
- Protective effect of LIF-huMSCs on the retina of diabetic model rats
- Therapeutic effect of a traditional Chinese medicine formulation on experimental choroidal neovascularization in mouse
