Aggregation-induced emission or aggregation-caused quenching?Impact of covalent bridge between tetraphenylethene and naphthalimide
2021-11-06XioxieWeijieChiXieHnChoWngShenghuLiuXiogngLiuJunYin
Xioxie M,Weijie Chi,Xie Hn,Cho Wng,Shenghu Liu,Xiogng Liu,*,Jun Yin,*
a Key Laboratory of Pesticide and Chemical Biology, Ministry of Education, Hubei International Scientific and Technological Cooperation Base of Pesticide and Green Synthesis, International Joint Research Center for Intelligent Biosensing Technology and Health, College of Chemistry, Central China Normal University,Wuhan 430079, China
b Singapore University of Technology and Design, Singapore 487372, Singapore
c School of Chemistry and Chemical Engineering, Wuhan University of Science and Technology, Wuhan 430081, China
1 These authors contributed equally to this work.
ABSTRACT Understanding the physical mechanisms governing aggregation-induced-emission (AIE) and aggregation-caused-quenching plays a vital role in developing functional AIE materials.In this work,tetraphenylethene (TPE, a classical AIEgen) and naphthalimide (NI, a popular fluorophore with ACQ characteristics)were connected through non-conjugated linkages and conjugated linkages.We showed that the nonconjugated-linkage of TPE to NI fragments leads to substantial PET in molecular aggregates and ACQ.In contrast, the conjugated connection between TPE and NI moieties results in the AIE phenomenon by suppressing twisted intramolecular charge transfer.This work provides an important guideline for the rational design of AIE materials.
Keywords:Aggregation-induced-emission Tetraphenylethene Naphthalimide Conjugated-linkage Mechanofluorochromism
Aggregation-induced emission luminogens (AIEgens) have been attracting increasing research interests for their broad range of applications (i.e., such as biosensors, photodynamic therapy agents,organic light-emitting diodes,and wave-guides)[1-10].As a popular AIEgen design strategy, tetraphenylethene (TPE) has been frequently attached to other fluorophores of diverse molecular structures and emission colors to achieve tailored functionalities [1].Yet, this strategy yielded varied successes,resulting in the creations of compounds with both aggregationinduced emission (AIE) and aggregation-caused quenching (ACQ)characteristics[11].To facilitate the effective development of such AIEgens, it is critical to reveal the relationship between their molecular structures and AIE characteristics.
To the end, significant research efforts have been devoted to design and synthesize novel TPE-based AIEgens[12-19].Tang et al.functionalized TPE to several planar ACQ fluorophores, such as anthracene and pyrene, and obtained new AIEgens (TPEpy and TPEAn) [20].The monomers of TPEpy and TPEAn exhibited weak fluorescence in tetrahydrofuran (THF), but their aggregation substantially enhanced the emission intensity.Our group also design a series of TPE-based AIEgens of various colors spanning across the entire visible to near-infrared spectrum,by linking TPE to different fluorophores, such as naphthalimide (NI) and borondipyrromethene (BODIPY) moieties [21].Choi et al.reported several NI-TPE isomeric conjugates, which were connected with single-bond, vinyl, and acetylene linkages between NI and TPE fragments[22].Their results showed that the 3-substituted and 4-substituted dyes with the single-bond linkage exhibited the AIE phenomenon, whereas 4-substituted dyes with vinyl and acetylene linkages showed ACQ.Besides this study,the ACQ phenomenon was noted in several TPE-perylenebisimide compounds[23].It is thus intriguing to understand both the structural factors and photophysical mechanisms governing the distinct AIE and ACQ phenomenon in TPE-fluorophore hybrid dyes, to better aid the rational creations of functional AIE materials.
In this communication, we reported the synthesis, characterizations, and theoretical investigation of the AIE/ACQ characteristics of sixteen TPE-NI derivatives with various substituents and substituent positions (Scheme 1 and Fig.1).Our analysis shows that the nature of the covalent bridges (in spanning the π-conjugation of the TPE-NI hybrid dyes) plays a decisive role in activating AIE in the resulted compounds.
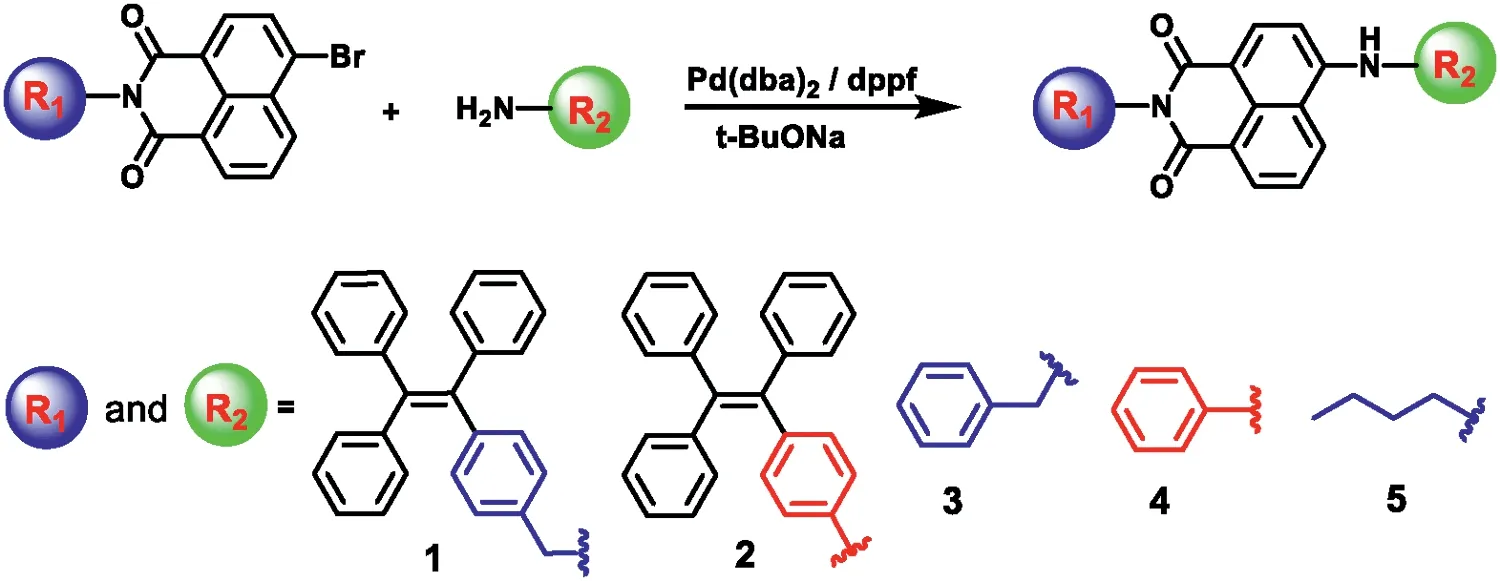
Scheme 1.Synthetic routes of TPE-NI derivatives.
Scheme 1 shows the synthesis route to obtain TPE-NI derivatives.The involved intermediates were synthesized according to previously reported methods with good yields.With the corresponding Br-substituted NI moiety and NH2-substituted aromatic moiety, the targeted compounds were prepared by a typical Pd-catalysed Buchwald reaction.All compounds were purified by silica gel columns, and their structures were well characterized by1H,13C NMR spectroscopy,and EI-MS.Fortunately,single crystals of 1-NI-3, 3-NI-4, and 3-NI-2 were obtained by diffusing hexane into DCM, THF, or EA solution at room temperature, respectively (Fig.S30 in Supporting information).The detailed synthesis procedures and characterizations are available in the supporting information (Figs.S32-S78 in Supporting information).
Many NI fluorophores exhibited ACQ characteristics, due to strong intermolecular π-π interactions[24],such as compounds 4-NI-3, 4-NI-5, 5-NI-3, and 5-NI-5 (Fig.1).In the tetrahydrofuran(THF)/water binary solution of these compounds, we noted a consistent drop in emission intensities due to the formation of molecular aggregates, as the volume ratio (fw) of water increased(Figs.S2-S5 and Table S1 in Supporting information).These results also suggest that a phenyl substituent is not bulky enough to effectively separate NI dimers and avoid strong intermolecular interactions.To further decrease intermolecular interactions and prevent ACQ,we decided to link a bulky TPE unit to NI.The UV-vis absorption and fluorescence spectra of the mixture of TPE and 5-NI-5 demonstrated that simple mixing of TPE and NI fluorophores could not change the ACQ phenomenon of NI fluorophores(Fig.S29 in Supporting information).Therefore,we covalently linked TPE to NI via two types of linkages: non-conjugated linkages and conjugated linkages.
We firstly connected TPE to NI via non-conjugated linkers.The non-conjugated covalent linkage could be realized either via inserting a -CH2- moiety between TPE and NI, such as in compounds 1-NI-3, 3-NI-1, 4-NI-1, and 1-NI-1 (Fig.1), or choose connection sites that disrupt conjugations(such as in 2-NI-1 and 2-NI-3 in Fig.1).In 2-NI-1 and 2-NI-3,the bond alternation breaks at the imide functionalization site of NI.
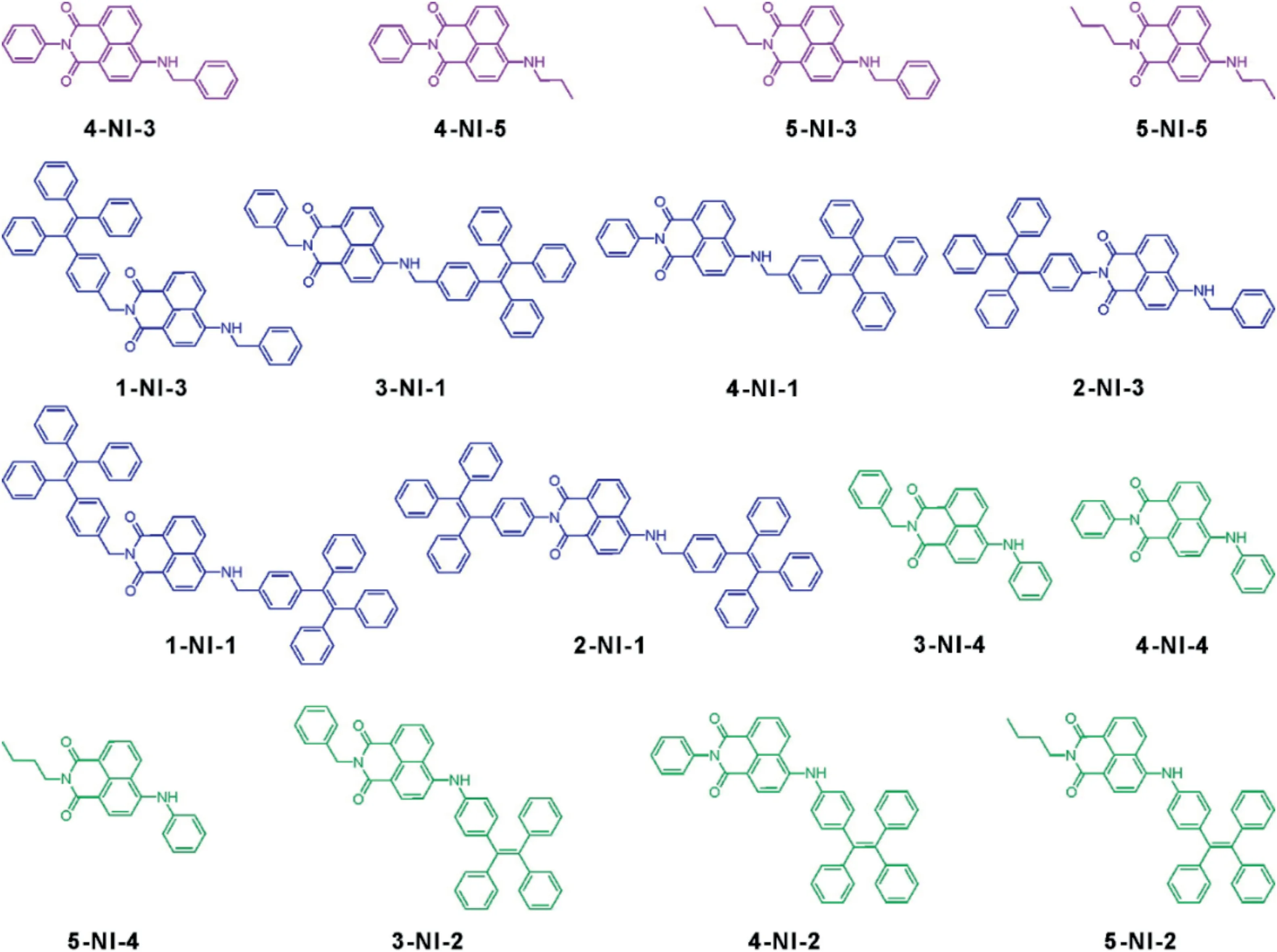
Fig.1.Compounds reported in this work.
We subsequently synthesized these six compounds and investigated their spectral properties (Fig.2 and Figs.S5-S10 in Supporting information).In these compounds,two distinct UV-vis absorption peaks are noted, registered at ~290 nm and ~440 nm,respectively (Fig.2a).These peaks matched very well with the reported data of TPE and NI derivatives, respectively [25,26].Subsequent quantum chemical calculations further support these assignments.Time-dependent density functional theory(TD-DFT)calculations show that the first and second absorption bands are contributed by the photoexcitation of the NI and TPE fragments,respectively (Fig.2b) [27,28].Moreover, the emission wavelength from the NI fragment showed an obvious redshift as the polarity of the solvent increases (Fig.2c).
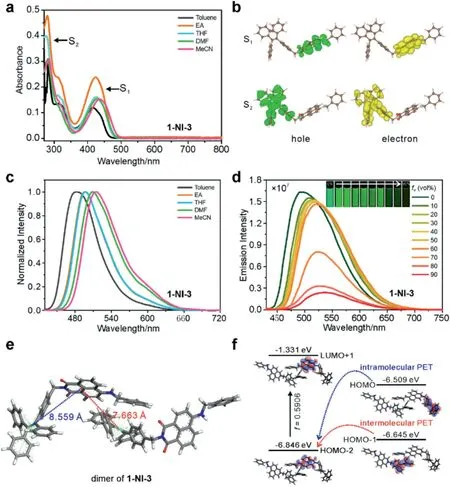
Fig.2.(a) UV-vis absorption spectra of 1-NI-3 in various solvents; [1-NI-3]=10 μmol/L.(b) Electron and hole distributions of 1-N1-3 during the S1 (the first absorption band) and S2 (the second absorption band) vertical excitation.(c)The normalized fluorescence spectra of 1-NI-3 in various solvents; [1-NI-3]=10 μmol/L;λex=420 nm.(d) The fluorescence spectra of 1-NI-3 in the binary mixture of THF/water as a function of the volume ratio of water(fw);the inset shows the photographs of the samples under UV radiations in a dark room; [1-NI-3]=10 μmol/L;λex=420 nm.(e) A dimer structure of 1-NI-3 as extracted from its single crystal structure.(f)Calculated frontier molecular orbitals of this dimmer in vacuo, which suggest both intramolecular and intermolecular PET from TPE to NI fragments in vacuo; the energy levels are not drawn in scale for clarity.
Next, we measured the fluorescence intensities of these compounds in the binary THF/water mixture.Taking 1-NI-3 as one representative example,as fwincreases from 0 to 90%to induce the formation of aggregates,the emission peak experiences a slight red shift from 491 nm to 535 nm (Fig.2d).This redshift is attributed to the enhanced ICT effect in the NI fragment(as solvent polarity increases).Notably,the corresponding quantum yield of 1-NI-3 considerably dropped from 66.5%to only 10.4%,exhibiting the ACQ characteristics.Other compounds in this group demonstrated similar ACQ effects, as reflected by a significant reduction of fluorescence intensity (Figs.S11-S15 in Supporting information).
To elucidate this ACQ mechanism, we performed DFT and TDDFT calculations on the dimer of 1-NI-3 at M06-2X/def2-SVP level[29].The initial structure is from the crystal structure of 1-NI-3(Table S2 in Supporting information).We showed that the photoinduced electron transfer (PET) process could be substantially activated as the distance between TPE and NI becomes smaller in the excited state,i.e.,via the formation of aggregation.For example,intramolecular TPE-NI distance amounts to 8.559 Å (relatively large).In contrast, the distance between NI and another TPE unit from a neighbouring molecule(in molecular aggregates)decreases to only 7.663 Å(Fig.2e).The reduced distance could substantially activate PET from TPE to NI, thus resulting in the ACQ of 1-NI-3[30,31].Indeed, our TD-DFT calculation on the dimmer of 1-NI-3 showed that S1photoexcitation is mainly from HOMO-2 to LUMO(in the NI fragment).The electron from HOMO-1 or HOMO sitting at the TPE fragments could transfer to HOMO-2 and quench the fluorescence via PET (Fig.2f) [32].
Next, we connected TPE to NI via conjugated linkages.We hypothesized that twisted intramolecular charge transfer (TICT)rotation could be employed in NI derivatives to enable AIE characteristics [33-35].During TICT rotation, the amino group at the 4-positon of NI experiences a ~90°rotation with respect to the NI scaffold,forming a non-emissive specie[36,37].However,upon molecular aggregation, TICT rotation could be effectively suppressed, thus recovering bright emissions from NI (provided that intermolecular π-π interactions are weak in molecular aggregates).Moreover,the TICT rotation could be activated by replacing the secondary amino group with either a dialkylated amino group or an amino-phenyl group [38,39].
To verify this speculation, we designed, synthesized, and characterized 3-NI-4, 4-NI-4, and 5-NI-4.We also successfully obtained the crystal structure of 3-NI-4 (Table S3 in Supporting information).Notably, as the solvent polarity increases (from toluene to acetonitrile),the emission intensity of 3-NI-4 and 4-NI-4 showed a large drop (Figs.S16 and S17 in Supporting information).This drop of emission intensities is attributed to the formation of TICT,as TICT is usually activated in polar solvents[21].Indeed,our viscosity dependent studies in the binary mixture of methanol/glycerol showed that increasing viscosity enhanced the emission intensities of 3-NI-4, corroborating the TICT mechanism (Fig.3a and Fig.S18 in Supporting information).Finally, the potential energy surface of excited-state calculations[38]also showed that the TICT state is energetically favorable for 3-NI-4 in polar solvents (Fig.3b).Similar polarity-dependent and viscosity-dependent emission results are also obtained in 4-NI-4,and 5-NI-4 (Figs.S19 and S20 in Supporting information).These results affirmed the TICT fluorescence quenching mechanism.Yet,the emission intensities of 3-NI-4,4-NI-4,and 5-NI-4 in molecular aggregates remained weak,presumably due to strong intermolecular interactions in molecular aggregates.
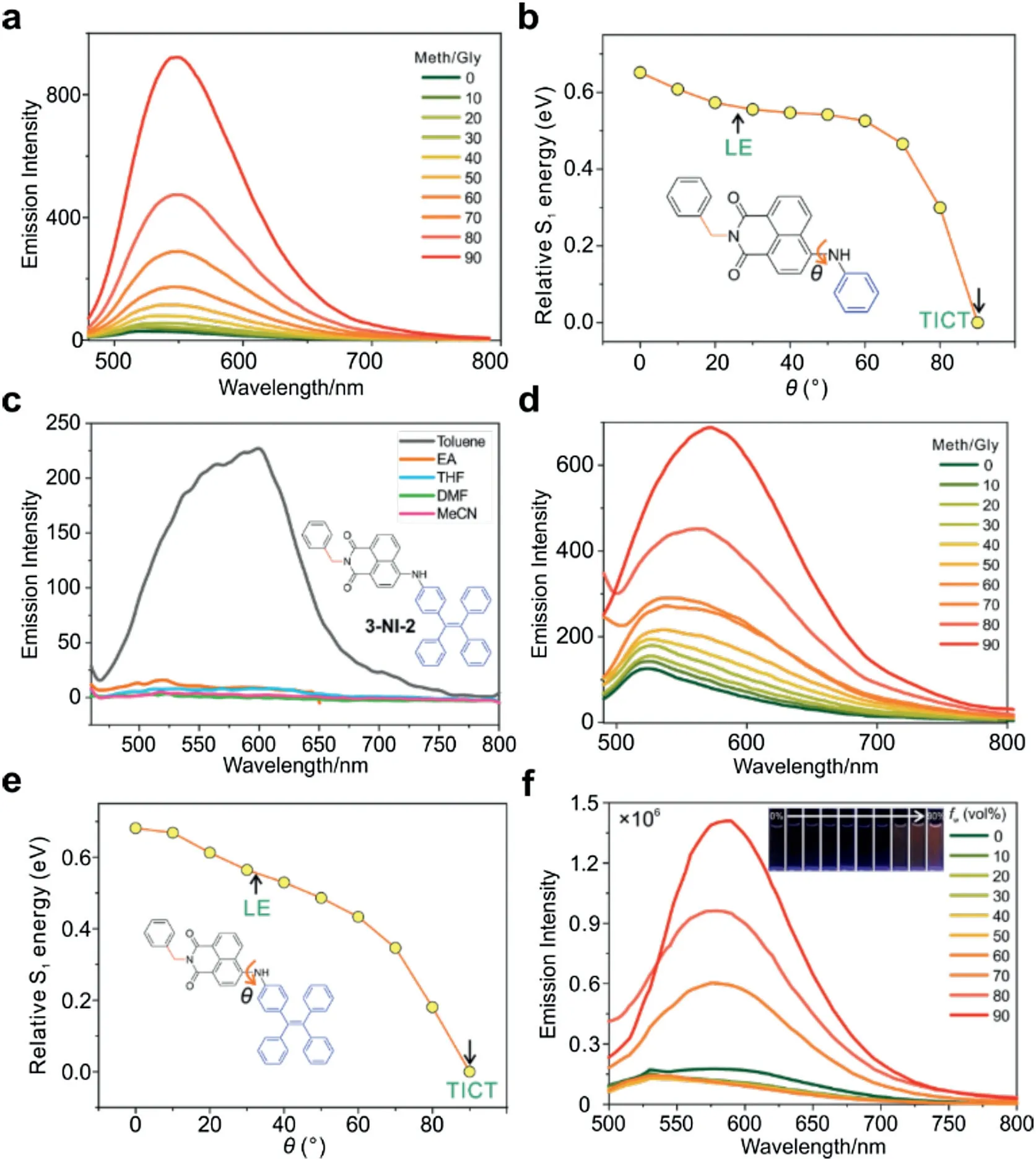
Fig.3.(a) The viscosity dependence of the emission intensities of 3-NI-4 in the binary mixture of methanol and glycerol, as a function of the volume ratio of glycerol;[3-NI-2]=10 μmol/L,λex=450 nm.(b)The potential energy surface in the S1 state of 3-NI-4 as a function of the amino group rotation in tetrahydrofuran.(c)The emission spectra of 3-NI-2 in various solvents; [3-NI-2]=10 μmol/L,λex=450 nm.(d)The viscosity dependence of the emission intensities of 3-NI-2 in the binary mixture of methanol and glycerol,as a function of the volume ratio of glycerol;[3-NI-2]=5 μmol/L,λex=450 nm.(e)The potential energy surface in the S1 state of 3-NI-2 as a function of the amino group rotation in tetrahydrofuran; (f) The fluorescence spectra of 3-NI-2 in the binary mixture of THF/water as a function of the volume ratio of water (fw); the inset shows the photographs of the samples under UV radiations in a dark room; [3-NI-2]=10 μmol/L,λex=450 nm.

Fig.4.(a)The emission spectra of 3-NI-2(λex=450 nm)in the crystalline(unground and annealed)and the amorphous(ground)states.(b)The photography of 3-NI-2 under daylight and UV light(in a dark room)upon grinding and annealing.(c)The powder X-ray diffraction patterns of 3-NI-2 in the crystalline(unground and annealed)and the amorphous (ground) states.
Inspired by the emission characteristics of 3-NI-4, we next connected TPE to NI via conjugated linkages for two considerations: (1) TPE is much bulky and could effectively suppress intermolecular interactions in aggregates; (2) Conjugated linkage of TPE preserves the π-conjugation and could retain TICT in the resulted compounds and introduce additional rotational modes due to the flexible structure of TPE.Collectively,these two factors could enable AIE characteristics based on the RIR mechanism[20].
We have thus designed and synthesized compounds 3-NI-2,4-NI-2,and 5-NI-2.We also obtained the crystal structure of 3-NI-2(Table S4 in Supporting information).As expected, when solvent polarity increases from toluene to acetonitrile, the emission intensities of these compounds experience a rapid reduction, due to the activation of TICT in polar solvents(Fig.3c and Figs.S21-S23 in Supporting information).In contrast,increasing viscosity greatly enhanced the emissions,indicating the inhibition of TICTand other rotation modes in these compounds (Fig.3d and Fig.S24 in Supporting information).Potential energy surface calculations further affirmed the presence of the TICT state in the monomers of these compounds (Fig.3e, Figs.S25 and S26 in Supporting information).
In contrast, in the binary mixture of THF and water,increasing fwis associated with a continuous and significant intensification of fluorescence by 11 times for 3-NI-2, 42 times for 4-NI-2, and 4 times for 5-NI-2, respectively (Fig.3f, Figs.S27 and S28 in Supporting information).The bright aggregate emissions suggest that TPE effectively avoid intermolecular interactions in the solidstate.It is thus clear that the conjugated connection of TPE to a fluorophore “kills two birds” (TICT+steric hindrance) with one stone, and represents a promising design strategy to develop AIEgens.In addition to the significant AIE characteristics, we hypothesized that conjugated linkages of TPE to NI might enable notable mechanofluorochromism.This is because the TPE moiety is highly flexible.Changing the conformation of TPE via mechanical grinding could greatly affect its electron-donating strength to the NI moiety, thus shifting the emission wavelength [40].Moreover,mechanical grinding may further modify intermolecular interactions due to transitions between the crystalline and amorphous phases.
Indeed,our analysis of the intermolecular contacts in the crystal structure of 3-NI-2 revealed multiple intermolecular N-H•••O, CH•••O, C-H•••π hydrogen bonds, and π-π stacking interactions(Fig.S31 in Supporting information).The alternation of these contacts (intermolecular interactions) along with the conformational changes in TPE is likely to cause interesting mechanochromic properties.
To verify this hypothesis,we tested 3-NI-2 and found that this compound indeed possesses favourable mechanochromic properties(Figs.4a and b).After grinding,the peak emission wavelength of 3-NI-2 exhibited a notable redshift from 582 nm to 605 nm.The peak emission intensity was also enhanced, with the absolute quantum yield changing from 4.16%in the pristine form to 14.02%upon grinding,or intensified by ~3 times.The spectral changes are reflected by significant colour changes both under ambient light and UV light (Fig.4b).Interestingly, upon annealing at 180°C for 5 min, the fluorescence properties reverted to the original state.
Along with these spectral changes, the diffraction peaks in PXRD results demonstrated that grinding effectively convert the powder from crystalline to amorphous phase, and the crystalline phase was recovered after annealing (Fig.4c).
In summary, through the systematic synthesis and characterizations of 16 compounds, we showed that the nonconjugatedlinkage of TPE to NI fragments leads to substantial PET in molecular aggregates,endowing these compounds with aggregation-causedquenching (ACQ) characteristics.In contrast, the conjugated connection of TPE to NI moieties not only electronically activates TICT in polar solvents in the resulting compounds but also provides steric hindrance to minimize intermolecular interactions as such compounds form aggregates.Accordingly,the inhibition of TICT in the molecular aggregates leads to significant AIE.This rational modulation of ACQ and AIE via linker engineering is likely to apply to many other fluorophores and will provide an important guideline for developing functional materials (i.e., for photothermal therapies and bioimaging applications).
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
J.Yin acknowledge financial support from the National Natural Science Foundation of China (Nos.21676113, 21772054), Distinguished Young Scholar Program of Hubei Province (No.2018CFA079),the 111 Project B17019,the Scholar Support Program of CCNU (No.0900-31101090002), and the Excellent Doctoral Dissertation Cultivation Grant of CCNU from the colleges’ basic research and operation grant(MOE,No.2019YBZZ029).The study was supported by Ministry of Education Key Laboratory for the Synthesis and Application of Organic Functional Molecules (No.KLSAOFM2012), Hubei University, China.And also supported by excellent doctorial dissertation cultivation grant of CCNU from the colleges’basic research and operation of MOE(No.2019YBZZ029).X.Liu is indebted to A*STAR under its Advanced Manufacturing and Engineering Program(No.A2083c0051).The authors would like to acknowledge the use of the computing service of SUTD-MIT IDC and the National Super-Computing Centre (Singapore).
Appendix A.Supplementary data
Supplementary material related to this article can be found,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.12.031.
杂志排行
Chinese Chemical Letters的其它文章
- Molecular recognition triggered aptazyme cascade for ultrasensitive detection of exosomes in clinical serum samples
- Electrosynthesis of 1-indanones
- Current signal amplification strategies in aptamer-based electrochemical biosensor: A review
- Photo-crosslinkable hydrogel and its biological applications
- STING-activating drug delivery systems: Design strategies and biomedical applications
- The carbon nanotubes-based materials and their applications for organic pollutant removal: A critical review
