Egg white as a natural and safe biomaterial for enhanced cancer therapy
2021-11-06JunHungXinruYouPeikunXinZhipengGuChunChenJunWu
Jun Hung,Xinru You,Peikun Xin,Zhipeng Gu,Chun Chen,*,Jun Wu,,*
a Department of Pediatrics, The Seventh Affiliated Hospital of Sun Yat-sen University, Shenzhen 518107, China
b School of Biomedical Engineering, Sun Yat-sen University, Guangzhou 510006, China
c College of Polymer Science and Engineering, Sichuan University, Chengdu 610065, China
ABSTRACT The last few decades have witnessed the emergence of a very large variety of engineered nanomaterials.However, it is far from to meet the growing clinical demand.Actually, nature itself is an excellent nanotechnologist,and provides us with a range of wonderful materials,from inorganic particles found in non-life bodies to biofilms, like platelets, erythrocyte membranes, produced by many bacteria or cells.These nanomaterials are entirely natural, and not surprisingly, there is a growing interest in the development of natural nanoproducts.Native components-inspired biomaterials have gained considerable attention owing to their safety and functions.In this study,egg white was developed as drug carrier to load PTX by a green and simple one-pot method,and systematic characterization was completed.The results indicated that PTX@EW NPs possess excellent biocompatibility, enhanced tumor targeting capability, effectively reducing the toxic side effects of PTX.The obviously enhanced antitumor effect further confirmed EW was a highly prospective biomaterial in the nano-carrier industry.
Keywords:Egg white Natural biomaterial Nanocarrier Biosafe Antitumor
Conventional therapeutic agents are often exposed to a number of issues, for example, poor water solubility, lack of targeting capability, nonspecific distribution, systemic toxicity, and low therapeutic index[1,2].Fortunately,positive changes are occurring with the rise of nanotechnology in medicine [3,4].The last few decades have witnessed the emergence of a very large variety of engineered nanomaterials [5,6].Currently many substances are still under investigation for drug delivery to reduce toxicity and side effects of drugs.For example, polymer nanoparticles could increase the aqueous solubility and improve the bioavailability of many therapeutic drugs, and even have demonstrated ability in protecting encapsulated components from enzymatic attack,controlling release rates [7].Protein-based drug delivery systems are also found to be ideal platforms [8,9].Multiple proteins have been applied for drug delivery due to their biocompatibility,biodegradability and low toxicity,including the apoferritin protein,viral capsids,albumin,soy and whey protein,and gelatin[10-12].
However,it is still far from to meet the growing clinical demand.Many types of polymer components are well-documented to work as drug carriers, but their monomers are usually not directly available,which require complex synthesis steps or consume much time during the extraction process.Additionally, some of the polymer components need to use toxic solvents or other extreme conditions to form microparticles, which might cause debatable biocompatibility problems [13].Actually, nature itself is an excellent nanotechnologist,and he offers a range of ideal candidate materials, from inorganic particles found in non-life bodies to biofilms,like platelets,erythrocyte membranes,produced by many bacteria or cells[14-16].These nanomaterials are entirely natural and with amazing features.Not surprisingly, there is a growing interest in the development of natural nanoproducts.Native components-inspired biomaterials have gained considerable attention owing to their safety and functions [17,18].
Egg white protein (EWP), consist of ovalbumin (OVA, 54%),ovotransferrin (OT, 12%) and other components, is a highly prospective biomaterial.Excellent nutritional value, digestibility,self-assembly and amphiphilic properties make it a candidate platform for drug delivery [19-22].There have been numerous published attempts in fabrication of nanoparticles using extracted proteins, but few studies have been performed to verify the feasibility to directly use natural raw proteins from food to work as drug carriers.
In this study, egg white was developed as drug carrier to load PTX just by ultrasonic dispersion at low temperature, without introducing any toxic organic solvents.It is a simple and green onepot method.The properties of the obtained nanoparticles(PTX@EW NPs)were systematically examined to verify our design.Undoubtedly, egg components-based drug delivery is more available due to at least the extremely low cost.Combined with the non-toxic and environmentally benign preparation process,we believed such natural nanoparticles will not only stimulate research and add a greener outlook to a traditionally high-tech field, and they will also provide solutions for a range of problems faced present in nanomedicine field.
As shown in Fig.1a, egg white-based PTX delivery system was prepared using ultrasonication nanotechnology.Compared to nanoprecipitation or other nano-formulation techniques, this method was simple and green.Briefly, an aqueous solution of egg white and PTX ethanol solution were mixed under ultrasonication,and ice bath was applied meanwhile to keep the system in low temperature.Transmission electron microscopy (TEM)images showed that both the EW NPs and PTX@EW NPs are with spherical shape structure.The particle size was evaluated using dynamic light scattering (DLS).Figs.1b and c showed that the average particle size of EW NPs or PTX@EW NPs are all sub-100 nm after systematic screening experiments (Table S1 in Supporting information).The small size and effective therapeutic drug loading will facilitate the accumulation of the therapeutic drug at tumor site by EPR effect.The PTX loading efficiency was 7.2%±0.54%,and the encapsulation efficiency was 69.4% ± 3.5%.The stability evaluation in vitro indicated that the particle size would not change obviously in PBS and 10% fetal bovine serum (FBS) solution over 72 h (Fig.1d), suggesting the obtained platform was a stable drug delivery system.The drug release profiles under different conditions(different pH buffer solutions,different concentrations of glutathione (GSH) solutions) were measured (Fig.1e), and the results displayed that acid environment is beneficial to the PTX release,and GSH has little effect to the release behavior.As known,the structural stability of proteins which are the main components of EW NPs was easily destroyed by acid,this resulted in the gradual collapse of EW NPs and the PTX release.However,the release rate was obviously slower than in acidic environment.Possible reasons for this are multifold,and the amount of cysteine which equip the GSH-sensitive disulfide bonds is not negligible.Reports have indicated cysteine was mainly concentrated in the yolk,in contrast,there was less in egg white.But this does not affect the functions of EW NPs to delivery drugs.It can be assumed that complex acidic environment in tumor cells will induce the PTX release from EW NPs and lead to local high concentration of PTX, which undoubtedly favor to the enhancing antitumor effect.
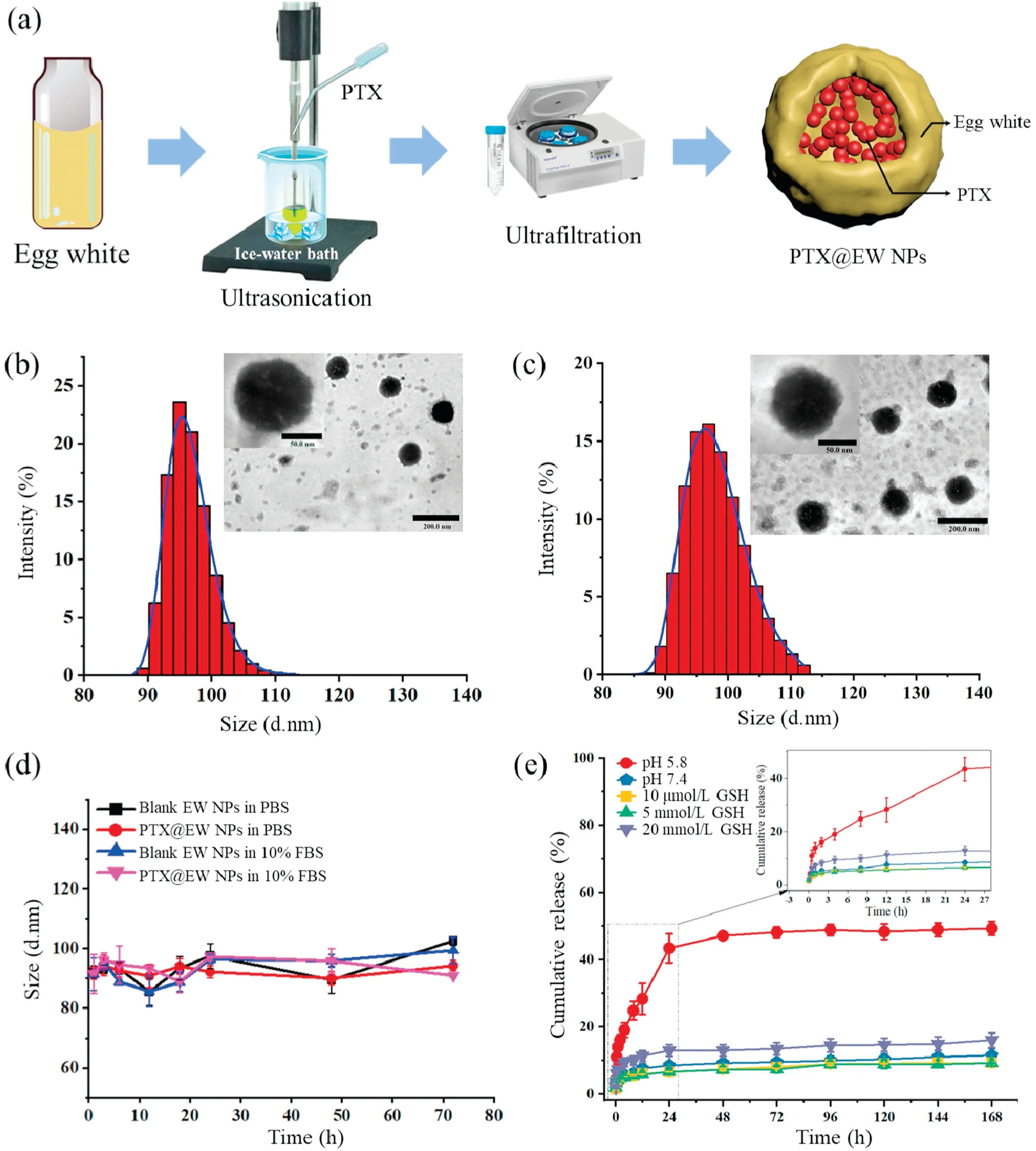
Fig.1.(a) Schematic of simplified steps to prepare PTX@EW NPs.(b) Representative TEM images and DLS size of EW NPs.(c) Representative TEM images and DLS size of PTX@EW NPs.(d)Time-dependent particle size distribution in PBS and 10%FBS solutions at 37°C.(e)PTX release profiles of formulations in different pH buffer solution(pH 5.8, 7.4) and different concentration GSH solution (10μmol/L, 5 mmol/L, 20 mmol/L) at 37°C.
To evaluate the biosafety of egg white nanoparticle, CT26.WT cells were selected to test the in vitro cytotoxicity.As shown in Fig.2a, the viability decreased obviously with PTX concentration increasing, while the blank EW NPs group displayed no declining trends incubating for 48 h.Moreover, apoptosis assays results(Figs.2b and c), there was no significant difference between Control and Blank EW NPs groups,further confirmed the excellent biocompatibility of EW NPs in vitro.While,working as drug carrier material, EWP could enhance the antitumor effect of PTX, which can be concluded by the 36.3% apoptotic rate of PTX@EW NPs comparing to the 23.4% apoptotic rate of PTX.
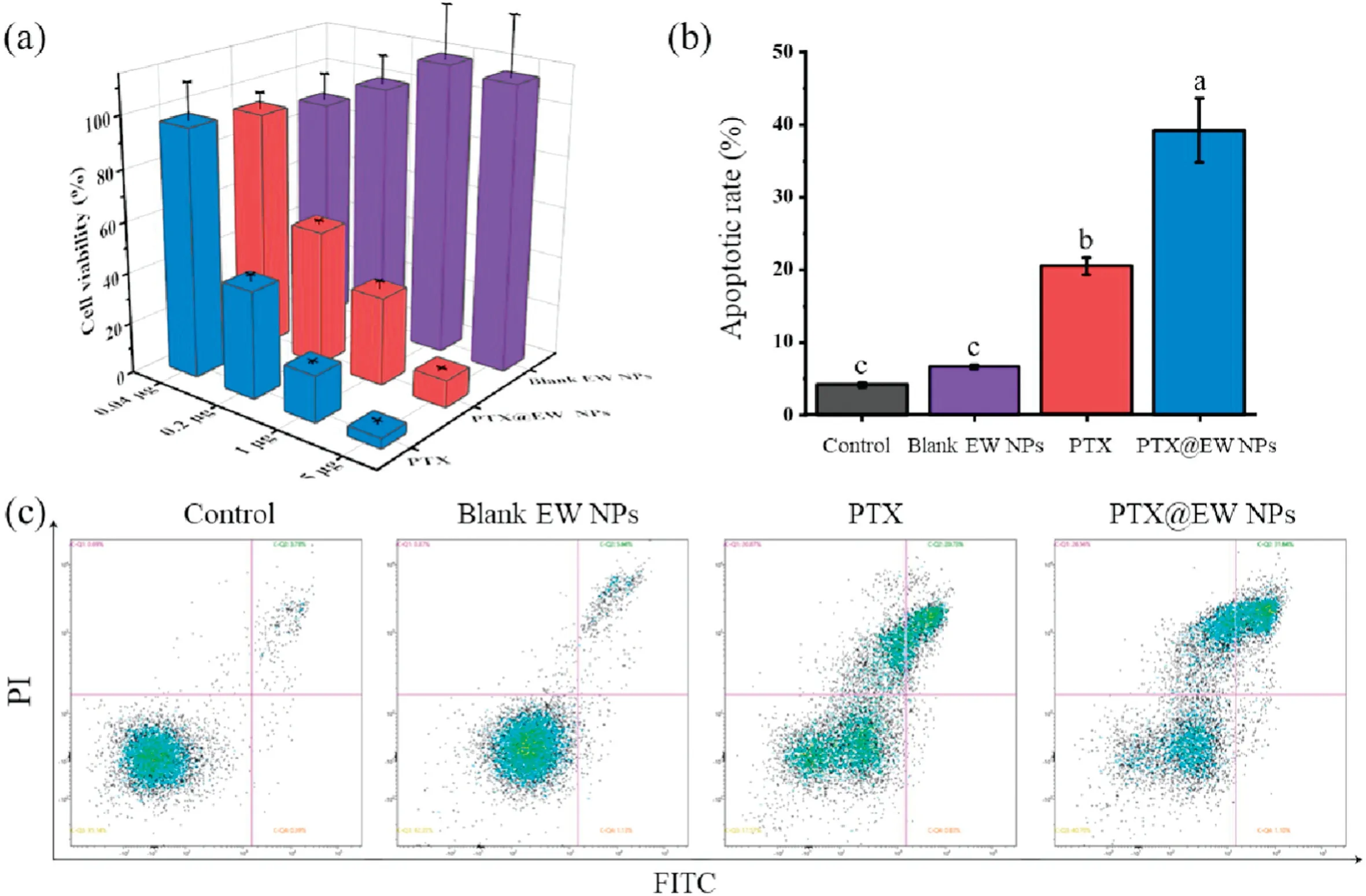
Fig.2.(a)In vitro cytotoxicity against CT26.WT cells after incubating with PTX,Blank EW NPs and PTX@EW NPs for 48 h.(b)Quantitative analysis of the in vitro apoptosis of CT26.WTcells exposed to PBS(control),Blank EW NPs,PTX(2 μg/mL)and PTX@EW NPs(PTX dose:2 μg/mL)for 24 h.Different letter in superscript on the bar graph indicate significant at 0.01 level by Tukey’s test.(c) In vitro apoptosis analysis of CT26.WT cells using Annexin V-FITC/PI staining method.
In order to analyze the behavior of EWNP sincells,Dilwasapplied to mark the nanoparticle and lysosome was labelled using green fluorescence dye.As shown in the CLSM images (Fig.3a), after incubation for 1 h,a lot of NPs distributed around cell nucleus,and the fluorescence of NPs located in the lysosome site,suggesting the successful internalization and captured by endo-lysosomes.Increased fluorescence intensity could be observed after incubating for 4 h,and then almost maintained at a relative stable status within 8 h.This could initially judge that the uptake balance was reached within 4 h.Additionally, the cellular uptake was quantitatively confirmed by flow cytometry(FCM).As illustrated in Figs.3b and c,uptake phenomenon occurred quickly after incubate, and the absorption equilibrium was reached within almost 2 h.
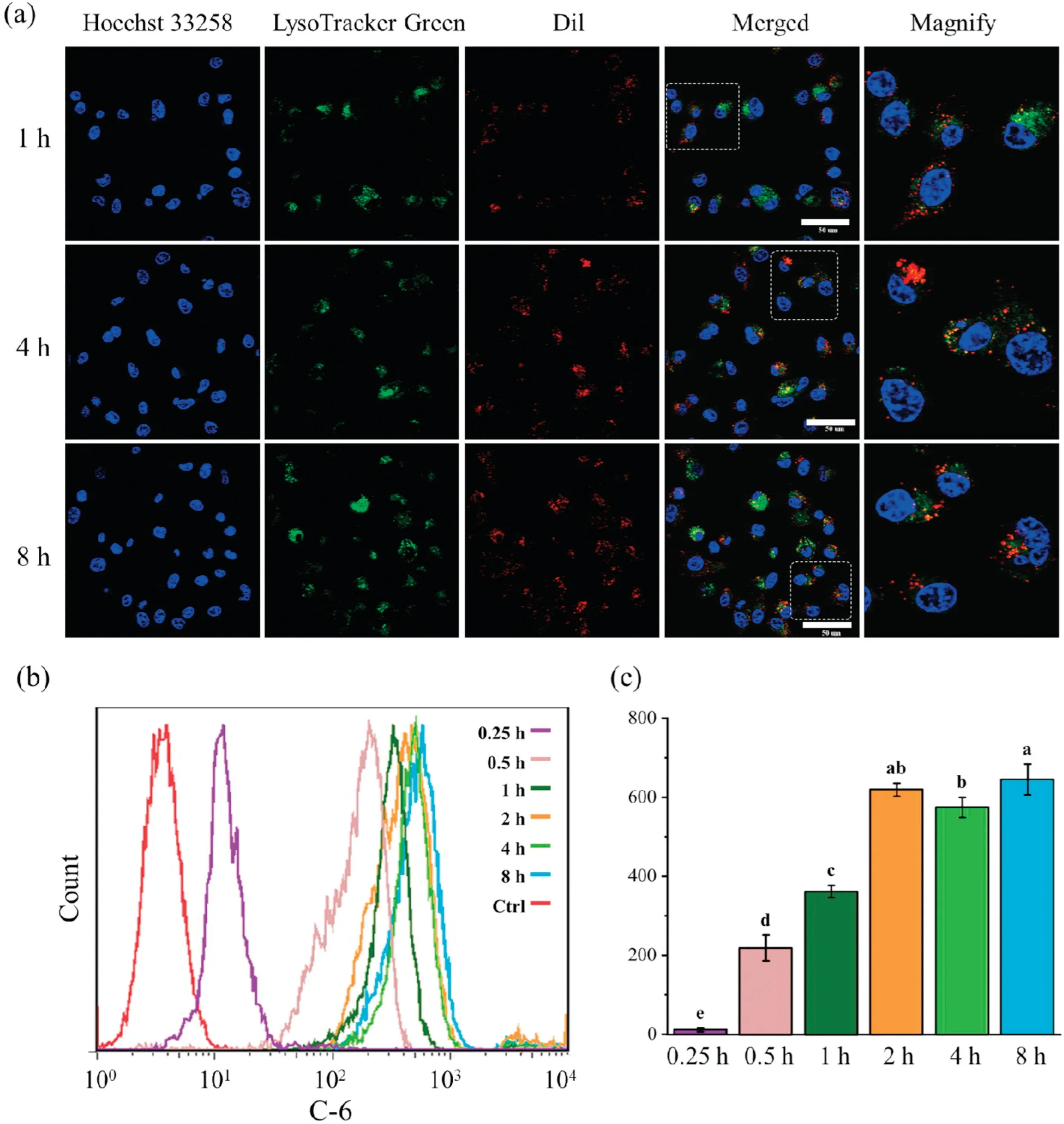
Fig.3.(a)Representative CLSM images indicating the location of Dil@EW NPs in CT26.WT cells(red)after incubation for 1,4 and 8 h.Nuclei were stained with hoechst 33258(blue),lysosomes were stained with lysotracker green DND-26(green).Scale bar:50μm.(b)In vitro uptake of C6@EW NPs by CT26.WTcells analyzed using FCM.(c)The mean fluorescent intensity of C6 after incubation with C@EW NPs for 0.25,0.5,1,2,4 and 8 h,respectively.Different letter in superscript on the bar graph indicate significant at 0.01 level by Tukey’s test.
As seen in Fig.4, Wound healing migration assay was carried out to evaluate the potential anti-migration activity of PTX@EW NPs.The PBS groups displayed strong migration abilities, the wound healing rate of scratch area have exceeded 80% after 72 h.While PTX@EW NPs groups showed significant antimigration effects, with 5.2% ± 0.5% wound healing rate.To verify this inhibition effect, invasion assays were further conducted.Similar trends to the wound healing assay results was observed,fewer cells in PTX or PTX@EW NPs groups migrated to the other side of the Transwell insert.While a visible area of invasive cells(stained with crystal violet)was observed in PBS and blank EW-NPs groups,and the average amount of invasive cells was 327.8±35.6 and 317.4±31, respectively.Migration and invasion assay results indicated that PTX@EW NPs could effectively inhibit the invasive activity of CT26.WT cells.

Fig.4.(a)Representative wound healing images after scratch incubation with PBS,PTX and PTX@EW NPs for 72 h.(b)Representative images of invasive CT26.WTcells treated with PBS, blank EW NPs, PTX and PTX@EW NPs, respectively.Scale bar: 100 μm.(c) Wound healing ratio after treatments with PBS, PTX and PTX@EW NPs for 72 h.(d)Quantitative analysis of the invasive CT26.WT cells treated with different formulations.Different letter in superscript on the bar graph indicate significant at 0.01 level by Tukey’s test.
To investigate the drug behavior in vivo,the PTX concentration vs.time profiles of different groups were analyzed (Fig.5), and pharmacokinetic parameters were also calculated.As shown in Table S2,the AUC value(area under the curve)of the PTX@EW NPs groups was 40.53 hμg/mL, which was almost 3 times that of the free PTX groups.The MRT (mean residence time) of the PTX@EW NPs groups were also obviously longer than that of free PTX groups (1.49 h vs.6.03 h), suggesting a prolonged PTX circulation time improved by EW NP.As known, upon injection into the bloodstream, nanomedicines are covered by a variety of proteins(protein corona) [23], this phenomenon might offer one explanation as to why EW NPs could avoid rapid clearance caused by immune system.Of course, the appropriate particle size distribution is mandatory to achieve outstanding long-circulating performance.This effect will significantly prolong the exposure time of tumor cells to PTX and lead to more apoptosis,undoubtedly caused an enhanced antitumor effect.
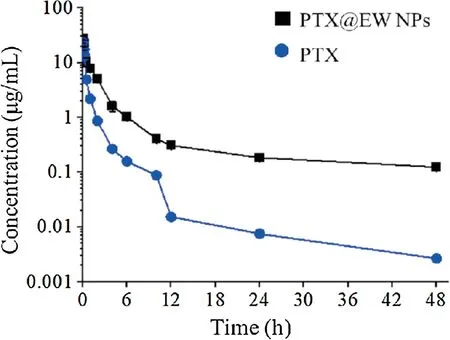
Fig.5.Time-concentration curves of PTX after intravenous injection with free PTX and PTX@EW NPs.
In vivo biodistribution of PTX@EW NPs is the key parameter linking to the clinical effect.Herein,Dir and Dir@EW NPs were used to compare the enhanced targeting to tumor,and the results were taken by IVIS imaging system.As shown in Fig.6, obvious fluorescence signals were observed in tumor regions in Dir@EW NPs,which confirming the significant targeting tumor property of Dir@EW NPs.Organs were excised at last to photograph, it was apparent that besides tumor, heart and spleen also displayed fluorescence signal for Dir@EW NPs.This phenomenon could be attributed to the rich blood perfusion of these organs.Overall,PTX@EW NPs displayed significant tumor targeting behavior after intravenous injection, which could speculate a better antitumor effect for Dir@EW NPs.
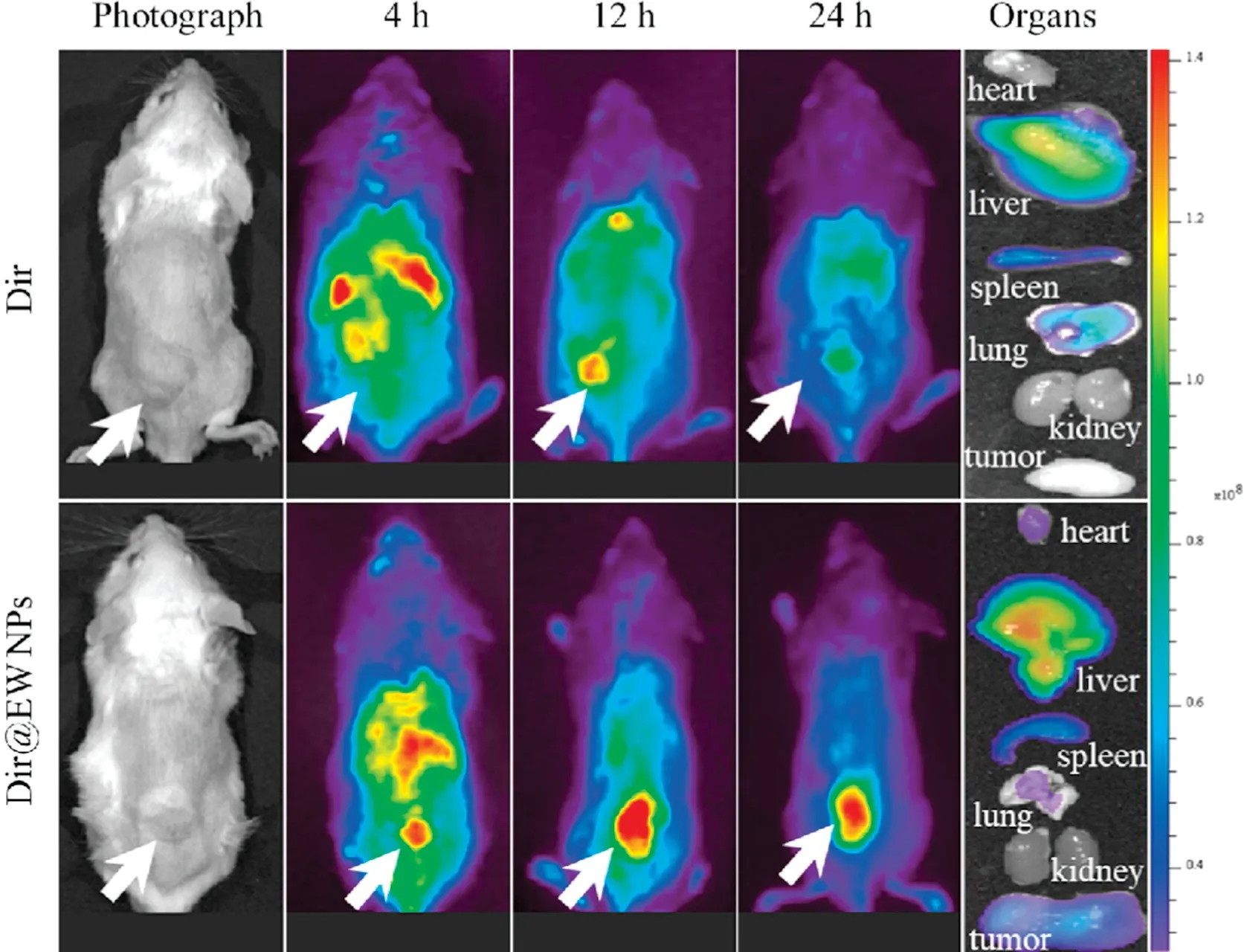
Fig.6.In vivo distribution of Dir at 4,12 and 24 h after administration of Dir and Dir@EW NPs, respectively.
The anticancer activity of PTX@EW NPs was further investigated in vivo.Mice were subcutaneously inoculated with CT26.WT cells,and then were administrated with the different formulations every 2 days.As shown in Fig.7a, mice treated with the PTX@EW NPs suffered almost no serious weight loss, further suggesting the reduced side effects of the EW NPs.Blood compatibility was verified byhemolysisassay.Theresults(Fig.S1)indicatedthatthehemolysis rate was slightly positively correlated with the NP concentration,but was generally maintained lower than the permissible levelof 5%across all the tested concentrations, demonstrating the good hemocompatibility of EW NPs.The variation of tumors volume had been compared in Fig.7b.Mice were sacrificed at last,and the tumors and other organs were excised for photographic and pathological analyses Fig.7c.Obviously smaller tumors was observed in PTX@EW NPs groups.Compared to PBS and blank EW NPs groups, the PTX@EW NPs groups exhibited obvious anticancer effect, which was consistent with the results of fluorescence imaging in vivo.This significant inhibitive effect to tumor growth was reached maybe due to improved targetingeffect,which could effectively increase the safe dosage of PTX.As shown in Fig.7d,therewere no visible pathological abnormalities in HE slices ofmainorgansinPTX@EWNPs groups,whileobviouscell shrinkage and absence could be read in tumors slices.These pathological differences were consistent with the results of the antitumor evaluation in vivo above.All these results demonstrated the good biosafety and antitumor efficacy of the PTX@EW NPs.
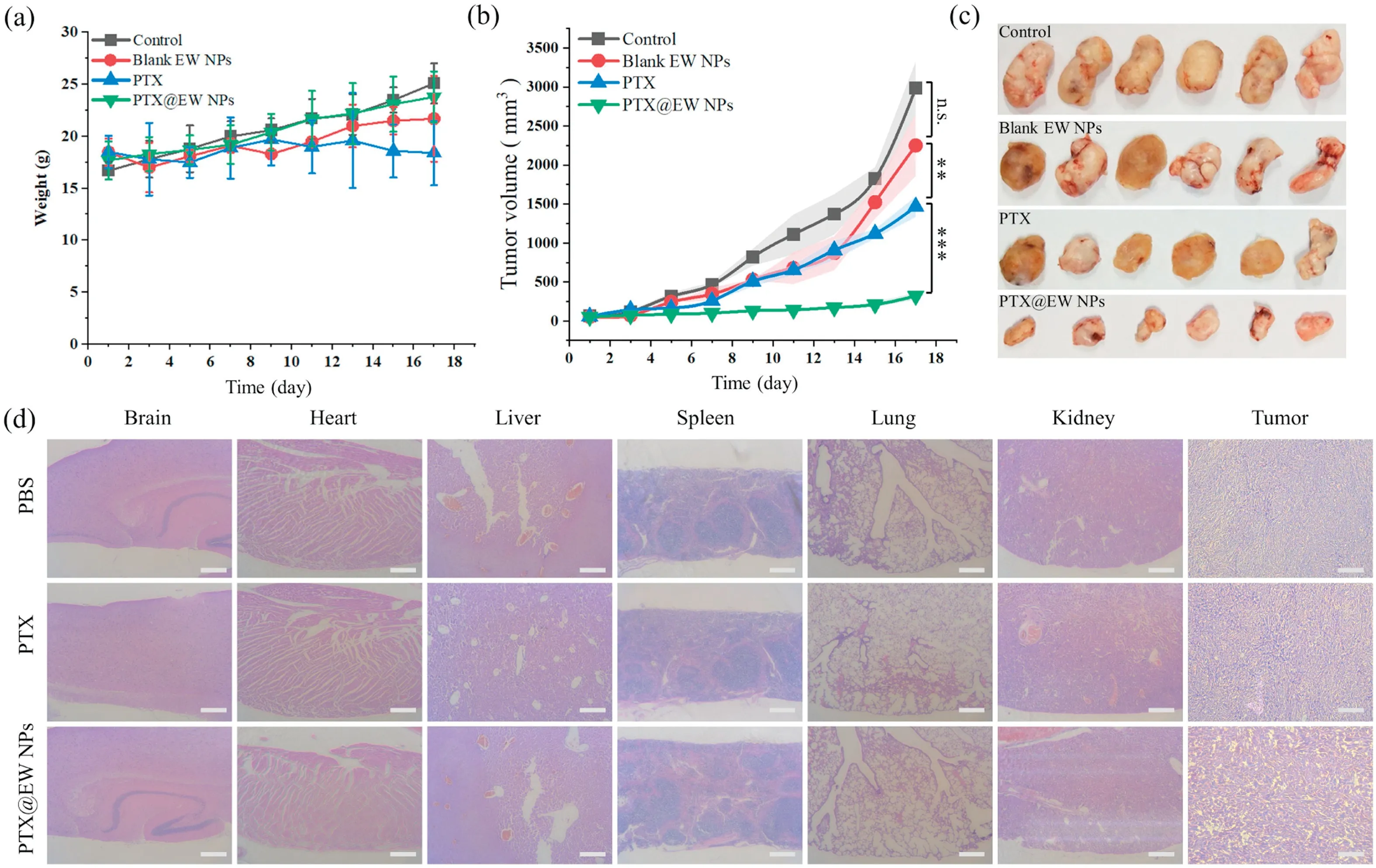
Fig.7.(a)Body weight trendy of mice of each group.(b)The profiles of tumor volume of different groups were recorded.(c)The tumors of different groups were collected and photographed after antitumor evaluation assay in vivo.(d)Representative histopathological images of major organs(brain,heart,liver,spleen,lung,kidney)and tumors from PBS, PTX and PTX/@EW NPs groups.** P< 0.01, *** P< 0.001, n.s.represents no significance.Scale bar: 100 μm.
In summary,by our work egg white was successfully developed as drug carrier by a green method to deliver PTX.The results confirmed that the optimized EW NPs drug delivery system have sub-100 nm size and good PTX loading efficiency.Low pH environment contributes to the drug release, leading to more drugs concentrated in tumor sites.For in vitro cellular studies PTX@EW NPs displayed significantly inhibitive effect against CT26.WT cells.In vivo,EW NPs significantly prolong the circulation time,and increased concentration of PTX at the tumor site brings obviously enhanced tumor inhibition effect.These properties indicate that EWP nanoparticles are promising candidates as highefficiency delivery systems for poorly water-soluble therapeutic drugs.This is an innovative try to search new biomaterials from natural world, which could provide a new solution for others.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work has been supported by National Science and Technology Major Project of the Ministry of Science and Technology of China (No.2018ZX10301402), International Cooperation and Exchange of the National Natural Science Foundation of China(No.51820105004), China Postdoctoral Science Foundation (No.2019M663246), the Fundamental Research Funds for the Central Universities (Nos.191gzd35 and 20ykpy15), Guangdong Basic and Applied Basic Research Foundation (No.2019A1515110686), the Seventh Affiliated Hospital of Sun Yat-sen University (Shenzhen)Research Startup Fund (No.ZSQYRSFPD0015), Science and Technology Program of Guangzhou (Nos.201707010094 and 201704020095), Guangdong Innovative and Entrepreneurial Research Team Program (Nos.2016ZT06S252 and 2016ZT06S029),Science and Technology Planning Project of Shenzhen(No.JCYJ20170307141438157),and Shenzhen Foundation of Science and Technology (No.JCYJ20170818103626421), National Natural Science Foundation of China (No.51973243).This study was approved by the Animal Ethical and Welfare Committee of Sun Yat-sen University.
Appendix A.Supplementary data
Supplementary material related to this article can be found,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.12.006.
杂志排行
Chinese Chemical Letters的其它文章
- Molecular recognition triggered aptazyme cascade for ultrasensitive detection of exosomes in clinical serum samples
- Electrosynthesis of 1-indanones
- Current signal amplification strategies in aptamer-based electrochemical biosensor: A review
- Photo-crosslinkable hydrogel and its biological applications
- STING-activating drug delivery systems: Design strategies and biomedical applications
- The carbon nanotubes-based materials and their applications for organic pollutant removal: A critical review
