Convenient synthesis of hexasubstituted benzene derivatives via DABCO promoted domino reaction of arylidene malononitrile and dialkyl but-2-ynedioate
2021-11-06HuiZhengYingHanJingSunChaoGuoYan
Hui Zheng,Ying Han*,Jing Sun,Chao-Guo Yan*
College of Chemistry & Chemical Engineering, Yangzhou University, Yangzhou 225002, China
ABSTRACT A convenient synthetic protocol for the hexasubstituted benzene derivatives was successfully developed by DABCO promoted domino reaction of arylidene malononitrile with two molecules of dialkyl but-2-ynedioates.The domino reaction resulted in tetraalkyl 6-cyano-[1,1′-biphenyl]-2,3,4,5-tetracarboxylates in good to high yields.This formal [2+2+2] cycloaddition was believed to proceed with sequential nucleophilic addition, Michael addition, annulation and aromatization processes.
Keywords:Hexasubstituted benzene Electron-deficient alkyne Malononitrile Annulation[2+2+2] cycloaddition
In recent years,nucleophilic tertiary amine catalyzed reactions has become one of the most efficient synthetic methodologies in organic chemistry [1-4].This convenient synthetic reaction usually started with the nucleophilic addition of tertiary amines such as pyridine, quinoline and isoquinoline as well as other tertiary amines to various electron-withdrawing group activated alkenes, alkynes and allenonates to generate active zwitterionic species.Subsequently, these active zwitterionic intermediates reacted further with various electrophiles and dipolarophiles containing C=C,C=O,C=N and N=N double bonds to give versatile acyclic,carbocyclic and heterocyclic compounds[5-11].As a result,a number of highly efficient synthetic reactions including new annulations to form various carbocycles and heterocycles have been successfully developed [12-24].
In this context, it has been reported that the nucleophilic catalyzed reaction of dialkyl but-2-ynedioate with readily available arylidene malononitriles afforded various acyclic or cyclic compounds under different reaction conditions.For examples, Nair and coworkers found that highly substituted buta-1,3-diene derivatives was formed with pyridine as catalyst(Scheme 1, Eq.1) [25].Similarly, this reaction in the presence of quinoline or isoquinoline gave functionalized tetrahydropyridoquinoline or tetrahydrobenzoquinolizine derivatives (Eq.2)[26-28].On the other hand, triphenylphosphine promoted reaction of dimethyl but-2-ynedioate and arylidene malononitrile afforded functionalized cyclopentenyl phosphoranes(Eq.3) [29].Although 1,4-diazabicyclo[2.2.2]octane (DABCO) and other tertiary amines have been widely used in the domino reaction of dimethyl but-2-ynedioate with diverse activated alkenes[30-52],there is no report about the DABCO catalyzed reaction of dimethyl but-2-ynedioate with arylidene malononitrile until now.In the study of nucleophilic tertiary amine catalyzed reaction of electron-deficient alkynes,we found that 1,4-diazabicyclo[2.2.2]-octane (DABCO) promoted domino reaction of two molecular dimethyl but-2-ynedioates with arylidene malononitriles in acetonitrile unexpectedly resulted in the formation of hexasubstituted benzene derivatives.Polysubstituted arenes including hexasubstituted benzenes are core frameworks occurring in various natural products, pharmaceuticals and functional materials [53,54].Polysubstituted arenes were mainly synthesized by traditional nucleophilic or electrophilic substitution, transition metal-catalyzed coupling reactions of the existing arene substrates[55,56].In recent years,domino benzannulation reactions have become the rapid construction methods for polysubstituted benzenes, in which two or more appropriate acyclic building blocks assembled into the arene rings through domino C--C bonds formation reactions [57-60].Herein, we wish to reveal a DABCO promoted benzannulation reaction for the efficient synthesis of tetraalkyl 6-cyano-[1,1′-biphenyl]-2,3,4,5-tetracarboxylates in atom-economy fashion.
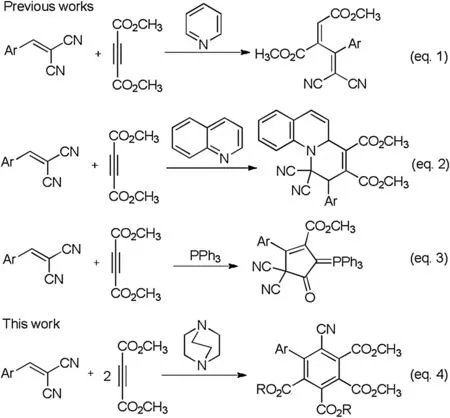
Scheme 1.Nucleophilic catalyzed annulation reactions.
Initially, the reaction conditions were examined with the reaction of 2-(4-methylbenzylidene)malononitrile and dimethyl but-2-ynedioate as standard reaction (Table 1).The reaction in CH2Cl2,CHCl3,toluene and THF in the presence of DABCO gave very quickly the polysubstituted benzene derivative 1a in moderate yields(Table 1,entries 1-4).However,no product was obtained in ethanol.The reaction in acetonitrile in the presence of DABCO afforded the product 1a in 70%yield.When DBU was used as base,no product was obtained.On the other hand, the reaction in acetonitrile in the presence of triethylamine gave the product in 65% yield.When the reaction was carried out at 0 or -5°C, the reaction was finished in 10 min to give the product 1a in 75% and 80% yields.However, the reaction at lower temperature (-10°C)did not increase the yield.On the other hand, excess amount of DABCO was needed for obtaining the product in high yields.When 1 equiv.of DABCO was used,the yield of the product 1a decreases Therefore,the desired polysubstituted benzene can be very conveniently prepared by carrying out the DABCO promoted domino reaction in acetonitrile at -5°C for 10 min.
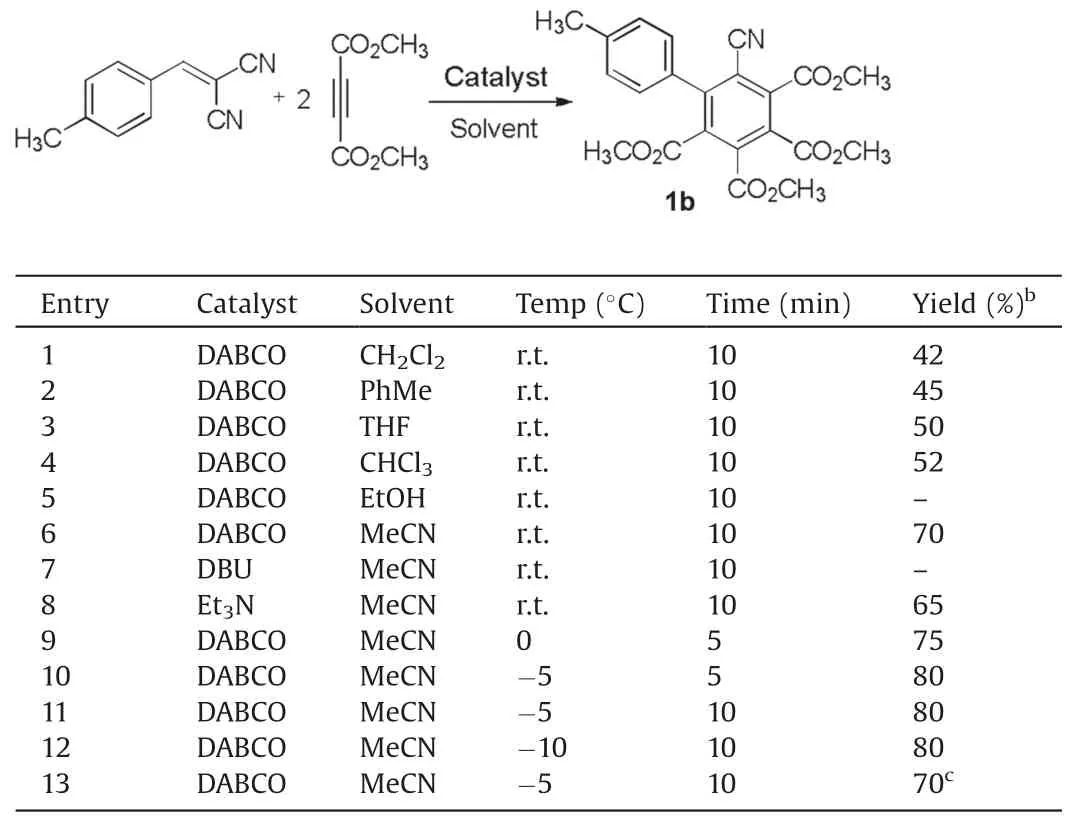
Table 1 Optimization of reaction conditions.a
With the optimized reaction conditions in hand, the scope of the reaction was developed by using various arylidene malononitriles in the reaction.The results are summarized in Table 2.It can be seen that all reaction proceeded smoothly to give the expected hexasubstituted benzene derivatives 1a-1z in moderate to good yields.The aryl group with electron-donating groups usually gave the products in higher yields than that of the aryl groups with electron-withdrawing groups (entries 1-13 in Table 2).However,the phenyl groups with stronger electron-donating 4-dimethylamino and 4-diethylamino groups gave the products 1n and 1o in lower yields, respectively (entries 14 and 15 in Table 2).The methylene malononitriles with heterocyclic 2-furyl,2-thiophenyl,3-pyridyl and 4-pyridyl group also gave the products 1o-1r in moderate yields(entries 16-19 in Table 2).The similar results were obtained when diethyl but-2-ynedioate was employed in the reaction(entries 19-26 in Table 2).These results showed that this reaction has a widely variety of substrates.In fact, this domino reaction provided an efficient method for the synthesis of polyfunctionalized biphenyl compounds.The obtained products 1a-1y were fully characterized by IR, HRMS,1H and13C NMR spectra.The single crystal structure of the compounds 1b,1d and 1n were successfully determined by X-ray diffraction method(Fig.1).The crystallographic data 1b (CCDC:2040759),1d(CCDC:2040760), and 1n (CCDC: 2045314) have been deposited at the Cambridge Crystallographic Database Centre.From Fig.1,it can beseen that the four methoxycarbonyl groups exist on the 2,3,4,5-position of the phenyl group.The two phenyl groups in the molecules of 1b and 1d have a dihedral angle of 66.678°and 61.817°, respectively.
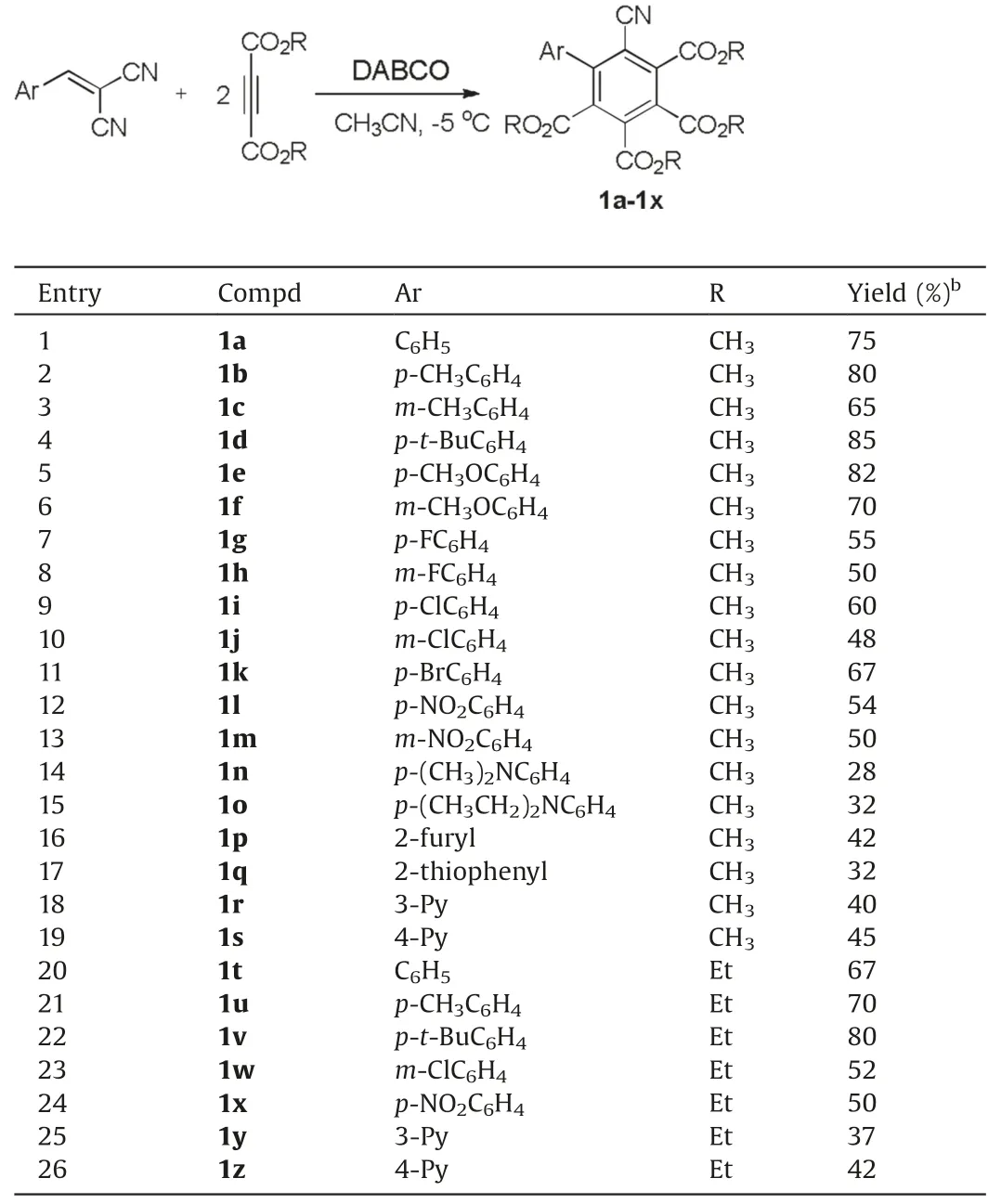
Table 2 Synthesis of hexasubstituted benzenes 1a-1x.a

Fig.1.Single crystal structures of the compounds 1b (left),1d (middle) and 1n (right).
For explaining this DABCO promoted reaction for the formation of the hexasubstituted benzene derivatives, a plausible reaction mechanism was proposed in Scheme 2 on the present experiments and previous related results[61].At first, nucleophilic addition of DABCO to but-2-ynedioate gave a zwitterionic intermediate (A).Secondly,the addition of carbanion in the zwitterionic intermediate (A) to the second molecular but-2-ynedioate afforded the intermediate (B).Thirdly, a Michael addition of the intermediate(B) to arylidene malononitrile resulted in a new zwitterionic intermediate(C),which was in turn converted to the intermediate(D) by 1,5-H shift process.Then, an intramolecular nucleophilic substitution gave a six-membered cyclic intermediate (D)with splitting of DABCO.At last, the base promoted elimination of hydrocyanic acid resulted in the hexasubstituted benzene 1.Therefore, this reaction can be regarded as an anion-relayed domino reaction.The driving force for the domino reaction was obvious the final aromatization process to give a stabilized benzene derivatives.
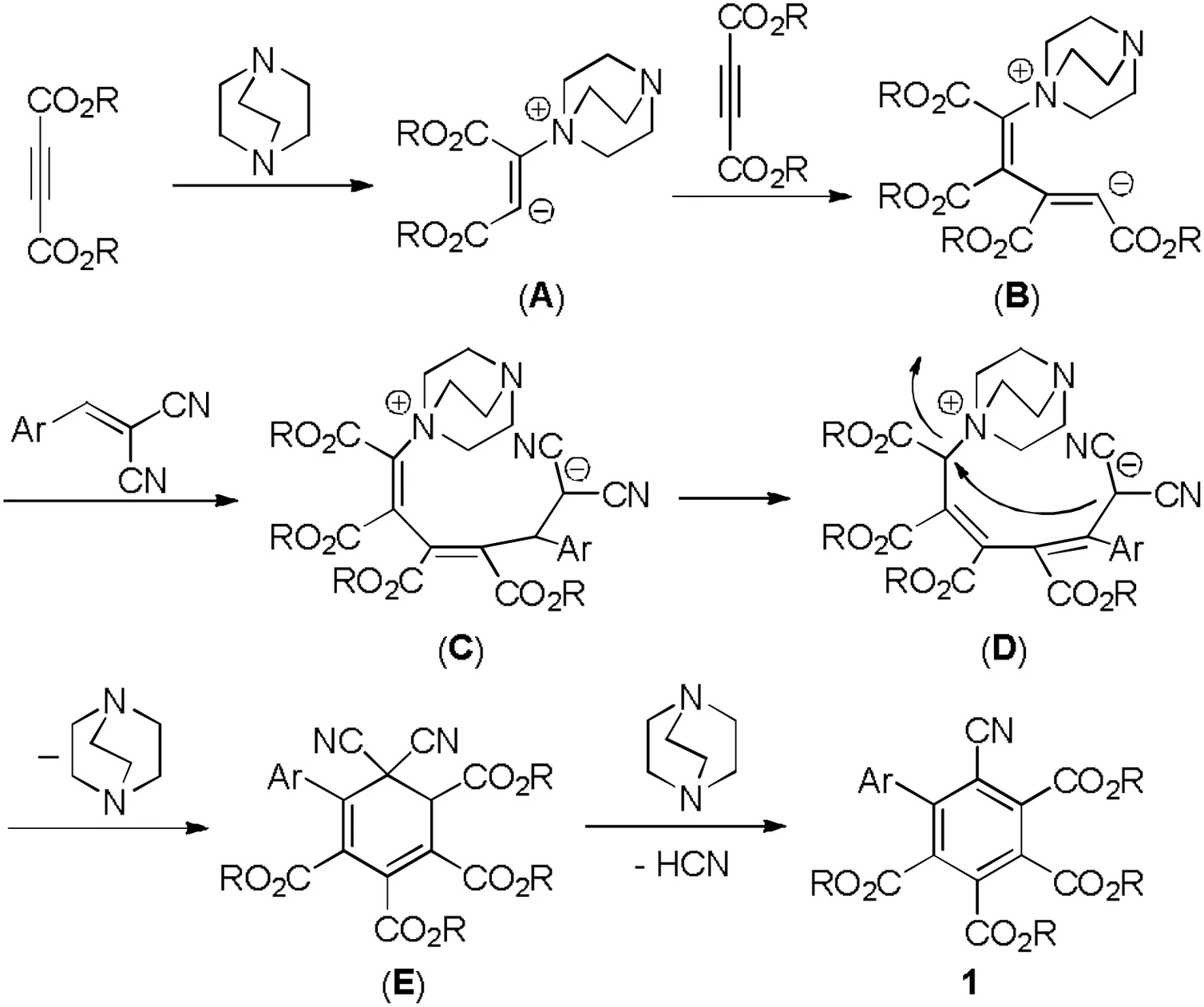
Scheme 2.Plausible reaction mechanism.
In order to develop the scope of the reaction,ethyl cyanoacetate and pivaloylacetonitrile was also used in the reaction.However,DABCO promoted reaction of ethyl cyanoacetate with dimethyl but-2-ynedioate in acetonitrile resulted in a complicate mixture of the products, from which no main product was obtained.The similar reaction of pivaloylacetonitrile with dimethyl but-2-ynedioate in acetonitrile in the presence of DABCO gave the known dihydropyran derivative 2a-2c in 75%-85%yield,which have been prepared by 4-(N,N-dimethylamino)pyridine catalyzed reaction of pivaloylacetonitrile with dimethyl but-2-ynedioate in dimethoxyethane (Scheme 3) [18].On the other hand, DABCO catalyzed reaction of methyl or ethyl propiolates with benzylidene malononitrile gave the known dimethyl or diethyl hex-2-en-4-ynedioates, which were derived from the base catalyzed dimerization of alkyl propiolates [62].These results indicated that formation of the hexasubstituted benzene derivatives required the precise matching of the reactivity of the substrates.
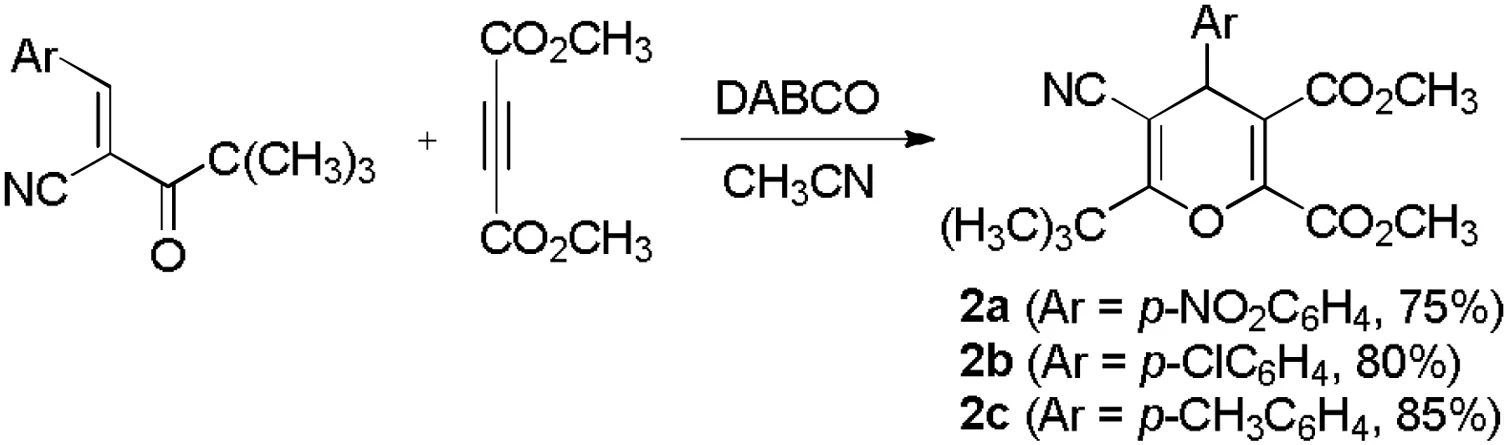
Scheme 3.The reaction with pivaloylacetonitriles.
In summary, we have successfully developed a convenient synthetic protocol for hexasubstituted benzene derivatives by the DABCO promoted domino reaction of arylidene malononitirles and dialkyl but-2-ynedioate in acetonitrile.This formal [2+2+2]cycloaddition was believed to proceed with sequential nucleophilic addition, Michael addition, annulation and aromatization processes.The advantages of the reaction included using readily available starting material, simple reaction conditions and high atomic economy.Additionally, this reaction also provided simple and efficient synthetic method for the important biphenyl compounds.The potential applications of this reaction in organic synthetic and medicinal chemistry might be significant.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No.21572196) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Appendix A.Supplementary data
Supplementary material related to this article can be found,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.12.024.
杂志排行
Chinese Chemical Letters的其它文章
- Molecular recognition triggered aptazyme cascade for ultrasensitive detection of exosomes in clinical serum samples
- Electrosynthesis of 1-indanones
- Current signal amplification strategies in aptamer-based electrochemical biosensor: A review
- Photo-crosslinkable hydrogel and its biological applications
- STING-activating drug delivery systems: Design strategies and biomedical applications
- The carbon nanotubes-based materials and their applications for organic pollutant removal: A critical review
