Curvature-regulated transmembrane anion transport by a trifluoromethylated bisbenzimidazole
2021-11-06XioQioHongYunYunXingZhongKunWngQinChoMoWenHuChen
Xio-Qio Hong,Yun-Yun Xing,Zhong-Kun Wng,Qin-Cho Mo,Wen-Hu Chen,d,*
a School of Biotechnology and Health Sciences, Wuyi University, Jiangmen 529020, China
b School of Pharmaceutical Sciences, Tsinghua University, Beijing 100084, China
c Guangdong Maoming Health Vocational College, Maoming 525000, China
d State Key Laboratory of Chemical Oncogenomics,Key Laboratory of Chemical Genomics,Peking University Shenzhen Graduate School,Shenzhen 518055,China
ABSTRACT In this paper, we demonstrate that modification of anion-transport active 1,3-bis(benzimidazol-2-yl)benzene with strongly electron-withdrawing trifluoromethyl and nitro groups leads to a dramatic increase in the anionophoric activity,and the activity may be greatly regulated by the curvatures of the liposomes used.
Keywords:Anion transporter Lysosomal curvature Anionophoric activity
During the past years,an increasing interest has been attracted in identifying small-molecule organic compounds that are capable of facilitating the transport of anions,in particular chloride anions across phospholipid membranes[1-5].Literature reports indicate that such compounds, namely anion transporters are able to disrupt the homeostasis of cellular anions [6] and as a consequence, they may be developed as a novel class of chemotherapeutic agents for cancers [7].
Because cellular membranes are rich in complexity and it has been reported that the bioactivity of anion transporters correlates with their activity on synthetic cellular models [8], many of the studies to date have used phospholipid vesicles of varying sizes as models of biological membranes for investigating anion transport processes.Transport of anions across phospholipid membranes is accomplished through the interaction of anion transporters with the membranes.This is a complex process, in particular for a mobile carrier, including the recognition of anions by anion transporters to form supramolecular complexes, diffuse within the membranes and release the anions on the opposite side of the membranes[9].As a consequence,anion transport efficiency may be affected by many parameters, such as phospholipid components and packing.For example, Gale et al.have investigated the anion transport properties of a range of alkyl-substituted phenylthioureas on vesicles formed from different types of phospholipids and found that changes in the compositions of lipid bilayers affected the rate of anion transport for all compounds in the series[10].In an earlier study, Regen et al.have reported that the curvature of lipid bilayers affects the lipid packing and mixing[11].Specifically,they have found that in cholesterol-poor membranes,where lipid interactions are relatively weak, curvature has little influence on sterol-phospholipid association.In sharp contrast,increased curvature significantly reduces the preference of sterols and high-melting-point phospholipids to become nearest neighbors in cholesterol-rich membranes, where lipid interactions are stronger.
These findings have raised one fundamental and crucial concern about how the anionophoric activity of an anion transport is affected by vesicular curvature.This issue is related to the assessment of the transport efficiency of anion transport systems on liposomal membranes of varying curvatures.To clarify this,we prepared one derivative of anion-transport active 1,3-bis(benzimidazol-2-yl)benzene(m-Bimbe,1,Fig.1),that is compound 2,and investigated the effect of vesicular curvatures on the anion transport efficiency.Compound 2 bears two trifluoromethyl groups on each benzimidazolyl subunit and one nitro group at the central phenyl subunit.This design was rationalized by our previous findings that introducing strongly electron-withdrawing groups into the benzimidazolyl and/or central phenyl subunits of m-Bimbe 1 leads to a significant increase in the anion transport efficiency [12-15].
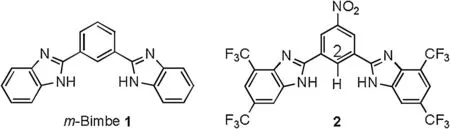
Fig.1.m-Bimbe 1 and its trifluoromethylated derivative 2.
Compound 2 was prepared from the condensation of 5-nitro-1,3-benzenedialdehyde [16] with 3,5-bis(trifluoromethyl)-1,2-benzenediamine (Scheme 1), and fully characterized on the basis of electrospray ionization mass spectrometry (ESI MS) [low resolution (LR) and high resolution (HR)] and nuclear magnetic resonance (NMR,1H and13C) data (Supporting information).
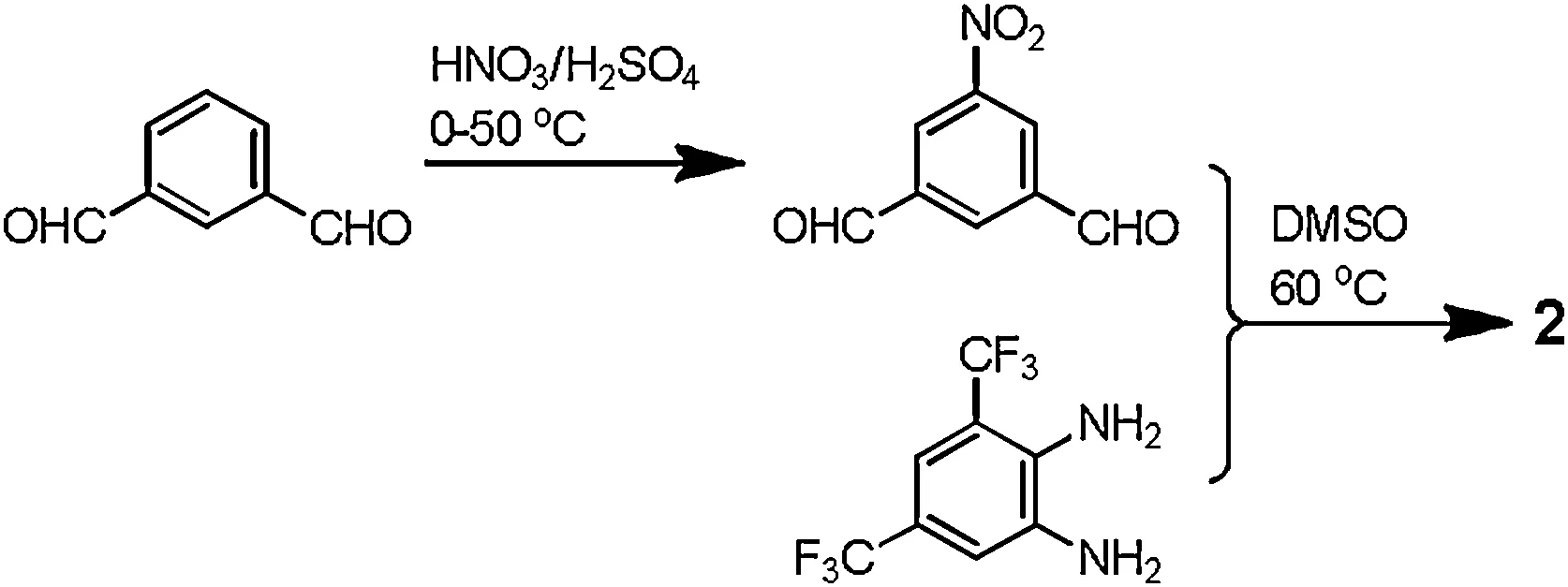
Scheme 1.Synthesis of compound 2.
As anion recognition is the initialstep foraniontransport[17],we first studied the complexation of compound 2 with chloride anions as a tetra(n-butyl)ammonium salt(TBACl),by means of ESI MS.As shown in Fig.2a,mixing of compound 2 with TBACl in CH3CN led to the presence of a new ion peak at m/z 662.63 as well as the ion peak at m/z 626.68 fromcompound 2 itself.This newpeak is assignable to a 1:1 complexof compound 2 with chloride anions.The binding site and affinity were established by means of1H NMR titrations(Fig.2b).Addition of TBACl toa solution of compound 2inCD3CNled to large down-field shifts of the C2H,indicating that chloride anions are complexed to the 1,3-bis(benzimidazol-2-yl)benzene core.Non-linearfitting analysis of these complexation-induced chemical shifts against the concentrations of TBACl gave the association constant (Ka) of compound 2 with chloride anions being(1.45±0.33)×104L/mol(Fig.S5 in Supporting information),about 4-fold greater than that of compound 1 with chloride anions[Ka=(3.51±0.09)×103L/mol]under the similar conditions[15].
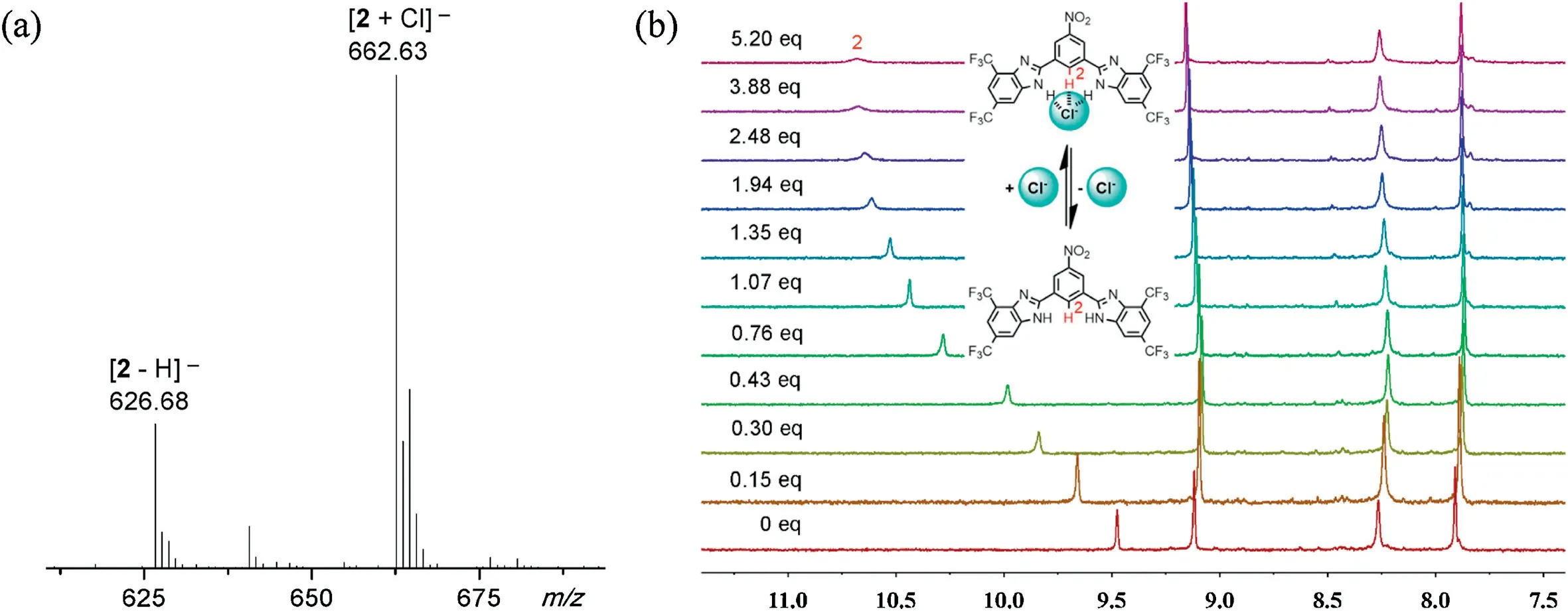
Fig.2.(a)ESI MS spectrum of compound 2(1.0×10-3 mol/L)mixed with 50 equivalences of TBACl in CH3CN;(b) 1H NMR titration of compound 2(1.0×10-3 mol/L)with TBACl of varying equivalences in CD3CN.
The anion transport activity of compound 2 was assessed on liposomal models using a conventional pH discharge assay based on sodium 8-hydroxypyrene-1,3,6-trisulfonate (HPTS, pKa7.2)[14,15].In these experiments, vesicles (100 nm, extrusion)encapsulated with an internal aqueous phase of pH of 7.0 and HPTS as a reporter for the intravesicular pH change,were prepared from egg-yolk L-α-phosphatidylcholine (EYPC) and suspended in an external aqueous phase of pH of 8.0.As shown in Fig.3a and Fig.S6(Supporting information),addition of compound 2,even at very low concentrations, led to an increase in the fluorescent intensity of HPTS, indicating that this compound is very active in discharging the pH gradient between the exterior and interior of the EYPC vesicles.The concentration dependent assays gave the 50% efficiency concentration (EC50) value that is defined as the effective transporter loading in molar percentage (mol%) of compound 2 to lipid when 50% of the maximum rate is reached,being(2.76±0.36)×10-3mol%(or 4.86 nmol/L).Under the same conditions,this EC50value was 11.08±1.36 mol%(or 19.5 μmol/L)for compound 1.These results represent 4.0×103-fold enhancement in the transport efficiency upon the modification of compound 1 with trifluoromethyl and nitro groups (to give compound 2).Calcein leakage assay confirmed that the observed powerful activity of compound 2 was authentic rather than due to the disruption of the EYPC liposomal membranes (Fig.S7a in Supporting information) [18].
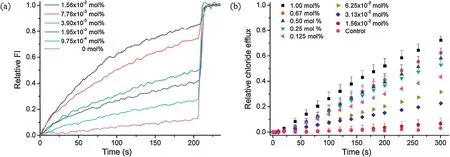
Fig.3.(a)Typical plots of the relative fluorescent intensity(FI,ex/em 460/510 nm)of HPTS against time in the presence of compound 2 of varying concentrations in EYPCbased liposomes(100 nm),under the conditions of intravesicular 0.1 mmol/L HPTS in 25 mmol/L HEPES buffer(50 mmol/L NaCl,pH 7.0)and extravesicular 25 mmol/L HEPES buffer (50 mmol/L NaCl, pH 8.0).(b) Relative efflux of chloride anions out of EYPC liposomes (100 nm) enhanced by compound 2 of varying concentrations, under the conditions of intravesicular 500 mmol/L NaCl in 25 mmol/L HEPES buffer (pH 7.0) and extravesicular 500 mmol/L NaNO3 in 25 mmol/L HEPES buffer (pH 7.0).
Studies to date have indicated that the anion binding affinity and lipophilicity of an anion transporter are two key parameters that play a critical role in the anion transport process [19].The above-mentioned finding that compound 2 exhibits ca.4-fold greater anion binding affinity toward chloride anions than compound 1, may rule out the possibility that the remarkably enhanced anionophoric activity of compound 2 was mainly due to the increase in the anion-binding affinity.As compound 2 (clog P= 8.05)is much more lipophilic than compound 1(clog P= 4.60),one may consider that the increased lipophilicity of compound 2 induced by the four trifluoromethyl substituents, is mainly responsible for the increase in the anion transport efficiency[20,21].
The anion-selective transport ability of compound 2 was confirmed by use of chloride selective electrode technique.As shown in Fig.3b, addition of compound 2 to vesicles containing intravesicular NaCl and extravesicular isotonic NaNO3led to the efflux of chloride anions out of the intravesicular media,indicating that compound 2 is able to mediate the transport of chloride anions.To clarify the probable transport mechanism of compound 2, we measured the efflux of chloride anions in the presence of different alkali metal cations or anions.As shown in Figs.4a and Fig.S7b(Supporting information),the efflux rate of chloride anions was regulated not by the alkali metal cations but by the anions,which suggests that compound 2 mediates the transport of chloride anions in an anion exchange fashion [22,23].U-tube experiments (Fig.4b) suggest that compound 2 functions as a mobile carrier [24], like a “ferryboat” diffusing back and forth within the interior of the membranes [25].
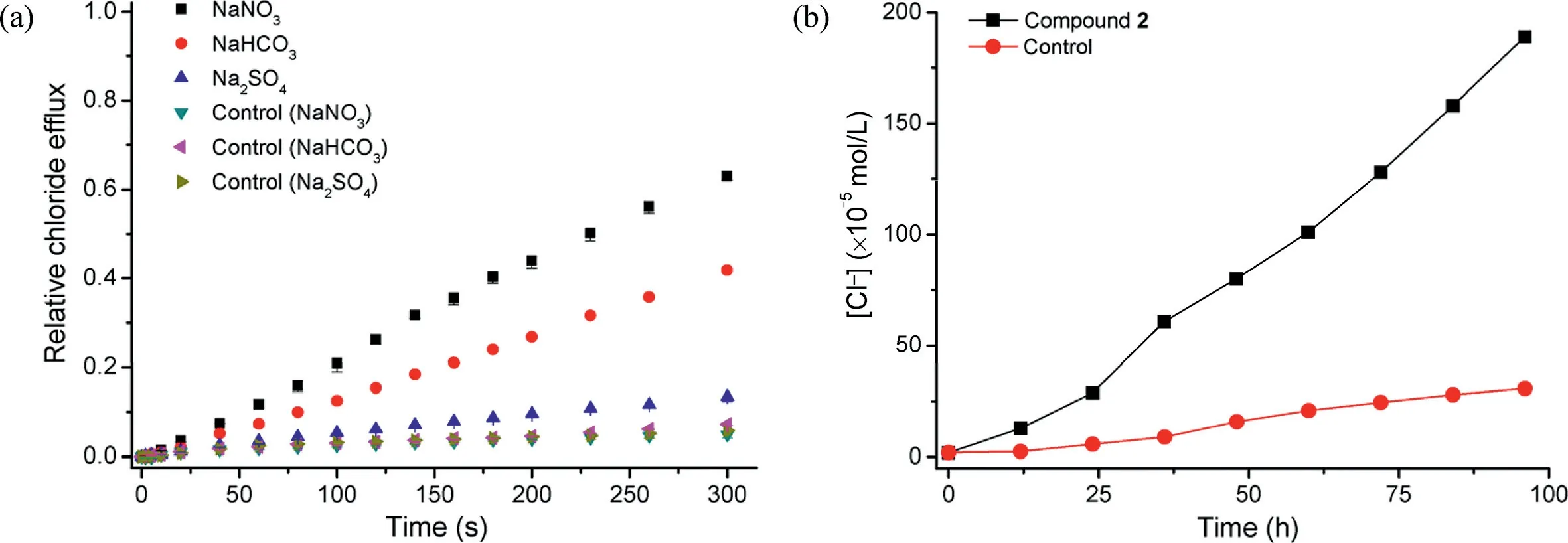
Fig.4.(a)Relative efflux of chloride anions out of EYPC liposomes(100 nm),enhanced by compound 2(0.1 mol%),under the conditions of intravesicular 500 mmol/L NaCl in 25 mmol/L HEPES buffer(pH 7.0)and extravesicular 500 mmol/L NaNO3,500 mmol/L NaHCO3 or 250 mmol/L Na2SO4 in 25 mmol/L HEPES buffer(pH 7.0).(b)Typical U-tube experiments in the presence of compound 2(1.0 mmol/L)in nitrobenzene containing 2 mmol/L TBAPF6.Measuring conditions:U-tube chloride-receiving phase,500 mmol/L NaNO3, 25 mmol/L HEPES buffer (pH 7.0); and U-tube chloride-donating phase, 500 mmol/L NaCl, 25 mmol/L HEPES buffer (pH 7.0).
To probe the effect of liposomal curvature on the anion transport efficiencyofcompound 2,we measured the effluxof chloride anions out of the EYPC vesicles with average liposome diameters from 51 nmto603 nm(Fig.S8andTableS1inSupportinginformation),by means of chloride ion selective electrode technique.These liposomes were prepared by extrusion methods.As shown in Fig.5a,the vesicular curvature has an obvious effect on the chloride efflux rate.To quantitatively characterize this effect,we measured the EC50values of compound 2 on EYPC liposomes of those diameters (Figs.S9-S13 in Supporting information and Table 1).As a result, the EC50values decrease with the decrease in the liposomal sizes, that is, increased curvature (i.e., liposomes of smaller diameters)led to an increase in the activity of compound 2.This effect becomes more evident when the diameters of the liposomes are reduced to ca 140 nm and below(Fig.5b and Table 1).Specifically,the activity increases by ca.102-fold when the vesicular diameters decrease from 603 nm to 51 nm.This effect is considered due to the less efficient packing of the lipids as a result of increased curvature [11], which may make compound 2 more ready to be inserted into the membranes to function as an anion carrier.
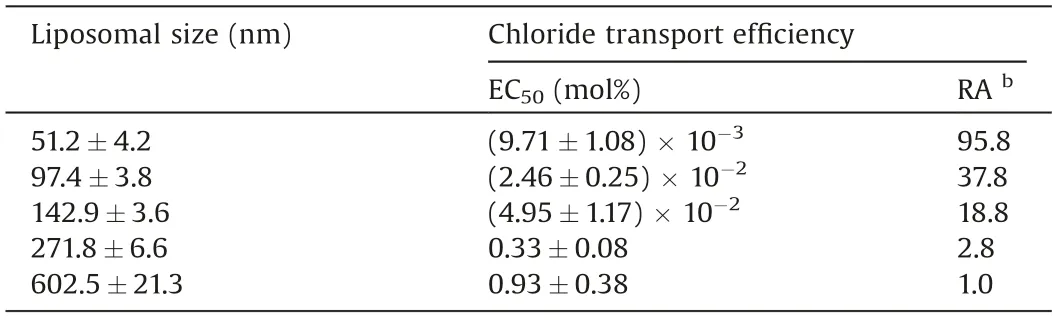
Table 1 Chloride transport efficiency (EC50, mol%) of compound 2 on EYPC liposomes of different sizes.a
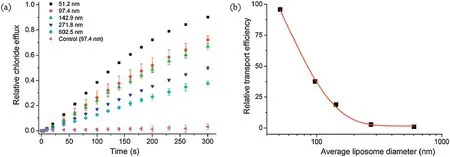
Fig.5.(a)Relative efflux of chloride anions out of EYPC liposomes of varying sizes enhanced by compound 2(1 mol%),under the conditions of intravesicular 500 mmol/L NaCl in 25 mmol/L HEPES buffer(pH 7.0)and extravesicular 500 mmol/L NaNO3 in 25 mmol/L HEPES buffer(pH 7.0).The experiments conducted on EYPC liposomes of 100 nm with DMSO were used as a control.(b) Plot of the relative transport efficiency against the average liposome diameters.
As high anion transport efficiency was observed on the EYPC liposomes that are comparable to cancer cells in sizes [26], wehypothesized that compound 2 may exhibit equally potent anionophoric activity in vitro and disrupt the homeostasis of anions in cancer cells, leading to cell death.To address this issue,we firstly measured the capability of compound 2 to facilitate the influx of chloride anions into HeLa cervical and A549 lung adenocarcinoma cancer cell lines, using a fluorescent assay based on a chloride anion sensitive fluorescent dye, N-(ethoxycarbonylmethyl)-6-methoxyquinolinium bromide (MQAE) [14].As shown in Fig.S14 (Supporting information), addition of compound 2 to HeLa and A549 cell lines led to a significant decrease in the fluorescence of MQAE,revealing the strong influx of chloride anions into the cells.MTTassays indicate that compound 2 exhibits potent cytotoxicity with the 50%inhibition concentration(IC50) values being 9.67±1.80 μmol/L toward HeLa cells and 10.48±1.20 μmol/L toward A549 cells, respectively (Fig.S15 in Supporting information).
In conclusion, we have shown that modification of 1,3-bis(benzimidazol-2-yl)benzene with strongly electron-withdrawing trifluoromethyl and nitro groups leads to a dramatic increase in the anionophoric activity and the activity is highly dependent on the curvature of the liposomes used,generally on liposomes of smaller sizes exhibiting better transport efficiency.The present finding suggests that particular cautions should be taken when one evaluates the transport efficiency of different anion transporters on liposomal membranes of varying curvatures.The potent anionophoric activity and in vitro cytotoxicity suggests that this compound is exploitable as an apoptotic agent,in particular for the cancer cells at early stages.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Financial support from the National Natural Science Foundation of China(No.21877057)and Jiangmen Program for Innovative Research Team,China(No.2018630100180019806)is acknowledged.
Appendix A.Supplementary data
Supplementary material related to this article can be found,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2021.01.005.
杂志排行
Chinese Chemical Letters的其它文章
- Molecular recognition triggered aptazyme cascade for ultrasensitive detection of exosomes in clinical serum samples
- Electrosynthesis of 1-indanones
- Current signal amplification strategies in aptamer-based electrochemical biosensor: A review
- Photo-crosslinkable hydrogel and its biological applications
- STING-activating drug delivery systems: Design strategies and biomedical applications
- The carbon nanotubes-based materials and their applications for organic pollutant removal: A critical review
