I-Assisted Synthesis Erythrocyte-like Bi2WO6with Excellent Adsorption and Photocatalytic Activity
2021-11-04SUNXinYuZHAOHuaLIHuiPengCAITianFengLIMingYueWANGYuCheng
SUN Xin-Yu ZHAO Hua LI Hui-Peng CAI Tian-Feng LI Ming-Yue WANG Yu-Cheng
(College of Petrochemical Technology,Liaoning Shihua University,Fushun,Liaoning 113001,China)
Abstract:Uniform erythrocyte-like Bi2WO6was obtained by means of I-assisted tactic without utilizing any organic capping reagents in the convenient hydrothermal process for the first time.In order to analyze the preconditions which affect the morphology of Bi2WO6,we tried to change the I-concentration,hydrothermal time and temperature,then put forward a possible growth mechanism.I-absorb to the surface of Bi2WO6 nanoplates to prevent excessive accumulation of nanoplates and guide them to form an erythrocyte-like structure.The unique layered structure,on the one hand,increases the specific surface area and provides more reaction sites,on the other hand,it also enhances surface acidity and heightens the adsorption capacity.
Keywords:Bi2WO6;erythrocyte-like structure;I-assisted;adsorption;photocatalysis
0 Introduction
Because of the consequences of traditional development method,environmental pollution has become an urgent problem to be solved,which not only affects the future sustainable development,but also poses a threat to human health.In recent years,people have put in a lot of effort to try to use semiconductor photocatalysts to achieve water purification,air purification and hazardous waste decomposition due to its cleanliness and sustainability[1-5].Nevertheless,a single semiconductor photocatalyst has difficulties of dealing with low visible light absorption and the fast recombination of photogenerated electron-hole pairs,which directly lead to the decrease of quantum efficiency and photocatalytic efficiency[6-8].In order to further improve the photocatalytic performance of semiconductors,many intensive efforts have been made,including element dop-ing[9],special morphology construction[10],and heterojunction fabrication[11].
The recent and rapid development of morphology modification offers a new opportunity to overcome the limited efficiency of photocatalysts.Many morphologies,such as zero-dimensional quantum dots[12],onedimensional nanowires[13],two-dimensional nanosheet[14],nanorods[15],three-dimensional microspheric[16],flowerlike spheres[17]and inverse opal structure[18],have been constructed.Construction of a special morphology often requires the participation of a template,which can be divided into soft templates and hard templates according to the state.Hard template is usually monodisperse spherical,such as polystyrene(PS)microspheres,SiO2microspheres,polymethylmethacrylate(PMMA)microspheres and so forth.The soft template is mainly organic capping reagent,such as polyvinylpyrrolidone(PVP),P123,cetyltrimethylammonium bromide(CTAB)and sodium citrate.
Among the various semiconductor photocatalysts,Bi2WO6emerged as an interesting candidate since it is stable and satisfying visible-light absorption property,and also it is low-cost and accessible[19].Meanwhile,Bi2WO6is an Aurivillius layered structure,which is composed of[Bi2O2]2+and[WO4]2-layers alternately arranged,in which oxygen atoms are shared between the two layers,thereby forming a chemical bond between the two layers.The layered structure is conducive to the effective separation of photogenerated electrons and holes[20-22].The morphology control of Bi2WO6has aroused great interest.For example,microspheric[23],nest-like[24],flower-like[25],and clew-like[26]Bi2WO6layered structure have all been synthesized.Especially,when 3D Bi2WO6layered structure as photocatalysts,its photocatalytic effect is often closely related to its morphology,structure and size[27-29].However,the control of morphology often needs the participation of soft template,or needs strong acid or base to adjust the pH.Increasing the complexity of the experiment,it may also cause the residue of the template,which will cause adverse effects in the subsequent reaction.
The photocatalytic degradation process is considered as a surface reaction,and only when the pollutant contacts the surface of the catalyst,the reaction can take place.Therefore,adsorption is first used to achieve the enrichment of pollutants on the surface of the catalyst,and then photocatalysis is used to degrade pollutants,which can increase the removal rate of pollutants theoretically.In fact,many studies have tried this strategy and the results are very satisfactory[30-33].
Here,in this work,we synthesized erythrocytelike Bi2WO6in the presence of structure-director I-which has never been reported before.We changed the I-concentration,hydrothermal time and temperature to analyze the conditions which affect the morphology.At the same time,proposing the formation process of erythrocyte-like Bi2WO6.Finally,the adsorption and photocatalytic properties of Bi2WO6were investigated.Our study demonstrates that by adjusting a series of parameters of the reaction,the morphology of the product can be effectively controlled,which provides inspiration for other 3D semiconductor materials.
1 Experimental
All chemicals used are analytical-grade reagents without further purification.Deionized water was prepared in our own laboratory.
1.1 Synthesis
The thin layer BiOI was synthesized by hydrothermal method.4 mmol Bi(NO3)3·5H2O was dissolved in 15 mL 0.3 mol·L-1HNO3,and stirred until it dissolved at room temperature.Meanwhile,dissolved 4 mmol KI in 50 mL water;then the two solutions were mixed under vigorous stirring.Afterwards,the obtained homogeneous solution was transferred into a Teflon-lined stainless steel autoclave and the reaction was carried out at a 170℃for 8 h.Cooled the autoclave down naturally until it reached room temperature,washing and drying to obtain red powdery BiOI.
Dissolved a certain amount of BiOI prepared in the previous step and 2 mmol Bi(NO3)3·5H2O in 40 mL ethylene glycol,stirred them until they dissolved.Dissolved 2 mmol of Na2WO6in 20 mL of water,added it to the mixed solution of Bi(NO3)3and BiOI drop by drop and stirred them for 30 min,the solution was placed in an autoclave and hydrothermally heated at 180℃for 12 h.Autoclave was cooled down to room temperature under ambient air,the product was separated by centrifuging,and washed with deionized water three times to remove excess reagent.The obtained samples were dried overnight in a vacuum drying oven.The products were synthesized by adding 0.4,1.0,or 1.6 mmol BiOI(labeled as BWI-x,x=0.4,1.0,1.6),BW product were obtained without BiOI.
1.2 Characterization
The X-ray diffraction(XRD)analysis was carried out by using a Bruker D8 Advance Diffractometer with CuKαradiation(λ=0.154 18 nm)at 40 kV and 40 mA,and the XRD patterns were recorded at 2θfrom 20°to 90°.The morphology and microstructure were characterized by using the SU8010 microscope(SEM)produced by Hitachi,Japan and the operating voltage was 15 kV.The UV-Vis diffuse reflectance spectroscopy(DRS)was measured with the Agilent Cary 5000 spectrometer from the United States.X-ray photoelectron spectroscopy(XPS,Thermo Escalab 250 XPS system)was used to analyze the surface state of the catalyst.AlKαradiation was used as the excitation source.
1.3 Adsorption and photocatalytic activity measurement
The photocatalytic performance was investigated by taking the degradation of rhodamine B(RhB)under the visible light.Similarly,through the adsorption experiment of RhB in the dark environment,the adsorption capacity of the photocatalyst was investigated as well.In a typical experiment,100 mg of photocatalyst was dispersed in 50 mL of 10 mg·L-1RhB aqueous solution,before illumination,the reaction mixture was magnetically stirred for 30 min to reach the adsorption/desorption equilibrium.Within the given time interval,3 mL samples were collected for centrifugation to remove the photocatalyst powder.An UV spectrophotometer(jascov-750)was used to record the variations of the absorption-band maximum(554 nm),and to monitor the adsorption and photodegradation process of RhB.
2 Result and discussion
2.1 Crystal structure and morphology
The phase structure and purity of as-prepared BW,BWI-x,and BiOI were characterized by powder XRD.The XRD patterns of as-prepared BWI-xshow the similar diffraction characteristics and can be wellindexed as orthorhombic Bi2WO6(PDF No.079-2381)(Fig.1).The strong and sharp diffraction peaks indicate that the synthesized samples are in highly crystallinity,which implies the addition of BiOI will not cause the change of BW crystal phase.For BWI,the peaks at 28.30°,32.93°,47.16°and 55.83°are assigned to the(113),(020),(220)and(313)planes.Surprisingly,there was no XRD pattern of BiOI,although more than 1 mmol of BiOI added to the samples.
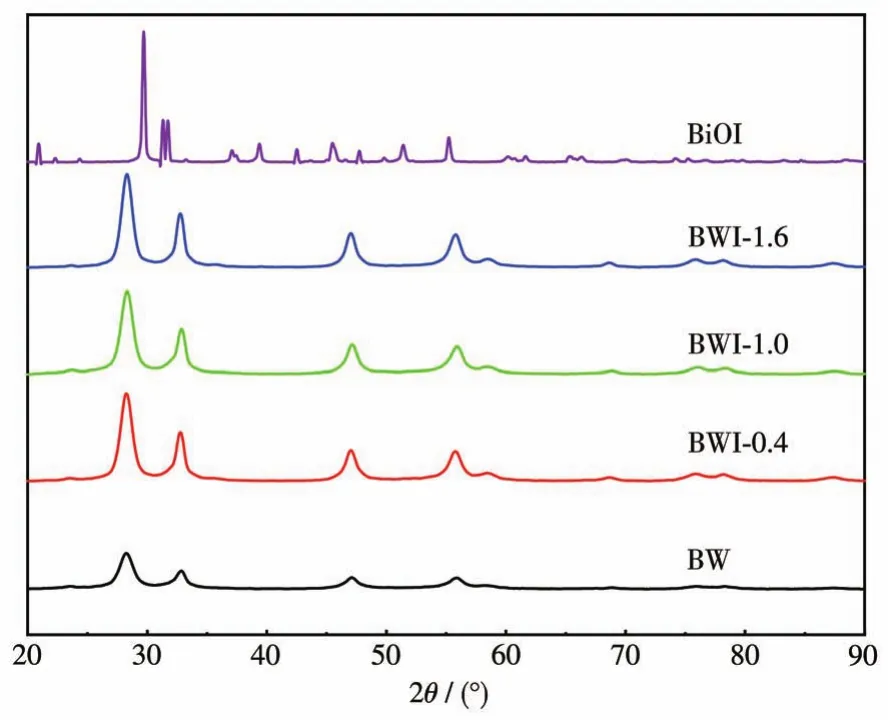
Fig.1 XRD patterns of BW,BWI-x and BiOI
In order to get insight into where BiOI going and the influence of BiOI on the chemical state of Bi,W,O in BW and BWI-1.6,XPS were carried out and the results are demonstrated in Fig.2.All of the data were calibrated by C as reference(284.6 eV).As shown in the Fig.2a,the XPS spectra of BW and BWI-1.6 were all composed of W4f,Bi4f,W4d,Bi4d,O1s,Bi4pand O auger state peaks,which revealing the presence of Bi,W,and O elements in the samples.BWI-1.6 also had obviousI3dpeaks,but no signal of I was detected on BW surface.In Fig.2b,159.4 and 164.8 eV are belong to Bi4f7/2and Bi4f7/2,respectively[34].The peak at 530.2 eV(Fig.2c)of BWI-1.6 was consistent with the O1speak in BW and is attributed to lattice oxygen[35].Fig.2d shows two peaks,35.80 and 37.9 eV,representing the W4f7/2and W4f5/2[36].In the I3dXPS spectra(Fig.2e),the double peaks with binding energies of 619.8 and 631.3 eV correspond to I3d5/2and I3d3/2,respectively[37].These peaks of I3d5/2and I3d3/2are different from that of I2at 618.3 eV,I5+at 624.7 eV or I7+at 627.1 eV obviously[38-39],suggesting no other iodine species existed.Compared with BW,the Bi4fand W4fpeaks of BWI-1.6 shifted to the high binding energy direction to varying degrees,but the O1speak shifted to the low binding energy direction.As in the previous reports,the change in binding energy is caused by the change in electron density around the element[40-41].Accordingly,in the XPS spectrum of BWI-1.6,the shifting of Bi and W to higher binding energy is due to the increase of electron concentration caused by I adsorption on the surface,the electronegativity of I in BWI-1.6 is stronger than that of Bi and W,and it is easier to attract electrons,but the electronegativity of I is weaker than that of O,causing some electrons to be attracted by O,which makes the peak of O shift to the direction of low binding energy.So we can conclude that the Bi2O22+forms by BiOI and the excess WO42-in the system preferentially form BW.The remaining I-will adhere to the surface of the formed BW nanoplates to prevent excessive accumulation of nanoplates and guide them to form an erythrocyte-like structure.
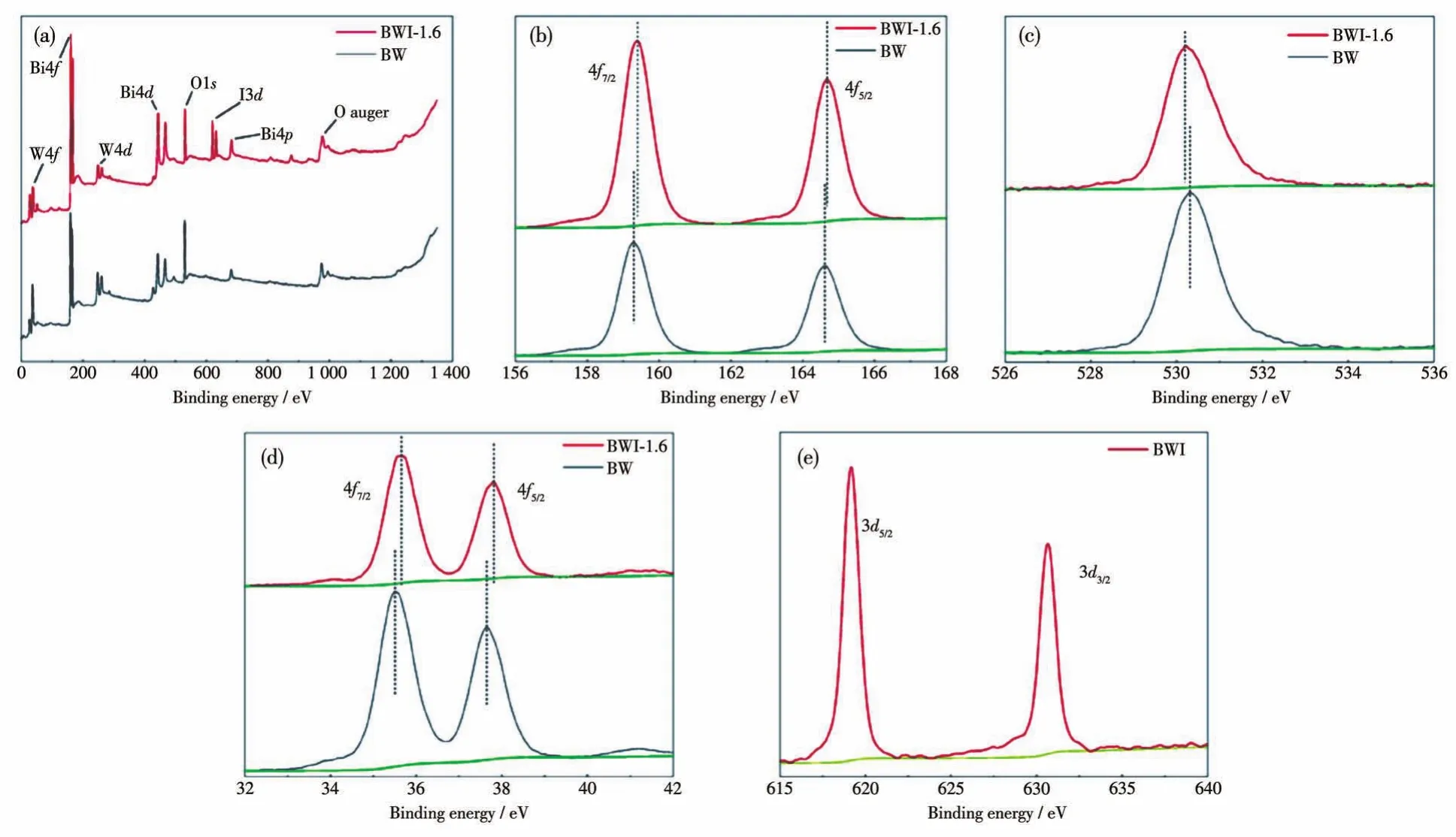
Fig.2 XPS spectra of BW and BWI-1.6
The morphology of the samples can be observed under SEM as shown in Fig.3.Fig.3a is SEM image of BiOI obtained with hydrothermal treatment at 170℃for 8 h.It is obvious that flower-like 3D BiOI hierarchical structure are constructed from many arranged 2D layers.Without the addition of BiOI,BW showed a flower-like structure assembled by some nanoplates,and was composed of many loosely arranged nanoplates(Fig.3b).When the amount of BiOI was adjusted to 0.4 mmol,BWI-0.4 showed an irregular erythrocyte-like structure(Fig.3c),but the morphology changed a lot compared with BW.There were nanoplates vertically standing in the center of the erythrocyte-like structure,and there were other structures crossing together.When the addition amount of BiOI increased to 1.0 mmol,the complete erythrocyte-like structure appeared(Fig.3d),although there were still small nanoplates attached to the edges of the structure.Compared with BWI-0.4,the stacking of nanoplates appeared tighter,the curvature of the concave surface of erythrocyte-like structure became larger.Further increasing BiOI amount to 1.6 mmol,a large number of erythrocyte-like structures can be observed from the Fig.3e,indicating that this method can be prepared on a large scale.Fig.3f revealed that BWI-1.6 also had erythrocyte-like structure,with an oblate shape and a depressed center.Each erythrocyte-like BWI-xwas scattered with each other,similar in appearance,uniform in size.As the amount of BiOI increased from 0.4 to 1.6 mmol,the diameter of erythrocyte did not change significantly.However,the stack of nanoplates became denser,compared with the erythrocyte-like BW prepared by adding NaF[35],the sample prepared by using BiOI as a structure directing agent had a smaller diameter,a larger thickness,and a smaller spacing between nanoplates,which can bring a larger specific surface area,providing more attachment sites.This result shows that I-plays a very vital role in the formation of erythrocytelike structure.
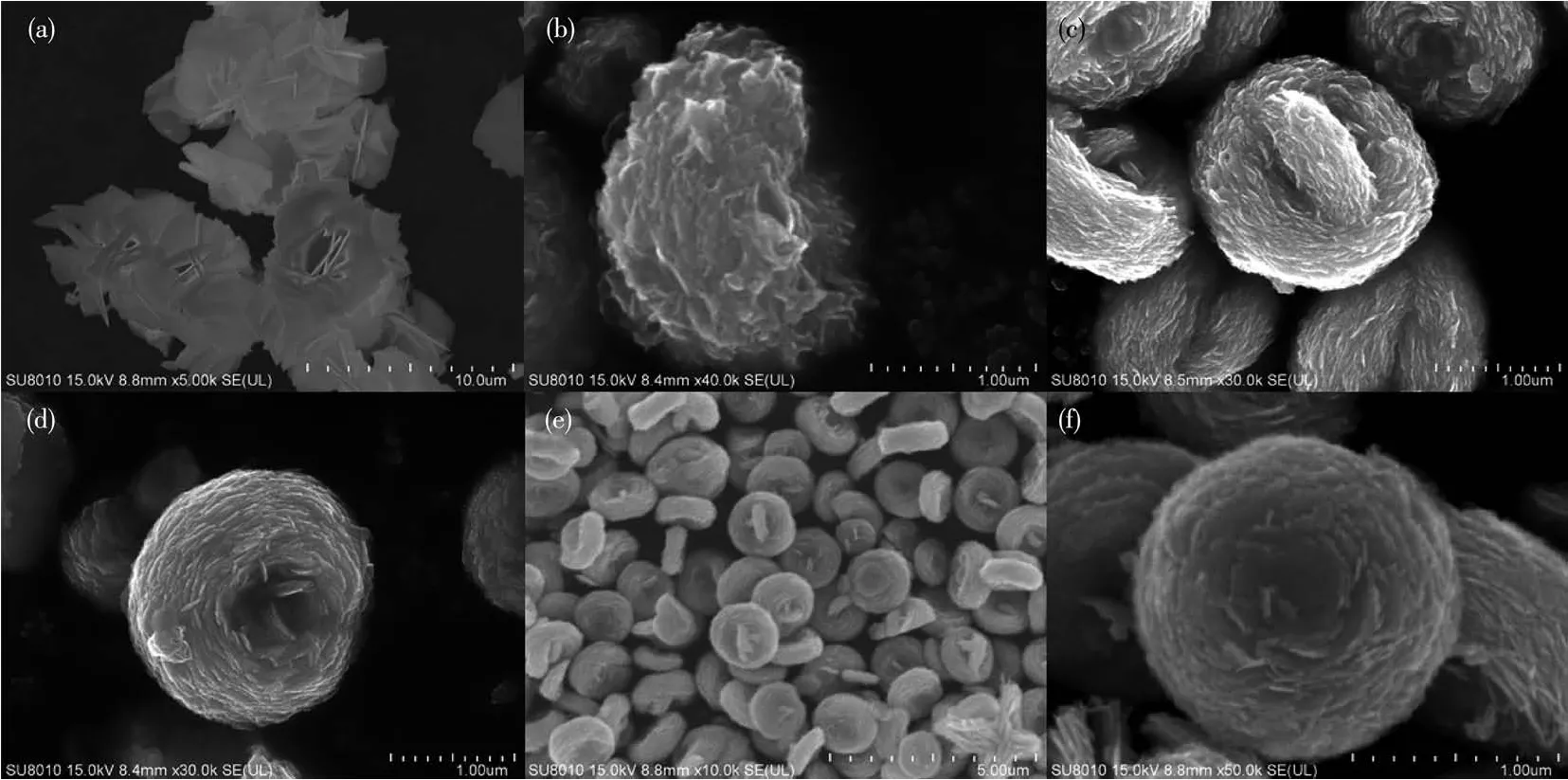
Fig.3 SEM images of(a)BiOI,(b)BW,(c)BWI-0.4,(d)BWI-1.0 and(e,f)BWI-1.6
2.2 Influence factors of morphology
In order to understand the process of I-guide BWI-xto generate erythrocyte-like structure,we changed the reaction time and temperature while ensuring that other conditions remained unchanged.The time dependence experiments showed the morphology change process of BWI-1.6 for reaction time of 3~24 h,as shown in Fig.4.As shown in the Fig.4a,in the 3 h hydrothermal reaction,only irregular clusters were obtained.By magnifying one of the nanoclusters,it can be found that its morphology is similar to that of pure BiOI.XRD analysis were applied to investigate further(Fig.4f),it can be concluded that there was only BiOI in the reaction system at this time,and BWI didn′t form.As the reaction time extended to 6 h,the erythrocyte-like structure formed,but there were still many nanoplates mixed in them,these nanoplates should be BiOI that decomposed,which is also consistent with the XRD results.When the reaction time was prolonged to 12 h(Fig.4e),BiOI nanoplates in the system disappeared,and only BWI-xwith erythrocyte-like structure existed,at this time,there were only BWI peaks in the XRD pattern.When the hydrothermal time was doubled to 24 h,the erythrocyte-like structure didn′t changed significantly,without any nanoplates which vertically standing in the center.
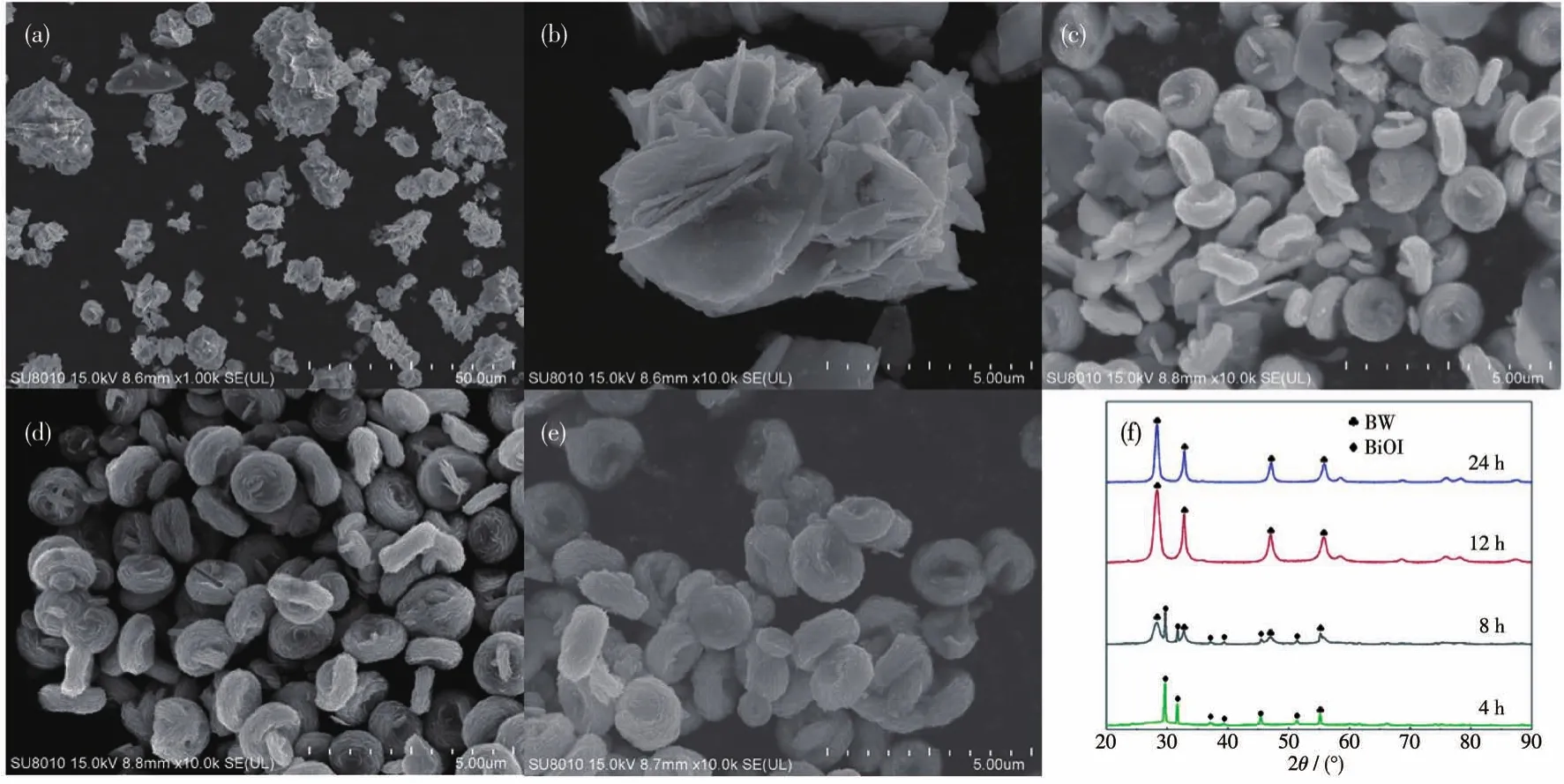
Fig.4 SEM images of BWI-1.6 for reaction time of(a,b)3 h,(c)6 h,(d)12 h and(e)24 h;(f)Corresponding XRD patterns
With the addition of 1.6 mmol BiOI,the influence of the hydrothermal temperature from 100 to 180℃on the layered structure of BWI-xis shown in Fig.5.When the hydrothermal temperature decreased to 100℃(Fig.5a,5b),130℃(Fig.5c,5d)and 150℃(Fig.5e),no 3D erythrocyte-like structure but flower-like structure which is similar to BiOI.Increasing temperature to 180℃,BWI-xdisplayed a 3D erythrocyte-like structure(Fig.5f).Thus,it can be concluded:compared with the effect of hydrothermal time,the effect of hydrothermal temperature on the formation of erythrocyte like structure is more serious.
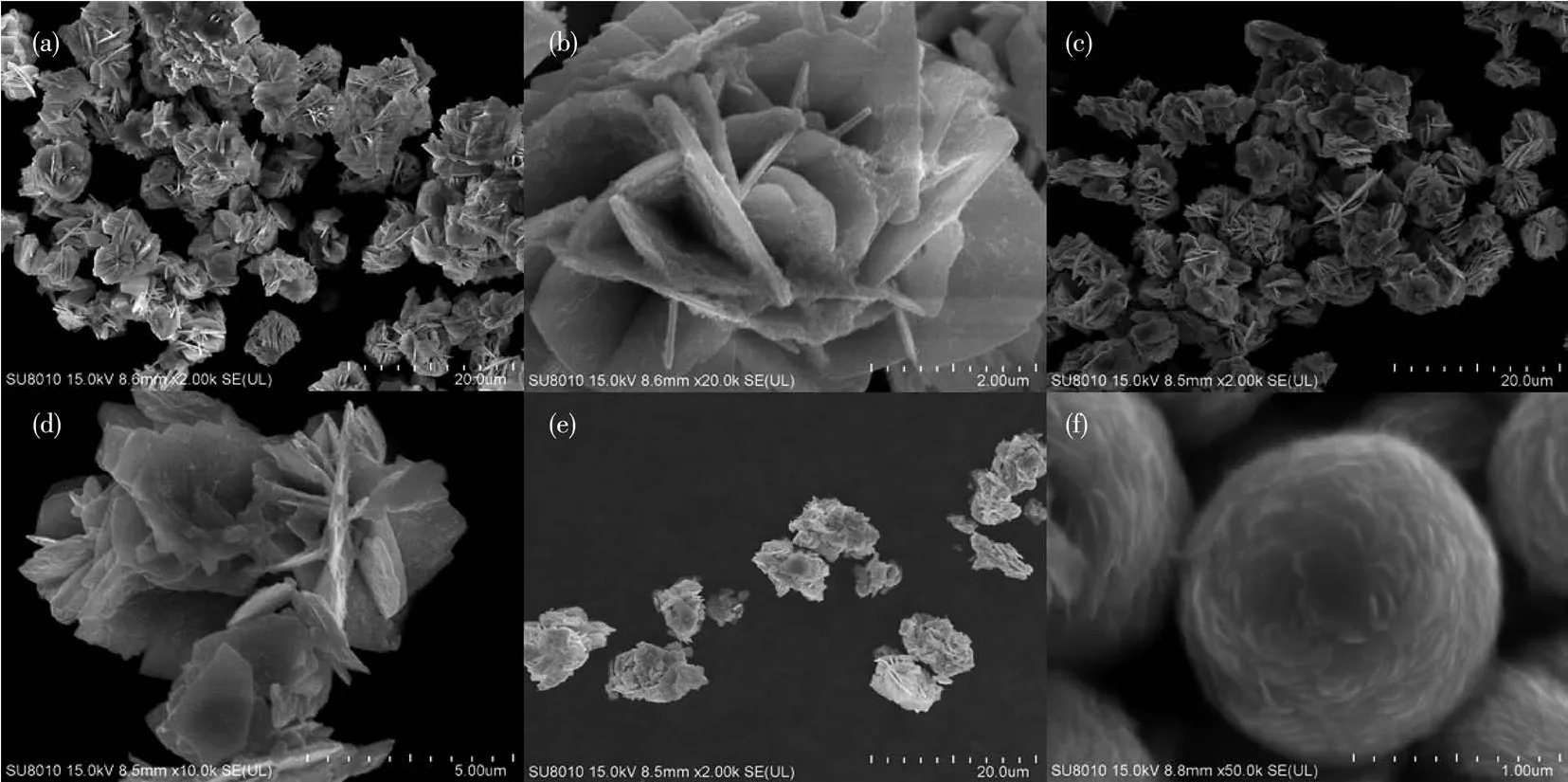
Fig.5 SEM images of BWI obtained by hydrothermal method with adding 1.6 mmol BiOI at different temperatures:(a,b)100℃;(c,d)130℃;(e)150℃;(f)180℃
2.3 Formation mechanism of BWI
After studying the factors that may affect the morphology and structure of the sample,the possible growth mechanism of BWI-xis shown in the Scheme 1.It involves the process of nucleation,growth,attachment and self-assembly in the construction of erythrocyte-like BWI[42-43].
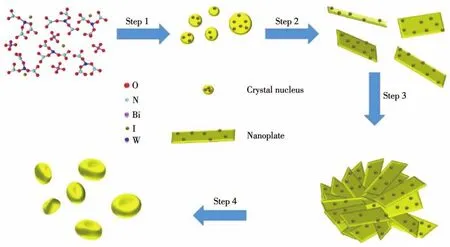
Scheme 1 Schematic diagram of crystal growth mechanism for BWI-x
The formation of BWI-xis a typical hydrothermal growth mechanism.In the initial stage of hydrothermal,because the solution is in a high supersaturated state,tiny particles are grown,and these small particles will continue to grow into BW nanoplates[44].In detail,Bi(NO3)3and BiOI will be hydrolyzed to produce Bi2O22+when dissolve in water.In the presence of excess WO42-,BW crystal nucleus are preferentially formed.According to the Gibbs-Thomson low,in a high supersaturated solution,in order to reduce the surface energy of tiny crystal nucleus,it will inevitably continue to grow,and will soon grow into small particles[45].Larger particles grow at the cost of the smaller particles and grow into nanoplates by dissolution-recrystallization(Ostwald ripening process)[42,46].The crystal growth of normal BW is anisotropic,but excessive I-will adsorb on the surface of the nanoplates,suppressing their intrinsic anisotropic crystal growth,and in the meantime,due to the existence of space steric hindrance,preventing the longitudinal growth of the nanoplates,ultrathin nanoplates are thus produced.The adsorption of I-will also cause the edge interaction between BW nanoplates and overlapping along the vertical direction.As more I-is adsorbed on the surface of BW nanoplates,greater space steric hindrance will be generated,and the nanoplates will be stacked preferentially in a direction perpendicular to the central axis,this stacking method is easy to produce a curved surface.During the oriented self-assembly process of nanoplates,the growth rate along the central axis is lower than the stacking velocity of perpendicular to the central axis which is due to the weaker adsorption force between I-on the nanoplates,which may be non-equilibrium kinetic growth process[47].At the same time,when the nanoplate units are stacked in a circle,due to the space steric hindrance at the center caused by the adsorbed I-,further stacking tends to stack outward.Based on the above analysis,I-can be regarded as the main reason for the formation of erythrocyte-like structure BWI[20].
2.4 Adsorption and photocatalytic performance
In order to evaluate the adsorption and photocatalytic performance of BWI-x,we tried to remove RhB in water at room temperature.Before photodegradation under visible-light irradiation,the samples were adsorbed in the dark for 30 min,as shown in the Fig.6.It can be clearly seen that after 30 min of dark reaction,the RhB removal rate of BWI-1.6 reached 94.7% ,and that of BWI-1.0 also reached 72.1% ,while unmodified BW can only adsorb 18.8% of pollutants.The adsorption performance was greatly improved.However,the adsorption capacity of BWI-0.4 was lower than that of BW,and the adsorption capacity of BiOI was relatively weak,which can only adsorb 10% of the pollutants.
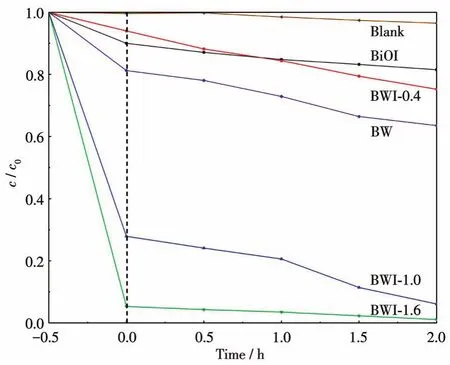
Fig.6 Degradation curves of RhB by BW,BWI-x,and BiOI
The photodegradation started after the visible-light was turned on.In contrast with BW,after 2 h of illumination,the RhB degraded by BWI-1.0 and BWI-1.6 were basically removed,and the removal rate was much higher than that of BW.Although the adsorption performance of BWI-0.4 decreased,it still retained the photocatalytic performance similar to that of BW.Pure BiOI also showed weak photocatalytic activity.The reason may be that the band gap of BiOI is too narrow,although it can absorb more visible light,but it leads to a serious recombination of photo-generated electrons and holes,resulting in low photocatalytic performance.
The photocatalytic reaction can be regarded as a first-order kinetic reaction,the kinetic constants of different BWI-xwere calculated by the equation,as shown in the Fig.7.After linear fitting,the fitting correlation valueRof each set of data was greater than 0.9,and the credibility of the trend line was high.Obviously,when the amount of catalyst was 0.1 g and the initial concentration of RhB was 10 mg·L-1,BWI-1.6 showed the highest kinetic constant.
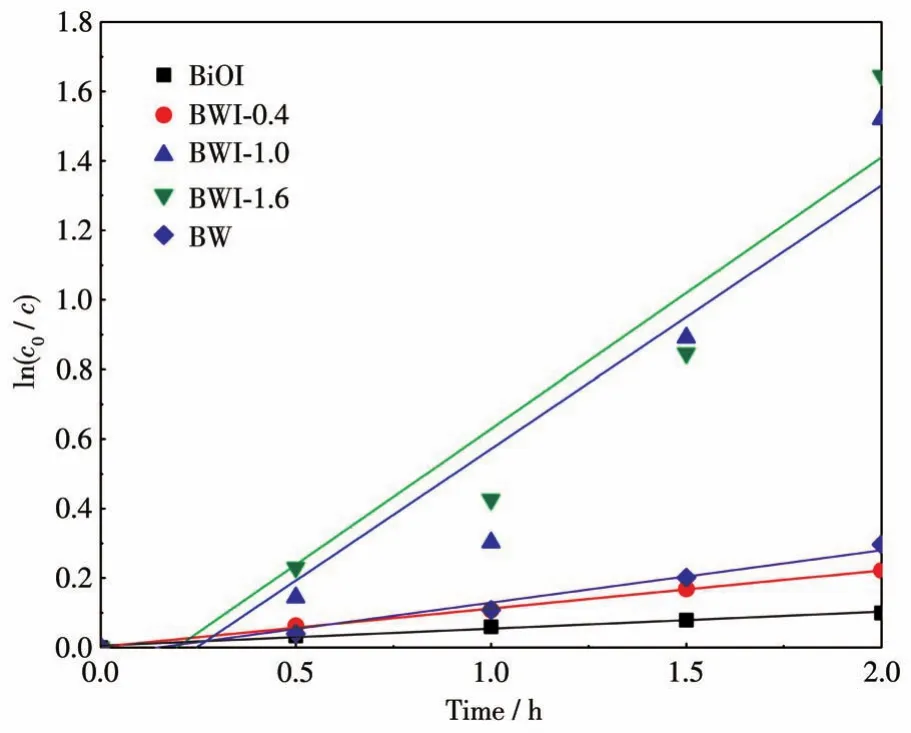
Fig.7 First-order kinetic plots of the samples
The optical properties and electronic band structure of samples were characterized by UV-Vis DRS.As shown in Fig.8a,each samples had strong absorption in the visible light region.The steep shapes indicate that the intense absorptions are not due to the transition from the impurity level but band-gap transition[48].When the amount of BiOI added was increased from 0 to 1.6 mmol,the absorption edge of the samples was redshift compared with that of BW.Indicating the ability to absorb light gradually increased.BW is generally an indirect band gap semiconductor[49],the band-gap energy(Eg)of samples can be estimated using a series of calculations.As shown in Fig.8b,the band gaps of BWI-0.4,BWI-1.0,and BWI-1.6 were 2.52,2.26 and 2.20 eV,respectively,which are significantly reduced compared to BW,indicating that the addition of BiOI makes the band gap of the material narrower.This is closely related to the control of BW morphology by BiOI[50].The band gap between BWI-1.0 and BWI-1.6 is significantly smaller than the gap between BWI-1.0 and BWI-0.4,which might be caused by the quantum size effect of the thinner nanoplates substructure in BWI-1.6[51].Interestingly,due to the addition of BiOI,the color of the samples also changed regularly,from the grayish green of BW to the bright yellow of BWI-1.6,which also demonstrate from the side that the optical properties of the samples have changed.
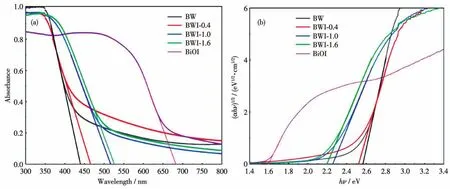
Fig.8 Plots of(αhν)1/2versushν (a)and UV-Vis DRS spectra(b)of samples
The generation and transmission of photogenerated charges will also affect the photocatalytic effect.So the PL emission spectra was measured at room temperature.It can be seen from the Fig.9 that both BW and BWI-1.6 exhibited emission peaks at 420 nm.Compared with BW,the emission peak intensity of BWI-1.6 was significantly lower,indicating that the recombination of photo-generated charges is suppressed,which is undoubtedly beneficial to the improvement of photocatalytic activity.
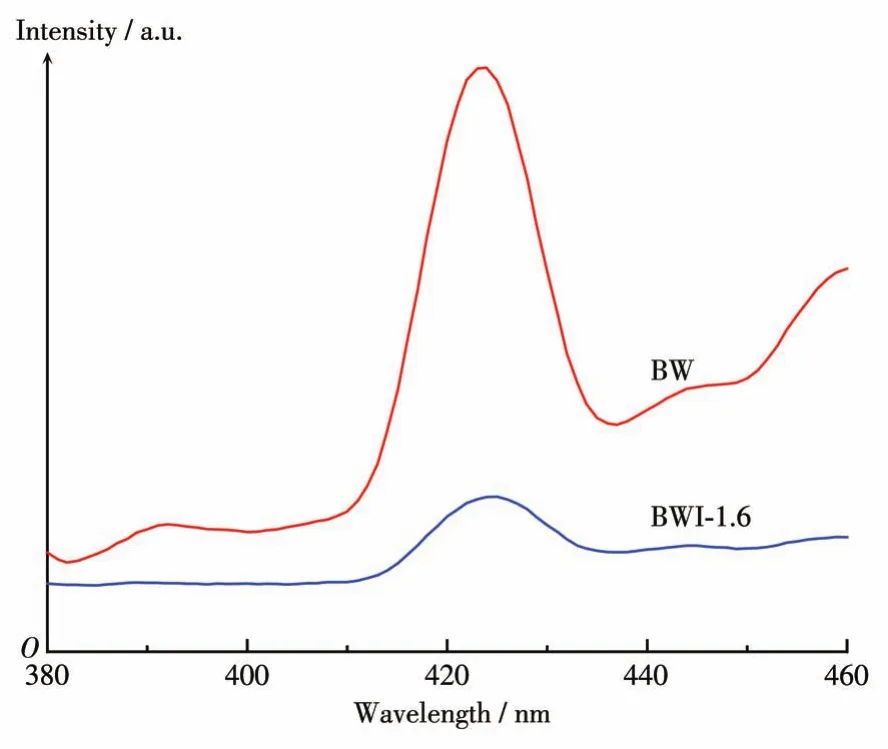
Fig.9 PL spectra of BW and BWI-1.6
Because BWI-1.6 had too strong adsorption performance,it failed to show complete photocatalytic per-formance in 10 mg·L-1RhB solution,so we adjusted the concentration to 30 mg·L-1and carried out repeated experiments(Fig.10).The results showed that BWI-1.6 not only had strong adsorption performance,and its photocatalytic performance was improved.
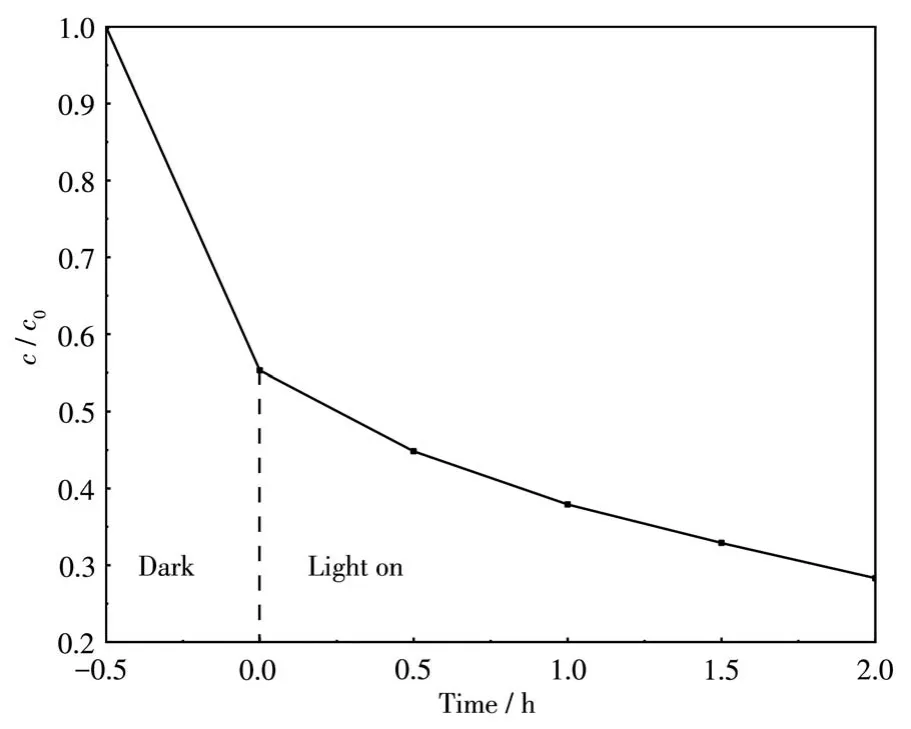
Fig.10 Adsorption and photocatalytic performance of BWI-1.6 for RhB with an initial concentration of 30 mg·L-1
In order to understand the reactive species of the BWI-1.6,trapping experiments were performed.Three different scavengers ethylenediaminetetraacetic acid disodium salt(EDTA-2Na),zoquinone(BQ)and isopropanol(IPA)were employed for trapping holes(h+),superoxide radicals(·O2-)and hydroxyl radicals(·OH),respectively.Fig.11 shows that when EDTA-2Na was added into the reaction system,the photocatalytic activity of BWI-1.6 experienced a remarkable decrease,indicating that h+is the main active species.However,only a slight decline was observed with the addition of BQ and IPA,suggesting that·O2-and·OH play small effects on the photocatalytic system.
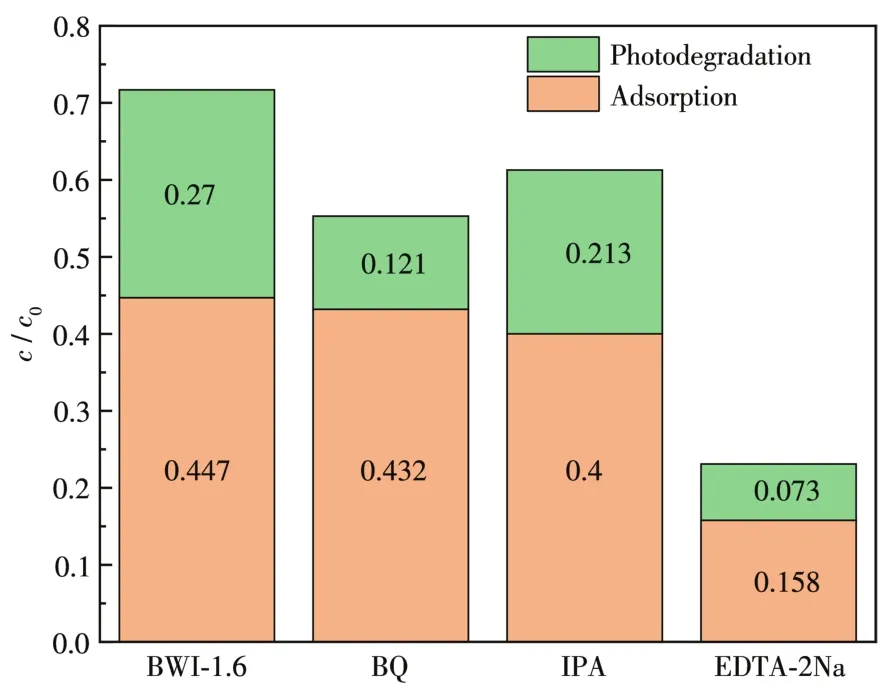
Fig.11 Photocatalytic degradation of RhB(30 mg·L-1)with trapping agents over BWI-1.6
Considering that BWI-1.6 has a strong adsorption effect on RhB,the adsorption kinetics of the material was studied by measuring the change of RhB concentration with time during the adsorption process.In order to ensure that the adsorption equilibrium can be fully achieved,the concentration of RhB solution was increased to 30 mg·L-1,and the adsorption process was completed in the dark.The adsorption kinetics diagram of BWI-1.6 is shown in Fig.11.Qt(mg·g-1)represents the amount of RhB adsorbed to the surface of the sample at timet,which can be calculated by the following equation:

Here,c0,ctandVrepresent the concentration of RhB solution(mg·L-1)at initial and at timet,and the volume of solution(L)respectively,mis the amount of photocatalyst(g).The adsorption capacity and equilibrium concentration of the photocatalyst at adsorption equilibrium were defined asQeandce,respectively.
The adsorption of RhB on the catalyst was a rapid adsorption process within the first 5 min,as depicted in Fig.12a.When the time reached 20 min,the concentration of the solution tended to be stable and did not change significantly until 60 min,which means that the adsorption-desorption equilibrium was achieved.After the adsorption,the color of the photocatalyst changed to rose red,which means that a large amount of RhB had adsorbed on the catalyst.In addition,in order to study the adsorption kinetics,we tried to fit the secondorder kinetic equation,which is expressed as follows[54-55]:
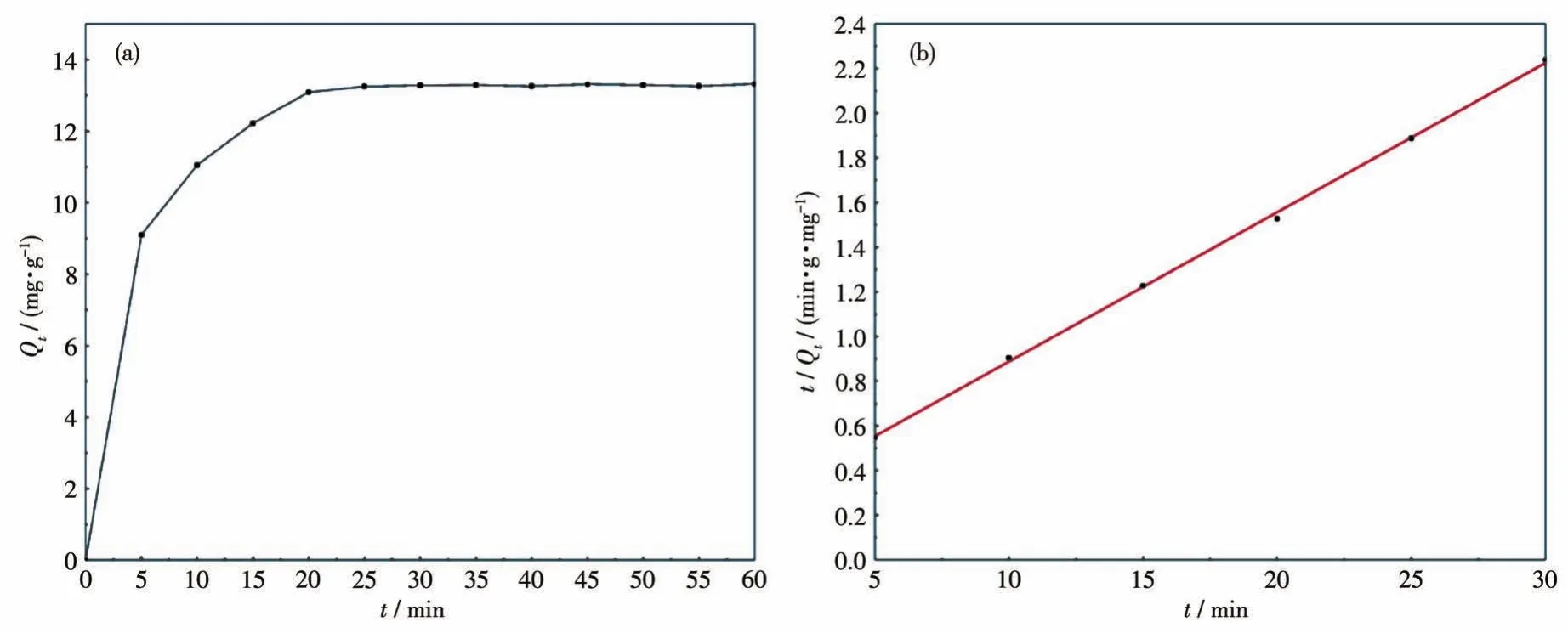
Fig.12 Adsorption kinetics curve(a)and second-order kinetics plot(b)for RhB at initial concentration of 30 mg·L-1on BWI-1.6

Here,K2(g·mg-1·min-1)is the rate constant for the second-order adsorption.Fig.12b shows the linear plot oft/Qtversust,and the slope and intercept represent theQeandK2,respectively.The final values ofQeandK2were 0.219 mg·g-1and 0.02.The adsorption results show that the process fitted to the second-order kinetic model,and the fitting correlation coefficientRwas greater than 0.999,indicating that the adsorption of RhB on the photocatalyst conforms to the second-order kinetic model.
In order to study the reason for the enhanced adsorption performance,N2adsorption-desorption isotherms of all samples are evaluated the surface area and pore sizes.The result was obtained and shown in Fig.13.Based on the Brunauer-Deming-assification,the isotherms of all samples are type Ⅳ,revealing the obvious characteristics of mesoporous structure.The obtained BET(Brunauer-Emmett-Teller)specific surface area,the corresponding pore-size distribution and micropore surface area of samples were summarized in Table 1.The BET surface area of BWI-xdecreased quickly from 74 to 55 m2·g-1along with increasing BiOI additional,when the addition of BiOI exceeded 1 mmol,the BET surface area increased again.According to the previous SEM images,we can see that as the amount of BiOI added increased,the stacking between the nanoplates will become more densified,which explains why the BET area decreases.It is noteworthy that the reduction of BET surface area is mostly caused by micropores,and micropores do not have a decisive effect on the adsorption of dyes.The change of the pore size follows the opposite rule,when the amount of BiOI added was less than 1 mmol,the pore size will continue to increase as the amount of BiOI added increased,once the amount of addition exceeded 1 mmol,the pore size decreased.The average pore sizes of BW and BWI-0.4 were small,which is not conducive to the adsorption of RhB.
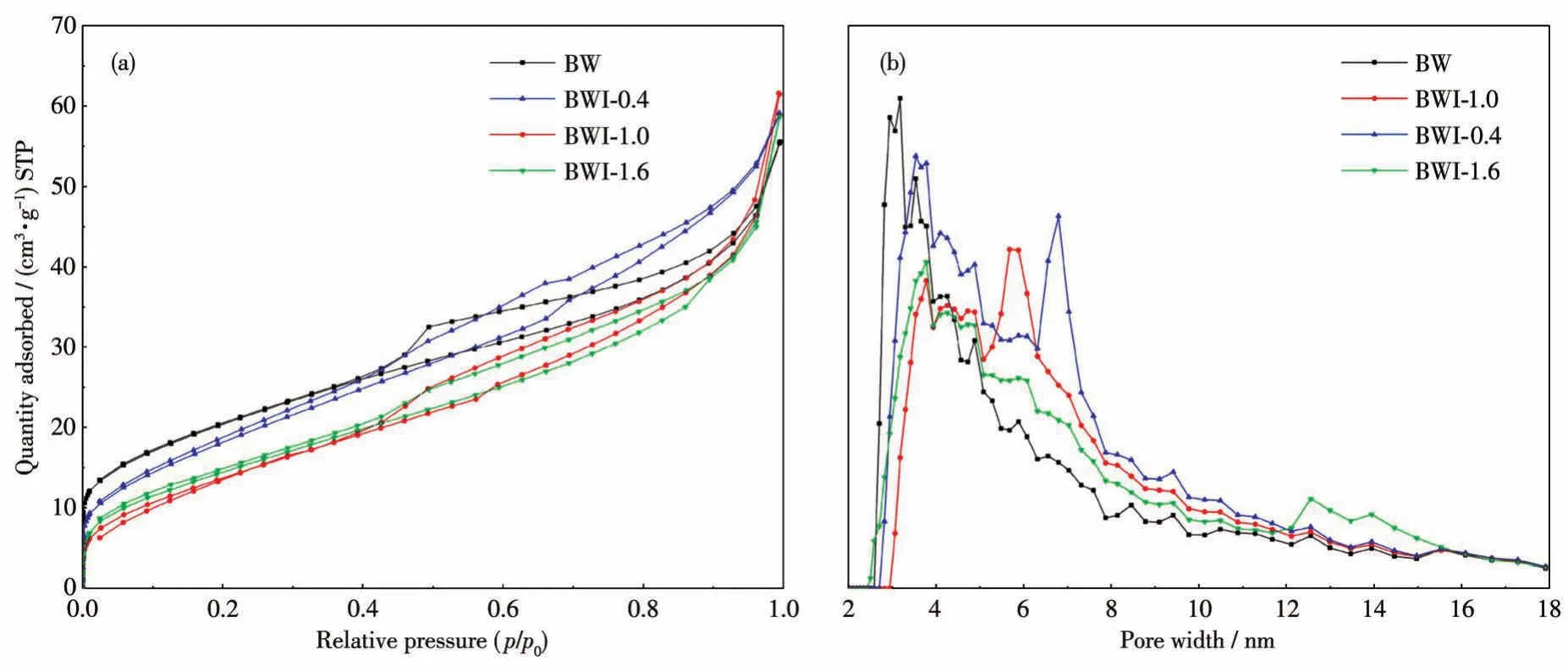
Fig.13 N2adsorption-desorption isotherms(a)and pore size distribution curves(b)of BW and BWI-x
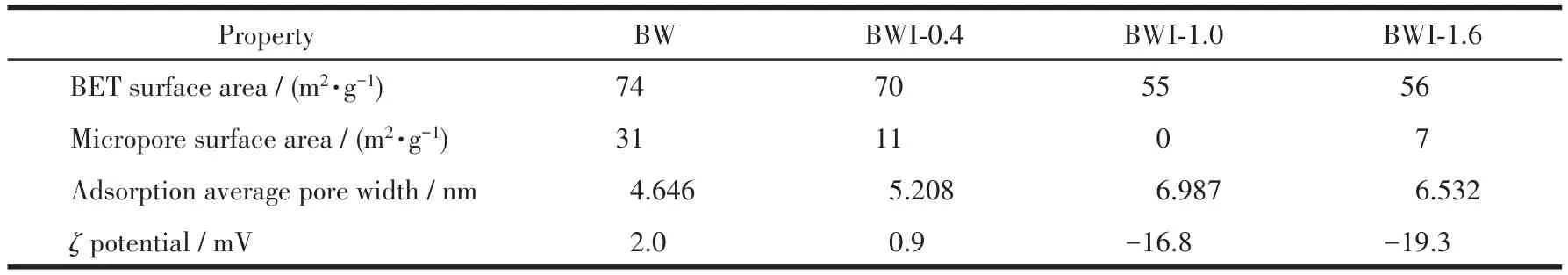
Table 1 BET specific surface area,micropore surface area,adsorption average pore width andζ potential of BW and BWI-x
In addition to surface area,surface charge is also an important factor affecting physical adsorption.As a kind of cationic dye,RhB has a stronger adsorption capacity for materials with negative charges on its surface.In the previous XPS analysis,it was found that the excess I-would be adsorbed on the surface of the BWI-x,so it is reasonable to believe that the surface of BWI-xwould be negatively charged.Theζpotential measurement confirms this idea,theζpotentials of BWI-1.0 and BWI-1.6 in water were both negative.Although theζpotential of BWI-0.4 was positive,the value was smaller than BW,which shows the addition of BiOI can significantly change the surface properties of BW.Combining the characterization results of BET andζpotential measurement,we can infer that electrostatic adsorption is the main reason affecting the physical adsorption of RhB,despite the specific surface area of BW and BWI-0.4 was large,theζpotential was too positive and the adsorption effect was not ideal.BWI-1.6 had a larger specific surface area and the most negativeζpotential,so it had the strongest RhB adsorption capacity.
In order to further prove the role of electrostatic interaction in the adsorption process,an experiment on the influence of pH on the adsorption process was carried out(Fig.14).The natural pH of RhB solution was about 7.When the pH value was less than 7,the adsorption capacity decreased.Because at low pH,the adsorption surface is protonated,and the adsorbent surface is positively charged,which reduces the electrostatic interaction to the cationic dye.When the pH value was slightly greater than 7,the adsorption capacity gradually increased because of the electrostatic attraction between the negatively charged sites on the adsorbent and the dye cations.But when the pH value was too high,the adsorption capacity decreased.BWI-1.6 particles are not chemically stable in stronger alkaline solutions as they tend to dissolve due to alkaline corrosion.
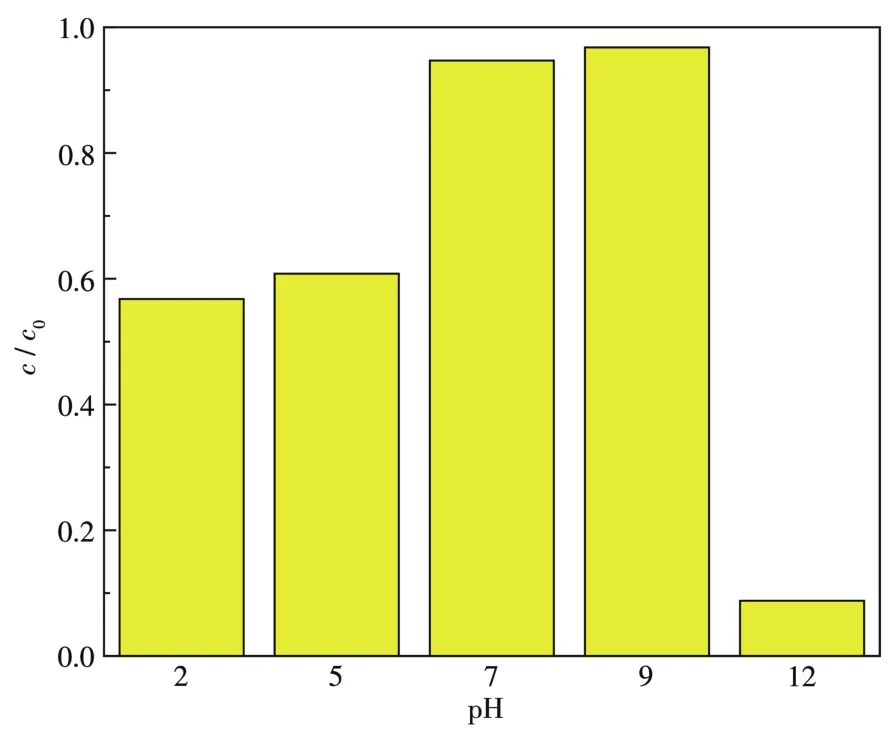
Fig.14 RhB adsorption behavior on BWI-1.6 under different pH values
Considering that the strong adsorption of BWI-1.6 may not only be caused by physical adsorption,the surface acidity of BW and BWI-1.6 have been characterized by NH3-TPD(temperature programmed desorption),as shown in Fig.15a.Combining the previous BET data,concluding that the surface acidity per unit area of BWI-1.6 is higher than that of BW,indicating that there is a stronger chemical adsorption between BWI-1.6 and RhB molecules.Generally,the acidic sites on the surface of catalyst were observed through interaction with basic probe molecules.In infrared spectroscopy,the most widely used basic probe is pyridine.By pyridine adsorption infrared(PY-IR),both Lewis acid(L acid)sites and Brønsted acid(B acid)sites were found on the surface of BWI-1.6,as shown in Fig.15b.Studies have shown that pyridine was adsorbed on the B acid site,and the characteristic absorption peak of the infrared spectrum was located near 1 580 cm-1;pyridine was adsorbed on the L acid site,and its characteristic peak appeared at about 1 450 cm-1.However,in BWI-1.6 spectrum,we found that the characteristic peaks of the two acid sites shifted to the higher wavenumber direction,which means that the surface acidity increased[54].And even if the temperature rose to 150℃,pyridine was still adsorbed,therefore,it must be at least 150℃to desorb pyridine completely.Therefore,we can infer that the adsorption of RhB on BWI-1.6 surface includes not only physical adsorption,but also chemical adsorption.Due to the low coupling between the electron levels of dye and semiconductor,the electron injection rate decreases with the distance between the sensitizer and the surface of metal oxide[55-57].Obviously,the force between RhB molecule and BWI-1.6 is stronger,and this interaction is related to the acidity of the catalyst surface.Since N atoms are also present in the molecular structure of RhB,the interaction between RhB and BWI-1.6 is similar to that between pyridine and BWI-1.6,both through the interaction of lone pair electrons with the surface acid center of BWI-1.6.The strength of RhB molecule on the surface will increase with the increase in acidity.This strong chemical effect facilitates the transfer of electrons on RhB to BWI-1.6,which is more conducive to photosensitization and charge separation.
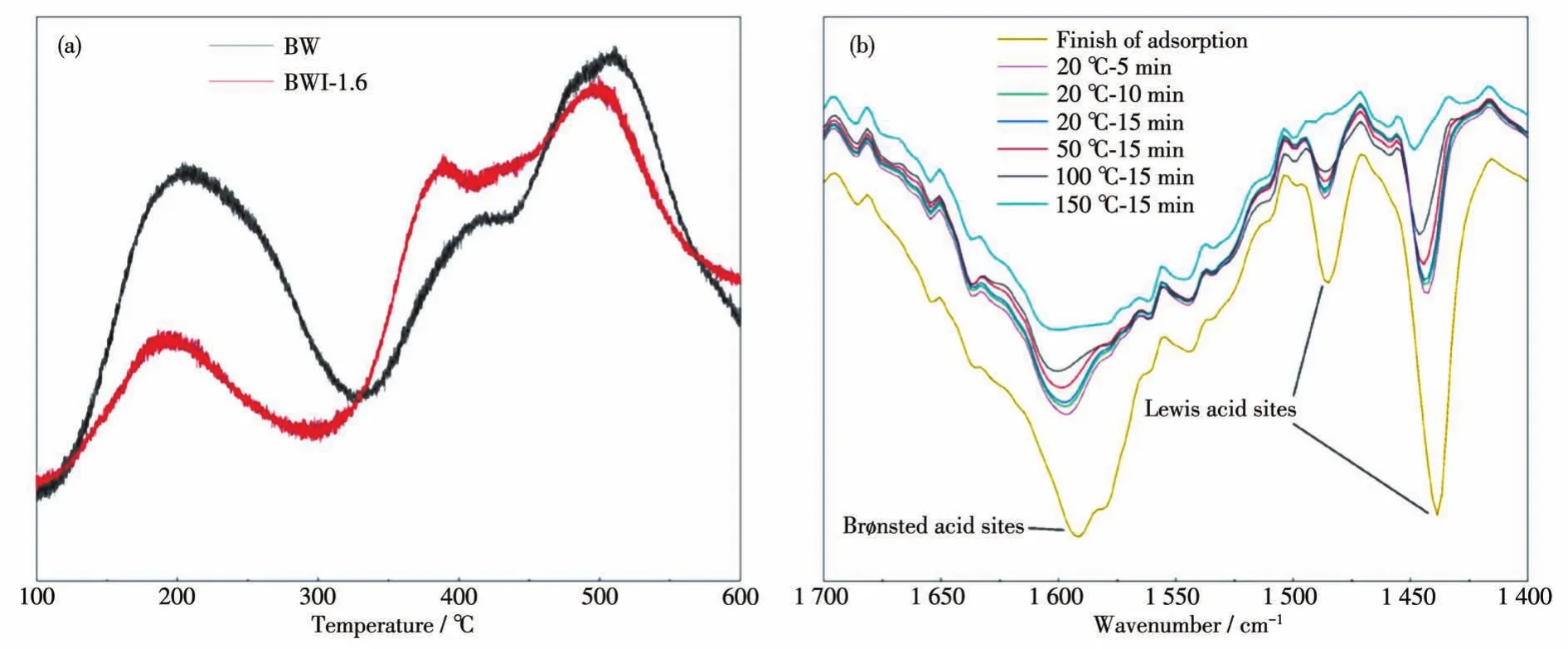
Fig.15 NH3-TPD curves for BW and BWI-1.6(a)and infrared spectra of pyridine adsorbed on the surface of BWI-1.6(b)
2.5 Mechanism of synergistic removal of RhB by adsorption and photocatalysis
By studying the optical and adsorption properties of BWI-1.6,it is found that BWI-1.6 is a kind of catalyst with both adsorption and photocatalysis functions,and it has a significant effect on the removal of RhB in solution.Summarizing the removal mechanism of RhB as Scheme 2.Because the adsorption process is fast,the RhB in the solution is first adsorbed to the surface of BWI-1.6,forming a lower concentration RhB solution.When exposed to visible light,the photocatalytic effect will further remove the RhB that don′t be adsorbed.A possible adsorption enhancement mechanism was proposed,which may be due to the increase in external surface area and average pore diameter,and the enhancement of surface acidity.Since the adsorption kinetic curve conforms to the second-order kinetic equation,chemical adsorption might be significant[58].Meanwhile,the ability of absorb light of BWI-1.6 enhanced significantly than BW,the recombination rate of photogenerated electrons is also significantly reduced.The trapping experiments shows that the main active species of photoreaction is h+.Above all,BWI-1.6 shows excellent RhB removal effect through the synergy of adsorption and photocatalysis.

Scheme 2 Removal mechanism of RhB by BWI-x
3 Conclusions
Erythrocyte-like structure Bi2WO6with excellent adsorption capacity and visible light catalytic activity be synthesized from BiOI by hydrothermal treatment.The adsorption of I-on the surface of the 2D nanoplates causes the nanoplates to produce directional self-assembly,forming this special structure,the concentration of I-,reaction time and temperature will all affect the morphology.At the same time as the morphology was changed,the visible light absorption capacity and adsorption capacity were improved,which is attributed to the narrowing of the band gap and enhancement of surface acidity.These results show that I-assisted construction of erythrocyte-like structure can effectively improve the performance of Bi2WO6to remove RhB.
Acknowledgments:This work was supported by the Guidance Plan of Liaoning Provincial Natural Foundation(Grant No.2019-ZD-0057),Introduction of Talent Research Start-Up Foundation of Liaoning Shihua University.
杂志排行
无机化学学报的其它文章
- Synthesis,Structures and Catalytic Activity in Knoevenagel Condensation Reaction of Cu(Ⅱ)/Co(Ⅱ)/Ni(Ⅱ) Coordination Polymers Based on Ether-Bridged Tetracarboxylic Acid
- Synthesis and Luminescence Properties of Double Perovskite Ca2Gd1-xTaO6∶xTb3+Green Phosphors
- Carbonized MoS2/S-Doped g-C3N4Heterojunction:Synthesis and Catalytic Degradation Mechanism of Rhodamine B under Visible Light
- Preparation of Li2Ni2(MoO4)3@C Composite as High-Performance Anode Material for Lithium-Ion Batteries with High Initial Coulombic Efficiency
- 单分散共价有机框架纳米颗粒的室温快速制备
- 含三苯胺基团的菲咯啉铁(Ⅱ)配合物电致变色材料的合成及性质
