Surface treatment of titanium dioxide nanopowder using rotary electrode dielectric barrier discharge reactor
2021-10-31NawRuthaPAWTakumaKIMURATatsuoISHIJIMAYasunoriTANAKAYusukeNAKANOYoshihikoUESUGIShioriSUEYASUShuWATANABEandKeitaroNAKAMURA
Naw Rutha PAW, Takuma KIMURA, Tatsuo ISHIJIMA,Yasunori TANAKA, Yusuke NAKANO, Yoshihiko UESUGI,Shiori SUEYASU, Shu WATANABE and Keitaro NAKAMURA
1 Faculty of Electrical Engineering and Computer Science, Kanazawa University, Kanazawa 920-1192,Japan
2 Research Center for Production & Technology, Nisshin Seifun Group Inc., Fujimino 356-8511, Japan
Abstract Titanium dioxide (TiO2) nanopowder (P-25; Degussa AG) was treated using dielectric barrier discharge (DBD) in a rotary electrode DBD (RE-DBD) reactor.Its electrical and optical characteristics were investigated during RE-DBD generation.The treated TiO2 nanopowder properties and structures were analyzed using x-ray diffraction (XRD) and Fourier-transform infrared spectroscopy (FTIR).After RE-DBD treatment, XRD measurements indicated that the anatase peak theta positions shifted from 25.3° to 25.1°, which can be attributed to the substitution of new functional groups in the TiO2 lattice.The FTIR results show that hydroxyl groups (OH) at 3400 cm−1 increased considerably.The mechanism used to modify the TiO2 nanopowder surface by air DBD treatment was confirmed from optical emission spectrum measurements.Reactive species, such as OH radical, ozone and atomic oxygen can play key roles in hydroxyl formation on the TiO2 nanopowder surface.
Keywords: dielectric barrier discharge, nanopowder, reactive species, rotary electrodes, surface treatment, titanium dioxide
1.Introduction
Among different semiconductors,titanium dioxide(TiO2)[1]has been regarded as a material with multifunctional applications [1–3] due to its diverse and unique properties.In particular, it is crucially important for photocatalytic applications due to its peculiar application prospects for humidity sensors [4], air treatment [5], air purification [6], water splitting [7], self-cleaning [8], super capacitors [9] and solar energy conversion [10, 11].However, a wide band gap and fast recombination of photo-generated electrons and holes are shortcomings that can reduce the efficiency of pure TiO2.To resolve this difficulty,one possible means of improving TiO2performance is efficient light harvesting.Another means is by obtaining a certain number of holes and photo-generated electrons on the surface before recombination.To achieve the desired performance using this technique, the surface structure is modified using metal doping and non-metal doping methods [12, 13].For metal doping methods, metallic elements, such as Fe [13], Ni [14] and Cu [15], are applied for surface treatment.When using the non-metal doping method,N [16], C [17] and S [13] are used as non-metallic dopants.For metal doping, some shortcomings related to thermal and chemical instability of TiO2remain.Although high doping enhances the band gap, the optical/photocatalytic performance is reduced due to increasing carrier recombination centers [18].Another technique for surface treatment is adding Ti3+[19] and defects of oxygen vacancies [20] in the band gap.In contrast to the case of high doping,instead of the recombination center,this method uses oxygen vacancies and Ti3+to construct a trap center to enhance the band gap.To create a trap center using defects of oxygen vacancies in the lattice of TiO2,vacuum activation[18],surface hydrogenation[9, 21, 22] and plasma treatment [23] have been reported as effective methods for TiO2surface treatment.Using the hydrogenation method, photocatalytic activity can be enhanced because the TiO2surface is functionalized by hydroxyl groups.However, a disadvantage of the hydrogenation method is that it necessitates the use of high temperatures.Moreover, the obtained sample becomes black,which is unsuitable for various optoelectronic applications.The vacuum activation method also increases absorption, but also color effect [18], which is not suitable for transparent electrode applications.
Among the treatment methods, the plasma treatment has also been reported as an effective method for TiO2surface modification due to its diverse and beneficial properties[18, 24, 25].Recently, Liet al[26] modified anatase-TiO2using dielectric barrier discharge (DBD) argon plasma with 20 min treatment time to enhance photocatalytic degradation.Nevertheless, no report in the relevant literature describes a study of the application of air DBD treatment for commercial TiO2nanopowder modification.Moreover, some problems remain for powder and particulate materials.In the case of a fixed sample treatment method using nonthermal plasma, the process effectiveness is expected to decrease due to the shadow effect as a result of overlapped powders [27].To overcome powder surface overlapping and to treat it uniformly with nonthermal plasma, a rotary electrode dielectric barrier discharge reactor (RE-DBDR) was developed to treat and modify the particulate powder material uniformly during nonthermal plasma exposure on the powder surface.
As described herein, we propose new devices to implement a simple method with low costs that reduce the treatment time for surface modification.With regard to cost effectiveness, gas treatment was conducted by air.Rotary electrodes were placed while dispersing the powder for uniform treatment.It has been reported that the chamber temperature increases concomitantly with increasing operational frequency [28], which would cause thermal damage to the particulate material and raise the cost of power sources and electrical assemblies.To overcome this difficulty, instead of high frequency,low operation frequency was used to generate nonthermal plasma during treatment.We treated the commercial TiO2nanopowder by taking advantage of the REDBDR using air DBD.The plasma treatment time was set to 3 min for this study.To clarify the time effect, the treatment time was varied with it being set at 3 and 10 min.A marked change in the surface properties was observed after treatment using air DBD.The results indicated that the DBD-treated TiO2nanoparticles enhanced hydroxyl groups on the TiO2nanopowder surface compared with the pure TiO2.Lowtemperature DBD might not only provide a new strategy for surface modification as our newly developed RE-DBD reactor can also engender fast and efficient treatment time for additional industrial applications.
2.Materials and methods
2.1.Experiment setup and procedures
The RE-DBDR experiment setup for powder treatment is presented in figure 1.Two rectangular thin metal plates(68 mm in length, 20 mm in height and 1 mm in thickness)were mounted on a rotating axial rod placed at the center of a cylindrical chamber (70 mm in inner diameter, 80 mm in height and 2 mm in thickness) made of polyoxymethylene(POM) material.The two thin metal plates, with upper and lower sides separated by a distance of 20 mm, are connected to a motor axis using an insulated rod, which allows it to act as a floating electrode in a reactor.Two cylindrical aluminum electrodes (74 mm in diameter, 30 mm in height and 0.2 mm in thickness)were mounted on the outside wall of the reactor with the upper and lower electrodes separated by 10 mm by the cylindrical POM insulator to prevent surface discharge on the external surface between the outside electrodes.For this study,DBD was produced using a cylindrical POM reactor at a high voltage (Vpp=30 kV, frequency=60 Hz).The AC voltage power supply was controlled by a 0–130 V voltage regulator.A high-voltage power supply (Neon Transformer,
Vop=15 kV;LECIP Holdings Corp.)was used to step up the AC voltage power supply.For voltage measurements, two high-voltage probes (P6015A, 40 kV, 4 ns; Tektronix Inc.)were used.High-voltage probe I was used to measure the output voltage of the transformer.Probe II was used to measure the discharge voltage; for discharge current measurement, a current probe (2877; Pearson Electronics) was used.A monitoring capacitor,Cm=2000 pF,was connected in series with the reactor to measure the transferred charge.Electrical characteristics were observed using a digital oscilloscope (MSO6054A, 500 MHz, 4 GSa/s; Agilent Technologies Inc.).An air compressor (10 L, ACP-10A; Earth Man)introduced air into the reactor.The gas flow rate was controlled to 1.5 slm using a mass flow controller(SEC-E40,Air,10 slm; Horiba Stec, Co., Ltd).A DC high-power motor(12 V, 18 800 rpm) was used for rotating and controlling the high-speed floating electrodes, to provide rotational speed of approximate 5000 rpm.Configurations of the RE-DBDR and an image of the DBD during electrode rotating are depicted in figure 2.
2.2.Sample preparation
For sample preparation, titanium dioxide (TiO2) nanopowder(CAS: 13463-67-7, P-25; Degussa AG) was used.The powder had an anatase and rutile ratio of 85:15 and purity of 99.9%.Two treatment samples of the TiO2nanopowder were prepared to study fluctuation of the DBD treatment effect.For DBD treatment, 300 mg of TiO2nanopowder was used for each sample.After the nanopowder was introduced to the inner wall of the cylindrical POM reactor, the reactor was enclosed by a PMMA flange with an O-ring seal and a filter for the powder.Then, the electrodes were rotated by the motor at 5000 rpm, while introducing the operational gas species.Before generating DBD, TiO2nanopowder was dispersed by the electrode rotation for about 30 s.The DBD treatment times of the TiO2nanopowder were,respectively,3 and 10 min.After DBD treatment, the DBD-treated TiO2nanopowder samples were prepared to investigate the crystal structure and surface functional groups using x-ray diffraction(XRD), and Fourier-transform infrared (FTIR) analysis.
2.3.Crystal characterization
To analyze the structure and composition of the crystalline phase of the treated TiO2, XRD measurements were taken.The crystal structure of the TiO2nanopowder was identified using an x-ray diffractometer (Miniflex600; Rigaku Corp.).The sample powder was scanned using Cu-Kα radiation with operating voltage of 40 kV and operating current of 15 mA.The surface functional groups of the TiO2nanopowder were characterized using an FTIR spectrometer (Nicolet iS5;Thermo Scientific).The scan number was 16.The resolution was 4.To specify the functional groups on the TiO2nanopowder surface, a transmission sampling method was used.The standard powder ratio (100:1) was prepared for thin transparent pellets.For each measurement, 30 mg of TiO2powder sample was compressed using a hydraulic press to produce a 7 mm diameter thin pellet sample.Three sample pellets were replicated to observe the reproducibility.The error bar was approximately 3%.
3.Results and discussion
3.1.Electrical characteristics
The static electrode configuration and an image of its discharge generation during rotation are shown in figure 2.Electrical characteristics of the RE-DBDR are shown in figures 3(a)and(b).DBD was generated between the edges of the rotary plates and the inner wall surface of the reactor.The waveforms of the applied voltageVa(t),discharge currentI(t) and capacitance voltageVc(t) atfapp=60 Hz andVpp=30 kV and rotational speed=∼5000 rpm are shown in figure 3(a).DBD in air is generally influenced by filamentary discharge in nature.Discharges can generate effective reactive species, such as atoms, radicals and excited species with high electron densities (1014–1015cm−3)[24, 29].These species are important for surface treatment.The formation mechanism and distribution of current filaments as surface discharge were discussed in earlier reports[24, 29, 30].The transported charge was measured using a 2000 pF monitoring capacitor connected in series with the reactor to calculate the discharge area.The charge voltage(Q–V)area,also known as the Lissajous figure,was obtained by plotting the applied voltage of the reactor on theX-axis and the transferred charge on theY-axis.In figure 3(b), the Lissajous figure atfapp=60 Hz,Vpp=30 kV and rotational speed=∼5000 rpm is shown.Power consumption during the DBD generation can be estimated from the energy by multiplying the frequencyfappand area of the Lissajous figure:Pdis=fapp×area of the Lissajous figure.The discharge power consumption during the powder treatment was 0.75 W.The Lissajous figure resembles an ellipse, indicating the presence of residual ions at all times.The shape effect and characteristics of the Lissajous figure were discussed in the report of an earlier study [29].
3.2.Optical characteristics
The optical emission spectra (OES) at 5000 rpm rotational speed are shown in figure 4.To identify the reactive species present in the DBD air plasma, the optical emission spectra were observed at wavelengths of 300–450 nm,as presented in figure 4.The main contributions to the emission spectrum of the air DBD produced by rotary floating electrodes are the nitrogen molecular band spectra of the second positive system(C–B).Moreover, a small peak of OH radical at 308 nm wavelength can also be investigated.The SPS of nitrogen can engender the formation of oxygen atom, producing ozone[29,31,32].Ozone is an active species due to its long lifetime and high oxidation potential.The ozone concentration was monitored using a UV O3analyzer (Model 49i; Thermo Scientific, Inc.) during DBD air discharge.Results show that the average ozone concentration by DBD air plasma was 250 ppm.In general, the initial ozone can be created according to the following equation [32]:

Therein, M is the third collision molecule, which can be O2,O3or N2.In addition, high UV radiation, oxygen atoms and nitrogen oxides contribute to the decomposition of ozone in the reactor [24, 32].Reactive atomic oxygen can be created from the ozone decomposition process, the surface layer of TiO2.The rotational and vibrational temperatures were 300 and 2500 K,respectively,based on the massive OES[33–35].Based on these results, the surface layer functional group mechanism can be changed by reactive species, such as OH radicals, ozone and atomic oxygen.These oxidative species can play a key role in improving surface properties.
3.3.XRD analysis
XRD patterns of pure TiO2nanopowder and DBD-treated TiO2are shown in figure 5(a).The phase theta structure in TiO2powder clearly illustrates the anatase and rutile phases.The phase peaks at 25.3°, 38°, 48°, 62.8° and 69° represent the anatase crystalline phase,whereas the peaks at 27.42°,36.2°,41.4°,54°and 55.4° are assigned to the rutile crystalline phase [36].The crystalline peaks remained the same,indicating that no heat effect caused by the DBD treatment exists on the crystalline structure.In figure 5(b), significant peak shift at anatase (101) and rutile(101) after DBD treatment is shown.Compared with the pure TiO2,the phase theta of anatase shifted from 25.3°to 25.1°.The rutile phase structure peak shifted in a similar way from 27.4°to 27.2° in the DBD-treated TiO2.However, no new peak can be observed in the XRD pattern.Because there was no change in the crystal phase of anatase and rutile peak performance,we assume that the peak shifting is attributed to the new functional group formed on the TiO2[37].
3.4.FTIR analysis
To confirm the fluctuation level, we produced three pellets of TiO2nanopowder using 0,3 and 10 min treatment.Figure 6(a)shows the FTIR spectra of pure TiO2at 3 and 10 min DBDtreated TiO2nanopowder at wavenumbers of 400–4000 cm−1.Figure 6(b) shows peaks at 1800–1200 cm−1.The obtained FTIR spectra had similar profiles for each treatment time,suggesting that uniform treatment of TiO2nanopowder surface was realized using RE-DBDR.Figure 6(a)shows transmittance in the vibrational band of 400–1250 cm−1, which is the result produced by the O–Ti–O lattice[38,39].Change in the O–Ti–O peak was observed after 3 min treatment.The peak at around 1630 cm−1was assigned to the Ti–O structure [36].Peaks at 350–3000 cm−1are denoted by bending vibration of the hydroxyl(OH)or H2O group.Formation of the OH groups has been reported [38, 39].This group has very high and strong oxidation capability.Results show that Ti–O–Ti and OH band did not appear clearly with increasing DBD treatment time,suggesting TiO2nanopowder modification.The DBD-treated TiO2nanopowder shows gas phase CO2band at 2348 cm−1,indicating CO2adsorption on the surface [40,41].Figure 6(b)shows a functional group peak at 1385 cm−1after DBD plasma treatment; the peak is assigned to C–H/COO groups.The C–H/COO group was detected due to the formation of CO2[26, 40, 42] on the TiO2surface.These results indicate a porous carbon layer formation on the TiO2nanopowder, presumably due to chemical and physical activation processes[42,43].The carbon source might be the air compressor or the reactor wall from the POM chamber by etching due to DBD.Identification of the carbon layer formation and its carbon source remains a subject for future work.Formation of the C–H/COO group and the gas phase CO2band at 2348 cm−1showed a stronger peak in both DBD treatments, indicating that the surface functional groups of commercial TiO2surface properties can be enhanced remarkably with the assistance of reactive species in DBD.

Figure 1.Experiment setup for a RE-DBDR.
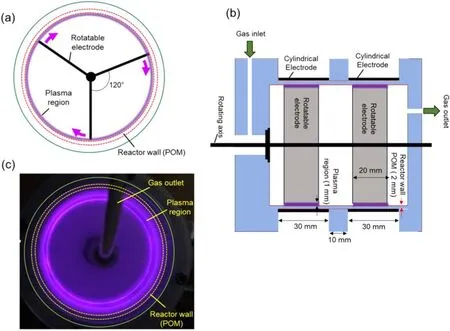
Figure 2.Schematic diagram of a RE-DBDR.(a) Top view, (b) cross-sectional view and (c) photograph of a RE-DBDR with DBD generation while rotating.
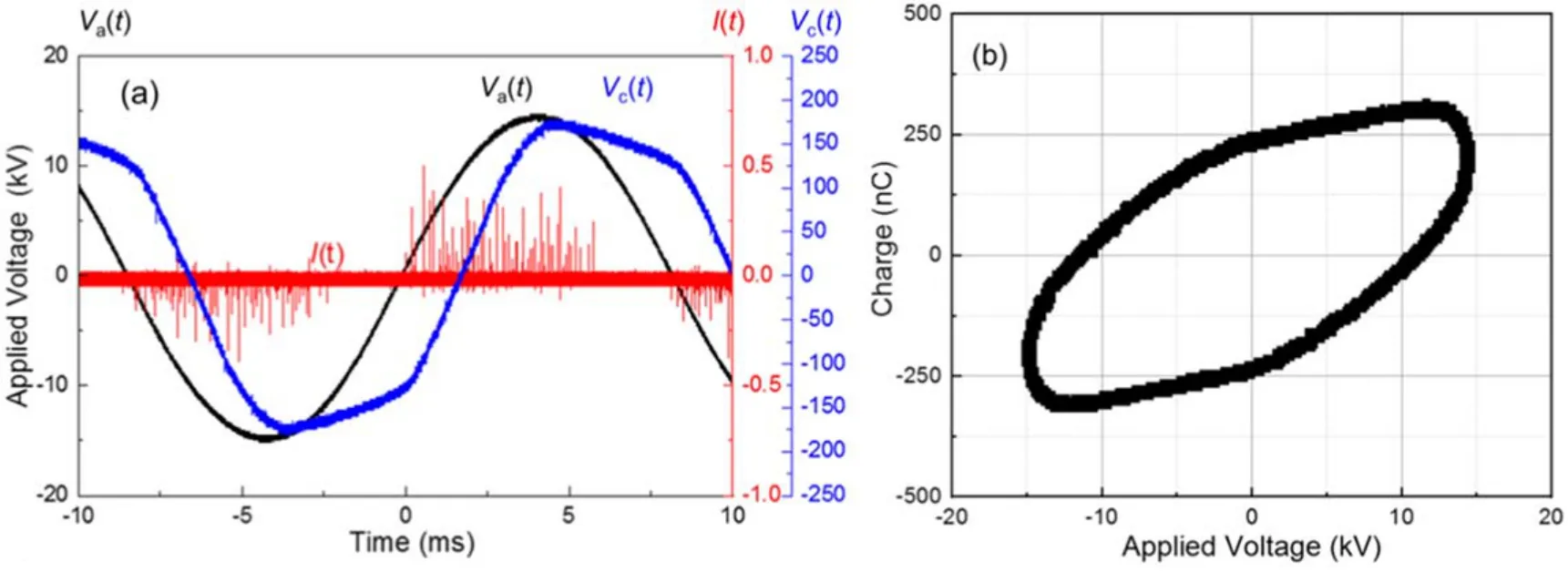
Figure 3.Electrical characteristic of RE-DBDR.(a) Voltage and current waveforms of RE-DBDR; I(t) is discharge current, Va(t) is applied voltage and Vc(t) is capacitance voltage; (b) Lissajous figure at fapp=60 Hz, Vpp=30 kV, rotational speed=5000 rpm.
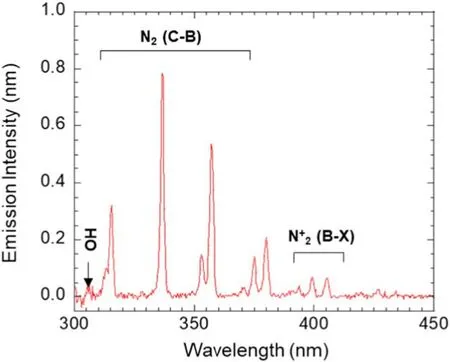
Figure 4.OES of RE-DBDR at fapp=60 Hz, Vpp=30 kV and 5000 rpm rotational speed.
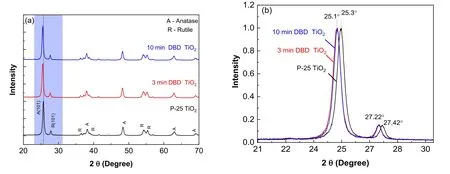
Figure 5.XRD patterns.(a)TiO2 nanoparticles,DBD-treated TiO2(3 min)and DBD-treated TiO2(10 min);(b)XRD pattern at anatase(101)and rutile (101) at fapp=60 Hz, Vpp=30 kV and 5000 rpm rotational speed.
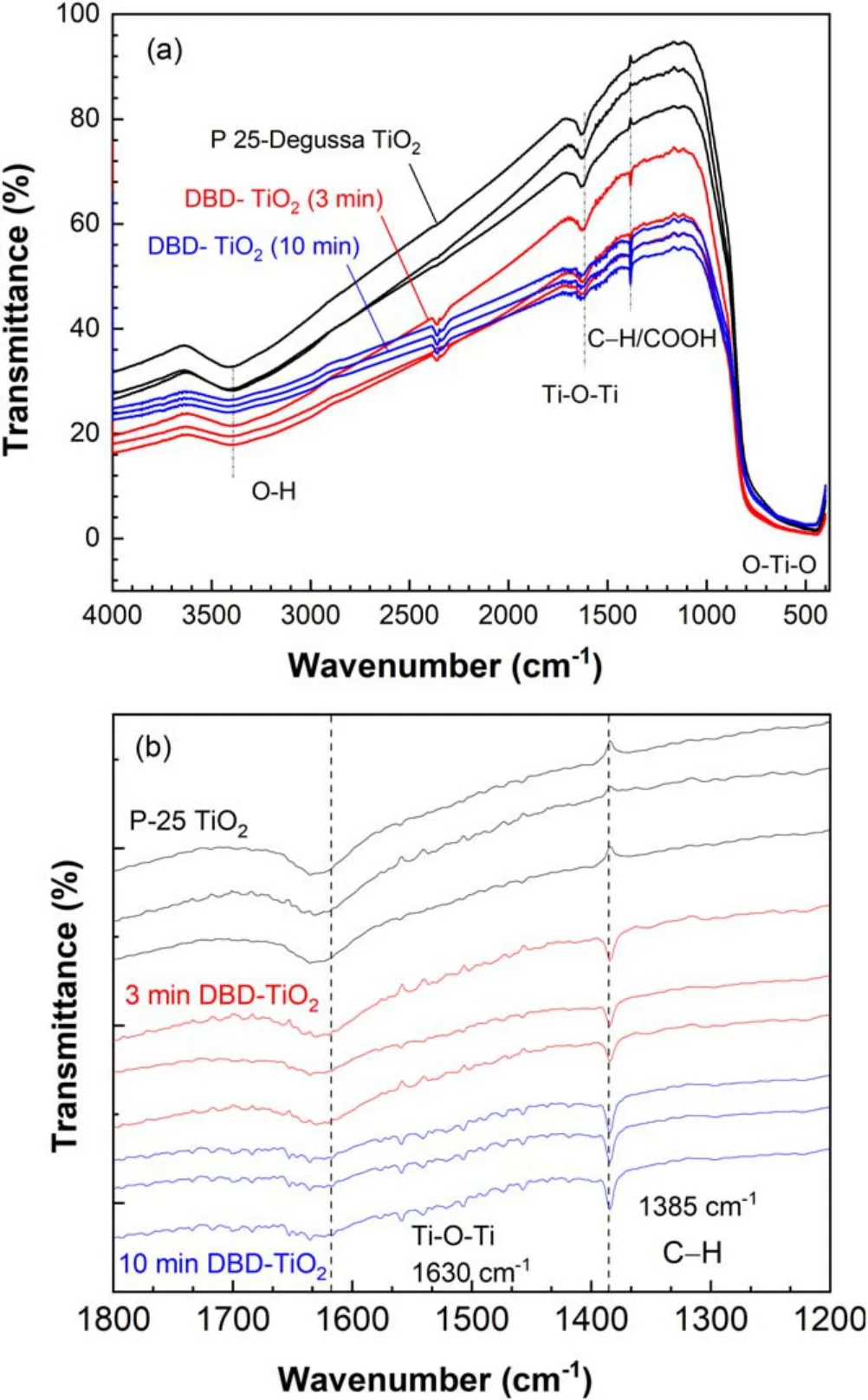
Figure 6.FTIR spectra of pure TiO2 nanoparticles.3 min and 10 min DBD-treated TiO2 in the wavenumber between (a) 400 cm−1 and 3800 cm−1 and (b) 1200 cm−1 and 1800 cm−1 at fapp=60 Hz,Vpp=30 kV and 5000 rpm rotational speed.
4.Conclusion
In conclusion, we processed and modified surface properties of RE-DBDR-treated P-25 TiO2nanopowders.Nonthermal barrier filamentary discharge was generated to treat and modify the TiO2nanoparticle surface in an air atmosphere using RE-DBDR.Using RE-DBDR, we treated the TiO2nanopowder at low temperatures for 3 and 10 min.It would be more interesting to apply RE-DBD in a continuous process, because the present study was conducted in a batch process.More detailed treatment time dependence is necessary as future work.Treatment with air DBD engenders remarkable modification of the TiO2nanopowder surface properties compared with untreated TiO2.The XRD patterns show that the peak shifted to a lower theta degree.Moreover,the FTIR results confirmed the surface functional groups and the porous carbon layer formation on the power surface.OES analyses elucidated the mechanism of the surface layer functional group.The main contributions to the emission spectrum of the air DBD produced by rotatable floating electrodes are the nitrogen molecular band spectra of the second positive system (C–B).Based on the OES results,reactive species, such as OH radical, ozone and atomic oxygen can play key roles in hydroxyl formation on TiO2nanopowder surface.These results constitute important information for improving nanopowder surface modification based on nonthermal DBD treatment for additional optoelectronic, environmental and energy applications.
Acknowledgments
The first author extends her sincere gratitude to her senior,Mr Kentaro Morimoto, for initiating the development of the rotary floating electrode reactor for particulate material treatment and another senior, Dr Mohammad Rasel Pervez,for valuable advice.
杂志排行
Plasma Science and Technology的其它文章
- Special issue on selected papers from CEPC 2020
- Application study on plasma ignition in aeroengine strut–cavity–injector integrated afterburner
- Microanalysis of a ductile iron by microchip laser-induced breakdown spectroscopy
- Study on water treatment effect of dispersion discharge plasma based on flowing water film electrode
- Decontamination of infected plant seeds utilizing atmospheric gliding arc discharge plasma treatment
- The regulation of memory effect and its influence on discharge properties of a dielectric barrier discharge driven by bipolar pulse at atmospheric-pressure nitrogen
