彭县雪胆中葫芦烷三萜类成分及其抗肿瘤活性研究
2021-10-25姚彩虹许旭东龚小妹周小雷罗祖良
姚彩虹 ,许旭东,龚小妹,周小雷*,罗祖良*
1.广西壮族自治区药用植物园,广西 南宁 530023
2.中国医学科学院 北京协和医学院 药用植物研究所,北京 100193
3.新疆大学生命科学与技术学院,新疆 乌鲁木齐 830046
彭县雪胆Hemsleya pengxianensisW.J.Chang为葫芦科(Cucurbitaceae)雪胆属HemsleyaCogn.ex F.B.Forbes & Hemsl.植物,主要分布于云南、贵州、四川、重庆等地[1]。现代研究表明,彭县雪胆主要含有葫芦烷三萜类、木脂素类、有机酸类等成分[2]。目前,以化学成分雪胆素为主的雪胆素胶囊以及“苗药”雪胆肠胃丸已在市场有售,主要用于治疗菌痢、肠炎、支气管炎、急性扁桃体炎[3],有着良好的开发前景。为进一步完善其药效物质基础,发现具有活性的化学成分,对瑶药彭县雪胆醋酸乙酯萃取部位进行了系统的分离,结合多种色谱分离方法,共分离得到17个葫芦烷三萜类化合物,分别鉴定为彭县雪胆萜A(hemsleyacin A,1)、jinfushanencin B(2)、短柄雪胆苷D(delavanoside D,3)、scandenogenin D(4)、jinfushanencin C(5)、jinfushanoside D(6)、hexanorcucurbitacin F(7)、短柄雪胆A(delavanoside A,8)、cucurbitacin V(9)、hemslepenside A(10)、jinfushanencin F(11)、scandenoside R3(12)、scandenoside R2(13)、巨花雪胆G(hemslecin G,14)、jinfushanoside A(15)、cucurbitacine F(16)、16-O-acetyl-cucurbitacin F(17),其中化合物1为新化合物,化合物2~9为首次从该属植物中分离得到。采用MTT法对以上所分离得到化合物进行了体外抗肿瘤细胞活性筛选。结果表明,化合物1、5、10、14、16~17对HeLa细胞具有良好的抑制作用,半数抑制浓度(median inhibitory concentration,IC50)为2.82~7.13 μmol/L。
1 材料与方法
BrukerAvanceⅢ600型核磁共振波谱仪(德国Bruker公司);HW-40C凝胶(Toyopearl公司);赛默飞世尔LTQ-Obitrap XL液质联用仪、LTQ-Obitrap XL型质谱仪(美国Thermo Fisher公司);制备型Aglient高效液相色谱仪(美国Aglient公司);MCI(日本三菱化学公司);柱色谱硅胶、薄层色谱用硅胶GF254(青岛海洋化工有限公司);RE-2000B型旋转蒸发仪(上海亚荣生化仪器厂);常规试剂均为分析纯,娃哈哈水。
药材于2017年采自于广西南宁,经云南省农业科学院高山经济植物研究所易思蓉教授鉴定为葫芦科雪胆属植物彭县雪胆H.pengxianensisW.J.Chang的干燥块根,标本(CS170925)保存于广西药用植物园实验室。
2 提取与分离
雪胆块根10.0 kg粉碎后,以95%乙醇回流提取3次,每次3 h,回收乙醇得浸膏约1.1 kg。以3 L水溶解此浸膏后,依次用石油醚、醋酸乙酯、正丁醇萃取3次,每次1 L,减压回收溶剂,得醋酸乙酯萃取物(350 g)。醋酸乙酯部位浸膏(350 g)经硅胶柱色谱以二氯甲烷-甲醇(100∶0~0∶100)进行梯度洗脱,经薄层色谱示踪合并相同组分,共得到6个部分Fr.1~6。Fr.1经 MCI柱色谱分离,30%~100%甲醇梯度洗脱,得到7个组分(Fr.1.1~1.7)。Fr.1.2经制备型HPLC(乙腈-水28∶72)制备得到化合物1(2.2 mg,tR=13.1 min)、2(1.8 mg,tR=15.1 min)。Fr.1.4经Sephadex LH-20柱色谱(甲醇-二氯甲烷1∶1)及半制备高效液相色谱(乙腈-水35∶65)制备得到化合物3(3.0 mg,tR=15.1 min)、4(2.3 mg,tR=17.8 min)、5(2.6 mg,tR=25.3 min)。Fr.2经Sephadex LH-20柱色谱(甲醇)及半制备高效液相色谱(乙腈-水25∶75)制备得到化合物6(2.1 mg,tR=15.6 min)、7(3.1 mg,tR=18.9 min)、8(3.1 mg,tR=24.6 min)。Fr.3经过反相RP-18柱色谱分离,30%~100%甲醇水梯度洗脱,得到6个组分(Fr.3.1~3.6)。Fr.3.3经制备型 HPLC(乙腈-水38∶62)制备得到化合物9(2.6 mg,tR=15.1 min)、10(1.8 mg,tR=19.1 min)、11(2.6 mg,tR=24.5 min)。Fr.4经硅胶柱(200~300目)色谱,二氯甲烷-甲醇(40∶1~0∶1)梯度洗脱得到6个流分Fr.4.1~4.6。Fr.4.2经制备型HPLC(乙腈-水37∶63)制备得到化合物12(2.6 mg,tR=13.6 min)、13(1.9 mg,tR=8.1 min)、14(2.1 mg,tR=25.5 min)。Fr.4.3经过反相RP-18柱色谱分离,40%~100%甲醇梯度洗脱,得到6个组分(Fr.4.3.1~4.3.6)。Fr.4.3.4经制备型HPLC(乙腈-水38∶62)制备得到化合物15(3.2 mg,tR=17.3 min)、16(1.9 mg,tR=20.5 min)、17(2.6 mg,tR=24.7 min)。
3 结构鉴定
化合物1:白色粉末,易溶于甲醇,[α]20D+19.1°(c0.1,MeOH)。HR-ESI-MS显示准分子离子峰m/z:641.332 0 [M+Na]+(计算值C34H50O10Na, 641.330 2),结合1H-NMR和13C-APT推断该化合物的分子式为C34H50O10,不饱和度为11。UV图谱显示化合物在222 nm处有较强吸收,说明结构中存在共轭体系。IR图谱显示结构中存在羟基(3404、3422、3437 cm-1),甲基(2942、2839 cm-1),羰基(1710 cm-1)。
化合物1的1H-NMR(表1)在高场区显示7个甲基信号δH1.18 (s), 1.24 (s), 1.37 (s), 1.40 (s),1.51(s), 2.13(s), 1.91 (s);1个烯烃的质子信号δH6.46(brs)。13C-APT(表1)谱给出36个碳信号,包括7个甲基信号δC20.3, 21.5, 19.6, 23.4, 25.2, 21.6,22.6,5个亚甲基信号δC35.6, 49.4, 44.3, 33.1, 35.6,7个次甲基信号70.3, 80.6, 124.6, 58.4, 36.7, 74.8,56.0,7个季碳信号δC44.7, 168.3, 48.1, 49.3, 50.4,80.1, 81.6,3个羰基信号δC216.2, 211.0, 199.7,2个酯基信号δC170.9, 170.6。化合物1的核磁数据与已知化合物巨花雪胆G[4]基本相似,不同之处在于化合物1额外存在1个羰基和乙酰基信号。进一步分析13C-APT数据发现,在羰基的影响下,化合物1的C-5 (δC168.3), C-6 (δC124.6), C-7 (δC199.7) 较巨花雪胆G的C-5 (δC145.7), C-6 (δC122.9), C-7 (δC66.4) 向低场位移;C-16乙酰氧基取代后,化合物1的C-16 (δC74.8) 较巨花雪胆G的C-16 (δC71.3)向低场位移。HMBC(图1)谱图中,H-6 (δH6.47)与C-7 (δC199.7) 相关,H-16 (δH6.07) 与C-16位羰基 (δC170.9) 相关,提示羰基和乙酰基分别存在于C-7和C-16位。1H-NMR谱中C-3氢信号表现为δH3.53 (1H, d,J= 2.4 Hz),提示A环中存在2,3位置的顺式二醇的结构。NOESY谱显示,H-3和CH3-24,H-16和CH3-27存在相关,提示2-OH,3-OH及16-OAc均处于α位。因此,化合物1的结构被鉴定为2α,3α,20-三羟基葫芦素-16,25-二乙酰氧基-5-烯-7,11,22-三酮,命名为彭县雪胆萜A。

图1 化合物1的1H-1H COSY、HMBC和NOESY相关Fig.1 Key 1H-1H COSY, HMBC and NOESY correlations of compound 1

表1 化合物1的NMR数据 (600 MHz for 1H-NMR, 150 MHz for 13C-APT, in pyridine-d5)Table 1 NMR data (600 MHz for 1H-NMR, 150 MHz for 13C-APT, in pyridine-d5) of compound 1
化合物2:白色粉末,易溶于甲醇。ESI-MSm/z:495.216 7 [M+Na]+。1H-NMR (600 MHz, pyridine-d5)δ: 0.69 (3H, s, H-18), 1.25 (3H, s, H-19), 0.86 (3H, d,J= 6.6 Hz, H-21), 1.15 (3H, s, H-28), 1.43 (3H, s,H-29), 1.01 (3H, s, H-30), 3.71 (1H, s, H-3), 5.70 (1H,d,J= 4.8 Hz, H-6), 5.91 (1H, t,J= 7.2 Hz, H-24),4.72 (2H, s, H-26), 4.73 (2H, s, H-27);13C-NMR (150 MHz, pyridine-d5)δ: 21.7 (C-1), 30.2 (C-2), 76.0(C-3), 42.3 (C-4), 141.9 (C-5), 119.4 (C-6), 24.6(C-7), 44.4 (C-8), 49.2 (C-9), 36.3 (C-10), 214.3(C-11), 49.5 (C-12), 49.5 (C-13), 50.0 (C-14), 34.9(C-15), 28.4 (C-16), 50.0 (C-17), 17.3 (C-18), 20.6(C-19), 36.4 (C-20), 18.3 (C-21), 36.4 (C-22), 24.9(C-23), 141.3 (C-24), 127.8 (C-25), 65.8 (C-26), 58.9(C-27), 18.5 (C-28), 26.7 (C-29), 28.3 (C-30)。以上数据与文献报道一致[5],故鉴定化合物 2为jinfushanencin B。
化合物3:白色粉末,易溶于甲醇。ESI-MSm/z:641.497 0 [M+Na]+。1H-NMR (600 MHz, pyridine-d5)δ: 0.68 (3H, s, H-18), 1.12 (3H, s, H-19), 0.83 (3H, d,J= 6.6 Hz, H-21), 1.83 (2H, s, H-27), 0.95 (3H, s,H-28), 1.10 (3H, s, H-29), 1.55 (3H, s, H-30), 3.62(1H, s, H-3) , 5.50 (1H, d,J= 6.0 Hz, H-6), 5.72 (1H,t,J= 6.6 Hz, H-24), 4.31 (2H, s, H-26), 4.89 (1H, d,J= 7.8 Hz, H-1′);13C-NMR (150 MHz, pyridine-d5)δ:22.3 (C-1), 28.9 (C-2), 87.6 (C-3), 42.4 (C-4), 141.7(C-5), 118.9 (C-6), 24.5 (C-7), 44.3 (C-8), 49.1 (C-9),36.3 (C-10), 214.1 (C-11), 49.4 (C-12), 49.5 (C-13),50.0 (C-14), 34.9 (C-15), 28.4 (C-16), 50.0 (C-17),17.3 (C-18), 20.7 (C-19), 36.2 (C-20), 18.6 (C-21),37.2 (C-22), 25.0 (C-23), 125.5 (C-24), 136.8 (C-25),68.5 (C-26), 14.4 (C-27), 19.2 (C-28), 28.7 (C-29),26.2 (C-30); 3-Glc: 107.8 (C-1′), 75.9 (C-2′), 79.1(C-3′), 72.2 (C-4′), 78.6 (C-5′), 63.4(C-6′),以上数据与文献报道一致[6],故鉴定化合物3为短柄雪胆苷D。
化合物4:白色粉末,易溶于甲醇。ESI-MSm/z:541.321 8 [M+Na]+。1H-NMR (600 MHz, pyridine-d5)δ: 1.28 (3H, s, H-18), 1.22 (3H, s, H-19), 1.46 (3H, s,H-21), 1.36 (3H, s, H-28), 1.29 (3H, s, H-29), 1.43(3H, s, H-30), 4.11 (1H, m, H-2), 3.42 (1H, d,J= 9.0 Hz, H-3), 5.69 (1H, d,J= 5.4 Hz, H-6), 5.11 (1H, m,H-16), 5.15 (1H, m, H-23), 6.71 (1H, d,J= 8.4 Hz,H-24), 4.73 (2H, s, H-26), 4.72 (2H, s, H-27);13C-NMR (150 MHz, pyridine-d5)δ: 34.4 (C-1), 70.7(C-2), 81.2 (C-3), 42.6 (C-4), 142.2 (C-5), 118.5(C-6), 24.0 (C-7), 42.6 (C-8), 49.0 (C-9), 34.0 (C-10),212.9 (C-11), 48.6 (C-12), 48.5 (C-13), 48.5 (C-14),41.5 (C-15), 70.3 (C-16), 55.8 (C-17), 19.9 (C-18),20.3 (C-19), 72.2 (C-20), 30.0 (C-21), 46.4 (C-22),70.6 (C-23), 128.3 (C-24), 142.1 (C-25), 65.3 (C-26),58.2 (C-27), 21.6 (C-28), 22.1 (C-29), 25.2 (C-30)。以上数据与文献报道一致[7],故鉴定化合物4为scandenogenin D。
化合物5:白色粉末,易溶于甲醇。ESI-MSm/z:615.420 7 [M+Na]+。1H-NMR (600 MHz, pyridine-d5)δ: 1.28 (3H, s, H-18), 1.30 (3H, s, H-29), 1.42 (3H, s,H-28), 1.51 (3H, s, H-26), 1.45 (3H, s, H-27), 1.54(3H, s, H-19), 1.51 (3H, s, H-30), 1.60 (3H, s, H-21),3.38 (3H, s, CH3-7), 3.49 (1H, d,J= 9.0 Hz, H-3),4.15 (1H, m, H-2), 3.67 (1H, d,J= 6.0 Hz, H-7), 4.94(1H, m, H-16), 6.10 (1H, d,J= 5.4 Hz, H-6);13C-NMR (150 MHz, pyridine-d5)δ: 34.8 (C-1), 71.2(C-2), 81.4 (C-3), 43.7 (C-4), 147.4 (C-5), 119.4(C-6), 76.7 (C-7), 48.7 (C-8), 48.9 (C-9), 35.6 (C-10),213.4 (C-11), 49.6 (C-12), 48.1(C-13), 50.8 (C-14),46.9 (C-15), 70.7 (C-16), 59.5 (C-17), 20.7(C-18),21.9 (C-19), 80.4 (C-20), 25.8 (C-21), 215.2 (C-22),32.6 (C-23), 35.7 (C-24), 82.2 (C-25), 26.3 (C-26),26.5 (C-27), 19.7 (C-28), 23.3 (C-29), 26.0 (C-30)170.6 (-OOCCH3), 22.4 (-OOCCH3)。以上数据与文献报道一致[8],故鉴定化合物5为jinfushanencin C。
化合物6:白色粉末,易溶于甲醇。ESI-MSm/z:497.370 1 [M+Na]+。1H-NMR (600 MHz, pyridine-d5)δ: 0.89 (3H, s, H-18), 1.46 (3H, s, H-29), 0.95 (3H, s,H-28), 4.71 (2H, s, H-26), 4.72 (2H, s, H-27), 1.37(3H, s, H-19), 1.22 (3H, s, H-30), 0.94 (3H, d,J= 6.0 Hz, H-21), 3.76 (1H, s, H-3) , 4.20 (1H, m, H-11),5.69 (1H, d,J= 5.4 Hz, H-6), 5.90 (1H, t,J= 6.0 Hz,H-24);13C-NMR (150 MHz, pyridine-d5)δ: 26.2(C-1), 31.3 (C-2), 76.7 (C-3), 42.8 (C-4), 144.8 (C-5),119.6 (C-6), 25.2 (C-7), 44.0 (C-8), 40.6 (C-9), 36.5(C-10), 78.3 (C-11), 41.3 (C-12), 47.8 (C-13), 50.3(C-14), 34.9 (C-15), 28.7 (C-16), 51.2 (C-17), 17.8(C-18), 26.8 (C-19), 37.5 (C-20), 19.9 (C-21), 37.4(C-22), 25.2 (C-23), 128.2 (C-24), 141.5 (C-25), 65.4(C-26), 58.9 (C-27), 19.0 (C-28), 27.3 (C-29), 27.5(C-30)。以上数据与文献报道一致[8],故鉴定化合物6为jinfushanoside D。
化合物7:白色粉末,易溶于甲醇。ESI-MSm/z:427.333 3 [M+Na]+。1H-NMR (600 MHz, pyridine-d5)δ: 1.22 (3H, s, H-18), 1.31 (3H, s, H-29), 0.79 (3H, s,H-28), 1.47 (3H, s, H-19), 1.54 (3H, s, H-30), 2.14(3H, s, H-21), 3.49 (1H, d,J= 6.6 Hz, H-3), 4.09 (1H,m, H-2), 3.43 (1H, d,J= 9.0 Hz, H-16), 5.72 (1H, d,J= 6.0 Hz, H-6);13C-NMR (150 MHz, pyridine-d5)δ:35.1 (C-1), 71.3 (C-2), 81.9 (C-3), 43.3 (C-4), 142.9(C-5), 118.9 (C-6), 24.6 (C-7), 43.8 (C-8), 49.5 (C-9),34.8 (C-10), 212.4 (C-11), 47.8 (C-12), 50.9 (C-13),49.6 (C-14), 46.5 (C-15), 71.7 (C-16), 68.4 (C-17), 19.6(C-18), 20.4 (C-19), 209.0 (C-20), 32.1 (C-21), 20.7(C-28), 22.8 (C-29), 25.9 (C-30)。以上数据与文献报道一致[7],故鉴定化合物7为hexanorcucurbitacin F。
化合物8:白色粉末,易溶于甲醇。ESI-MSm/z:803.458 0 [M+Na]+。1H-NMR (600 MHz,pyridine-d5)δ: 0.71 (3H, s, H-18), 1.24 (3H, s, H-19),0.83 (3H, d,J= 6.6 Hz, H-21), 1.01 (3H, s, H-28),1.14 (3H, s, H-29), 1.42 (3H, s, H-30), 3.71 (1H, brs,H-3), 5.67 (1H, d,J= 6.0 Hz, H-6), 5.40 (1H, t,J=7.2 Hz, H-24), 4.56 (2H, s, H-27); 4.93 (1H, d,J= 7.8 Hz, H-1′), 5.36 (1H, d,J= 7.8 Hz, H-1′′);13C-NMR(150 MHz, pyridine-d5)δ: 21.7 (C-1), 30.2 (C-2), 76.0(C-3), 42.3 (C-4), 141.8 (C-5), 119.4 (C-6), 24.6(C-7), 44.4 (C-8), 49.5 (C-9), 37.2 (C-10), 214.4(C-11), 49.2 (C-12), 49.5 (C-13), 50.0 (C-14), 35.0(C-15), 28.5 (C-16), 50.0 (C-17), 17.4 (C-18), 20.6(C-19), 36.4 (C-20), 18.9 (C-21), 37.2 (C-22), 25.3(C-23), 130.4 (C-24), 132.6 (C-25), 22.3 (C-26), 67.7(C-27), 18.6 (C-28), 20.6 (C-29), 26.7 (C-30);27-Glc(inner): 101.8 (C-1′), 84.2 (C-2′), 78.6 (C-3′),71.8 (C-4′), 78.4 (C-5′), 63.1 (C-6′); 26-Glc(terminal):106.8 (C-1′′), 77.3(C-2′′), 79.0 (C-3′′), 71.9 (C-4′′),78.7 (C-5′), 62.9 (C-6′′)。以上数据与文献报道一致[6],故鉴定化合物8为短柄雪胆苷A。
化合物9:白色粉末,易溶于甲醇。ESI-MSm/z:511.340 9 [M+Na]+。1H-NMR (600 MHz, pyridine-d5)δ: 1.24 (3H, s, H-18), 1.27 (3H, s, H-19), 1.62 (3H, s,H-28), 1.12 (3H, s, H-29), 1.41 (3H, s, H-30), 1.55(3H, s, H-21), 1.51 (3H, s, H-26), 1.51 (3H, s, H-27),3.71 (1H, s, H-3), 5.25 (1H, m, H-16), 5.66 (1H, s,H-6);13C-NMR (150 MHz, pyridine-d5)δ: 21.0 (C-1),29.7 (C-2), 75.3 (C-3), 41.7 (C-4), 141.2 (C-5), 118.8(C-6), 24.1 (C-7), 43.3 (C-8), 49.2 (C-9), 35.7 (C-10),214.6 (C-11), 49.8 (C-12), 50.9 (C-13), 48.5 (C-14),46.7 (C-15), 71.9 (C-16), 59.4 (C-17), 20.3 (C-18),20.1 (C-19), 74.3 (C-20), 27.0 (C-21), 47.9 (C-22),123.0 (C-23), 143.0 (C-24), 69.6 (C-25), 30.6 (C-26),30.5 (C-27), 18.8 (C-28), 27.7 (C-29), 26.1 (C-30)。以上数据与文献报道一致[9],故鉴定化合物9为cucurbitacin V。
化合物10:白色粉末,易溶于甲醇。ESI-MSm/z:639.472 5 [M+Na]+。1H-NMR (600 MHz, pyridine-d5)δ: 0.70 (3H, s, H-18), 1.16 (3H, s, H-19), 0.85 (3H, d,J= 6.0 Hz, H-21), 0.98 (3H, s, H-28), 1.10 (3H, s,H-29), 1.56 (3H, s, H-30), 3.63 (1H, s, H-3), 5.52 (1H,d,J= 6.0 Hz, H-6), 6.46 (1H, t,J= 7.2 Hz, H-24),9.55 (2H, s, H-26), 1.79 (3H, s, H-27), 4.87 (1H, d,J=7.8 Hz, Glc-H-1');13C-NMR (150 MHz, pyridine-d5)δ: 22.6 (C-1), 28.4 (C-2), 87.6 (C-3), 42.4 (C-4), 141.6(C-5), 118.9 (C-6), 24.5 (C-7), 44.3 (C-8), 49.5 (C-9),36.3 (C-10), 214.0 (C-11), 49.1 (C-12), 49.3 (C-13),50.0 (C-14), 34.9 (C-15), 28.9 (C-16), 49.9 (C-17),17.3 (C-18), 20.7 (C-19), 36.4 (C-20), 18.7 (C-21),35.2 (C-22), 24.8 (C-23), 145.6 (C-24), 139.7 (C-25),195.5 (C-26), 9.6 (C-27), 18.6 (C-28), 28.7 (C-29),26.3 (C-30), 107.8 (C-1′), 76.0 (C-2′), 79.2 (C-3′),72.1 (C-4′), 78.7 (C-5′), 63.4 (C-6′)。以上数据与文献报道一致[3],故鉴定化合物10为hemslepenside A。
化合物11:白色无定型粉末,易溶于甲醇。525.325 7 [M+Na]+。1H-NMR (600 MHz, pyridine-d5)δ: 1.26 (3H, s, H-18), 1.22 (3H, s, H-19), 1.42 (3H, s,H-21), 1.97 (3H, s, H-26), 1.36 (3H, s, H-28), 1.28(3H, s, H-29), 1.47 (3H, s, H-30), 4.10 (1H, m, H-2),3.40 (1H, d,J= 9.0 Hz, H-3), 5.71 (1H, d,J= 6.0 Hz,H-6), 5.14 (1H, m, H-16), 5.09 (1H, m, H-23), 6.68(1H, d,J= 6.0 Hz, H-24), 4.52 (1H, s, H-27a), 4.58(1H, s, H-27b);13C-NMR (150 MHz, pyridine-d5)δ:35.2 (C-1), 71.3 (C-2), 81.7 (C-3), 43.2 (C-4), 142.8(C-5), 119.0 (C-6), 24.6 (C-7), 43.3 (C-8), 49.8 (C-9),34.6 (C-10), 213.4 (C-11), 49.2 (C-12), 49.0 (C-13),49.1 (C-14), 42.2 (C-15), 70.8 (C-16), 56.5 (C-17),20.4 (C-18), 21.2 (C-19), 72.7 (C-20), 30.5 (C-21),47.2 (C-22), 71.4 (C-23), 129.4 (C-24), 139.1 (C-25),21.4 (C-26), 61.3 (C-27), 22.3 (C-28), 22.7 (C-29),25.8 (C-30)。以上数据与文献报道一致[8],故鉴定化合物11为jinfushanencin F。
化合物12:白色粉末,易溶于甲醇。ESI-MSm/z:819.926 3 [M+Na]+。1H-NMR (600 MHz, pyridine-d5)δ: 1.21 (3H, s, H-18), 1.23 (3H, s, H-19), 1.68 (3H, s,H-21), 1.89 (3H, s, H-27), 1.29 (3H, s, H-28), 1.31(3H, s, H-29), 1.52 (3H, s, H-30), 3.66 (1H, s, H-3),5.46 (1H, d,J= 6.0 Hz, H-6), 4.90 (2H, d,J= 7.8 Hz H-26), 5.48 (1H, t,J= 7.2 Hz, H-24);13C-NMR (150 MHz, pyridine-d5)δ: 22.3 (C-1), 28.6 (C-2), 87.2(C-3), 42.1 (C-4), 141.6 (C-5), 118.9 (C-6), 25.0(C-7), 43.8 (C-8), 49.0 (C-9), 36.1 (C-10), 214.2(C-11), 49.2 (C-12), 49.8 (C-13), 50.1 (C-14), 34.9(C-15), 22.4 (C-16), 51.1 (C-17), 19.1 (C-18), 20.4(C-19), 74.2 (C-20), 26.1 (C-21), 44.7 (C-22), 23.1(C-23), 130.3 (C-24), 132.3 (C-25), 75.2 (C-26), 14.2(C-27), 19.1 (C-28), 28.1 (C-29), 26.7 (C-30); 3-Glc:107.9 (C-1′), 75.9 (C-2′), 79.1 (C-3′), 72.1 (C-4′), 78.6(C-5′), 63.4 (C-6′); 26-Glc: 103.4 (C-1′′), 75.6 (C-2′′),79.1 (C-3′′), 72.1 (C-4′′), 78.2 (C-5′′), 63.2 (C-6′′),以上数据与文献报道一致[9],故鉴定化合物12为scandenoside R3。
化合物13:白色粉末,易溶于甲醇。ESI-MSm/z:657.401 2 [M+Na]+。1H-NMR (600 MHz, pyridine-d5)δ: 1.07 (3H, s, H-18), 1.26 (3H, s, H-19), 1.53 (3H, s,H-21), 1.85 (1H, s, H-26), 1.13 (3H, s, H-28), 1.34(3H, s, H-29), 1.46 (3H, s, H-30), 4.23 (2H, s, H-27),3.62 (1H, s, H-3) , 5.47 (1H, d,J= 5.4 Hz, H-6), 5.85(1H, t,J= 7.2 Hz, H-24);13C-NMR (150 MHz,pyridine-d5)δ: 22.5 (C-1), 29.0 (C-2), 87.7 (C-3), 42.2(C-4), 141.7 (C-5), 118.9 (C-6), 24.6 (C-7), 42.5(C-8), 49.2 (C-9), 36.1 (C-10), 213.6 (C-11), 49.2(C-12), 49.3 (C-13), 50.0 (C-14), 34.6 (C-15), 22.5(C-16), 51.8 (C-17), 18.2 (C-18), 20.5 (C-19), 72.9(C-20), 26.3 (C-21), 45.3 (C-22), 23.6 (C-23), 128.1(C-24), 136.5 (C-25), 21.0 (C-26), 61.3 (C-27), 18.4(C-28), 28.6 (C-29), 26.3 (C-30), 3-Glc: 107.9 (C-1′),75.9 (C-2′), 79.2 (C-3′), 72.9 (C-4′), 78.7 (C-5′), 63.4(C-6′),以上数据与文献报道一致[10],故鉴定化合物13为scandenoside R2。
化合物14:白色粉末,易溶于甲醇。ESI-MSm/z:601.416 7 [M+Na]+。1H-NMR (600 MHz, pyridine-d5)δ: 1.28 (3H, s, H-18), 1.52 (3H, s, H-19), 1.76 (3H, s,H-21), 1.50 (3H, s, H-26), 1.51 (3H, s, H-27), 1.48(3H, s, H-28), 1.34 (3H, s, H-29), 1.61 (3H, s, H-30),1.90 (3H, s, COCH3-25), 3.50 (1H, d,J= 9.0 Hz,H-3), 4.17 (1H, m, H-2), 4.51 (1H, s, H-7), 4.92 (1H,t,J= 7.2 Hz, H-16), 6.25 (1H, d,J= 5.4 Hz, H-6);13C-NMR (150 MHz, pyridine-d5)δ: 34.4 (C-1), 70.7(C-2), 81.0 (C-3), 42.8 (C-4), 145.0 (C-5), 122.2(C-6), 65.9 (C-7), 52.8 (C-8), 50.1 (C-9), 35.1 (C-10),213.3 (C-11), 49.2 (C-12), 47.7 (C-13), 50.1 (C-14),46.3 (C-15), 70.1 (C-16), 58.7 (C-17), 22.7 (C-18),21.8 (C-19), 79.9 (C-20), 25.1 (C-21), 214.9 (C-22),32.0 (C-23), 35.2 (C-24), 81.4 (C-25), 25.7 (C-26),25.8 (C-27), 19.3 (C-28), 20.1 (C-29), 25.3 (C-30)170.0 (-OOCCH3), 22.0 (-OOCCH3)。以上数据与文献报道一致[4],故鉴定化合物14为巨花雪胆G。
化合物15:白色粉末,易溶于甲醇。ESI-MSm/z:659.416 7 [M+Na]+。1H-NMR (600 MHz,pyridine-d5)δ: 0.85 (3H, s, H-18), 1.30 (3H, s, H-19),0.91 (3H, d,J= 6.0 Hz, H-21), 0.87 (3H, s, H-28),1.15 (3H, s, H-29), 1.56 (3H, s, H-30), 4.72 (2H, d,J=7.8 Hz, H-26), 4.72 (2H, d,J= 7.8 Hz, H-27), 3.66(1H, s, H-3) , 4.19 (1H, m, H-11), 5.49 (1H, d,J= 6.0 Hz, H-6), 5.90 (1H, t,J= 7.2 Hz, H-24);13C-NMR(150 MHz, pyridine-d5)δ: 27.2 (C-1), 30.0 (C-2), 88.4(C-3), 42.8 (C-4), 144.7 (C-5), 118.9 (C-6), 25.0(C-7), 43.9 (C-8), 40.5 (C-9), 36.6 (C-10), 78.6(C-11), 41.5 (C-12), 47.8 (C-13), 50.2 (C-14), 34.9(C-15), 28.7 (C-16), 51.0 (C-17), 17.4 (C-18), 26.7(C-19), 37.3 (C-20), 19.7 (C-21), 37.4 (C-22), 25.0(C-23), 128.1 (C-24), 141.2 (C-25), 65.8 (C-26), 58.9(C-27), 19.1 (C-28), 28.1 (C-29), 26.8 (C-30), 107.9(C-1′), 75.9 (C-2′), 79.1 (C-3′), 72.2 (C-4′), 78.2(C-5′), 63.4 (C-6′)。以上数据与文献报道一致[11],故鉴定化合物15为Jinfushanoside A。
化合物16:白色无定形粉末,易溶于甲醇。ESI-MSm/z: 541.500 0 [M+Na]+。1H-NMR (600 MHz, pyridine-d5)δ: 1.20 (3H, s, H-18), 1.45 (3H, s,H-29), 1.23 (3H, s, H-28), 1.60 (3H, s H-26), 1.50(3H, s, H-27), 1.29 (3H, s, H-19), 1.43 (3H, s, H-30),1.47 (3H, s, H-21), 3.43 (1H, d,J= 9.0 Hz, H-3), 4.16(1H, m, H-2), 4.92 (1H, m, H-16), 5.72 (1H, d,J= 5.4 Hz, H-6), 6.50 (1H, d,J= 15.6 Hz, H-23), 7.54 (1H, d,J= 15.6 Hz, H-24);13C-NMR (150 MHz, pyridine-d5)δ: 34.8 (C-1), 71.4 (C-2), 81.9 (C-3), 43.2 (C-4), 142.9(C-5), 119.1 (C-6), 24.6 (C-7), 43.6 (C-8), 49.6 (C-9),35.1 (C-10), 213.7 (C-11), 49.2 (C-12), 49.2 (C-13),51.6 (C-14), 43.2 (C-15), 70.9 (C-16), 59.6 (C-17),20.8 (C-18), 21.0 (C-19), 79.7 (C-20), 26.0 (C-21),204.7 (C-22), 121.2 (C-23), 156.0 (C-24), 70.7 (C-25),30.4 (C-26), 30.2 (C-27), 19.5 (C-28), 22.9 (C-29),25.9 (C-30)。以上数据与文献报道一致[12],故鉴定化合物16为cucurbitacine F。
化合物17:白色粉末,易溶于甲醇。ESI-MSm/z:583.416 7 [M+Na]+。1H-NMR (600 MHz, pyridine-d5)δ: 1.17 (3H, s, H-18), 1.32 (3H, s, H-29), 1.24 (3H, s,H-28), 1.53 (3H, s H-26), 1.52 (3H, s, H-27), 1.20(3H, s, H-19), 1.49 (3H, s, H-30), 1.50 (3H, s, H-21),3.43 (1H, d,J= 9.0 Hz, H-3), 4.14 (1H, m, H-2), 5.85(1H, t,J= 7.8 Hz, H-16), 5.73 (1H, d,J= 5.4 Hz,H-6), 7.46 (1H, d,J= 15.0 Hz, H-23), 7.54 (1H, d,J=15.0 Hz, H-24);13C-NMR (150 MHz, pyridine-d5)δ:35.1 (C-1), 71.4 (C-2), 81.8 (C-3), 44.0 (C-4), 142.9(C-5), 119.0 (C-6), 24.4 (C-7), 43.0 (C-8), 48.7 (C-9),34.7 (C-10), 213.0 (C-11), 49.1 (C-12), 49.4 (C-13),51.0 (C-14), 43.3 (C-15), 74.9 (C-16), 55.7 (C-17),20.8 (C-18), 20.6 (C-19), 79.3 (C-20), 25.0 (C-21),204.3 (C-22), 120.2 (C-23), 157.2 (C-24), 70.7 (C-25),30.3 (C-26), 30.3 (C-27), 23.0 (C-28), 26.0 (C-29),19.3 (C-30), 170.7 (-OOCCH3), 21.6 (-OOCCH3)。以上数据与文献报道一致[13],故鉴定化合物17为16-O-acetyl-cucurbitacin F。
4 抗肿瘤活性测定
将人宫颈癌HeLa细胞接种于96孔培养板中,每孔100 μL,37 ℃培养箱静置培养24 h。给药组加入100 μL不同质量浓度的样品,样品终质量浓度分别为100.0、50.0、10.0、5.0、1.0、0.5、0.1 μg/mL,每个质量浓度平行3个复孔。空白对照组给予含DMSO的完全培养基继续培养,多柔比星(IC50=0.24 μmol/L)为阳性对照组。培养24 h后,每孔加入5 mg/mL的MTT溶液20 μL,4 h后终止培养。将孔内培养液小心吸出,每孔加入150 μL DMSO,置摇床上低速振荡10 min,使结晶物充分溶解。用酶标仪在570 nm处测定各孔吸光度(A)值。
实验结果显示,化合物1、5、10、14、16~17对HeLa细胞有良好的细胞毒活性,IC50分别为5.07、7.13、3.53、3.79、6.49、2.82 μmol/L(表2)。
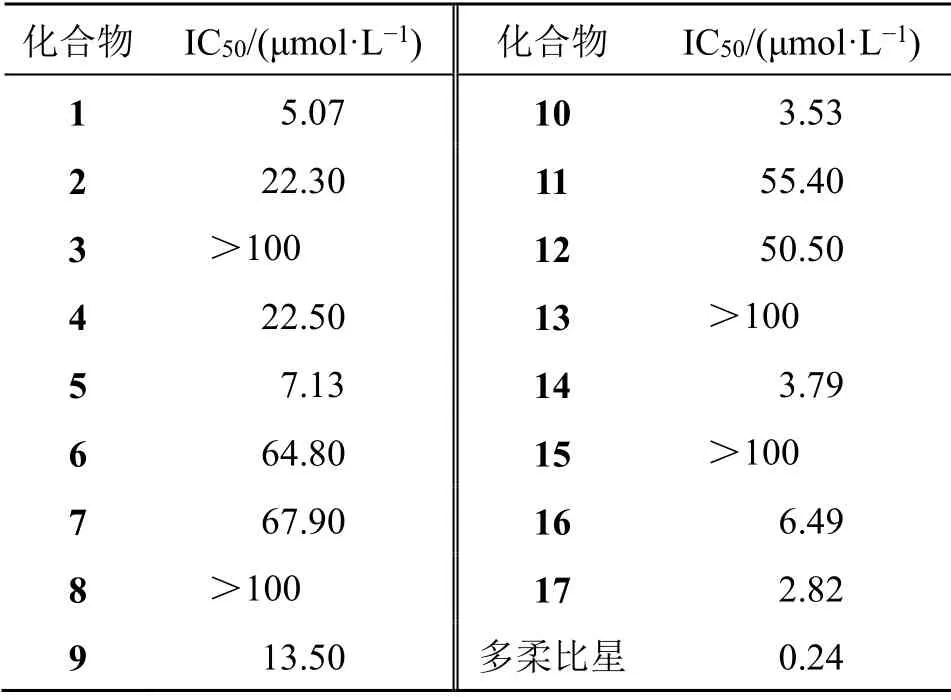
表2 化合物1~17对HeLa细胞的抗肿瘤活性Table 2 Antitumor activity of compounds 1—17 on HeLa cell lines
5 构效关系分析
雪胆单体化合物抗HeLa细胞增殖试验结果表明部分化合物具有抑制HeLa细胞增殖活性。通过对比试验数据,化合物的抗肿瘤活性与结构存在一定的构效关系。
葫芦烷三萜皂苷元的活性要强于单糖苷、双糖苷[14],如化合物1、14的IC50值小于化合物3、12等含糖单元化合物。当化合物C-16位与C-23位通过醚键成环时,细胞毒活性降低或消失。如化合物4、11,IC50值分别为22.5、55.4 μmol/L,化合物3、8的IC50值均大于100 μmol/L。当化合物C-11位取代基由羰基变为羟基时,化合物的细胞毒活性降低或消失[15],如化合物8、15的IC50值均大于100 μmol/L,化合物2、6的IC50值在20~100 μmol/L。当化合物C-2、C-3位有羟基时,细胞毒活性增强,如化合物6的活性强于15。当化合物C-26位存在醛基时,化合物的细胞毒性增强[16],如化合物10的IC50值为3.53 μmol/L。
利益冲突所有作者均声明不存在利益冲突
