Egg tanning improves the efficiency of CRlSPR/Cas9-mediated mutant locust production by enhancing defense ability after microinjection
2021-10-22ZHANGTingtingWENTingmeiYUEYangYANQiangDUErxiaFANSanhongSiegfriedROTHLlShengZHANGJianzhenZHANGXueyaoZHANGMin
ZHANG Ting-ting,WEN Ting-mei,YUE Yang,YAN Qiang,DU Er-xia,FAN San-hongSiegfried ROTH,Ll Sheng,ZHANG Jian-zhenZHANG Xue-yaoZHANG Min
1 Research Institute of Applied Biology,Shanxi University,Taiyuan 030006,P.R.China
2 School of Life Science,Shanxi University,Taiyuan 030006,P.R.China
3 State Key Laboratory of Cotton Biology/Key Laboratory of Plant Stress Biology,School of Life Sciences,Henan University,Kaifeng 475004,P.R.China
4 Guangdong Provincial Key Laboratory of Insect Developmental Biology and Applied Technology,Institute of Insect Science and Technology,School of Life Sciences,South China Normal University,Guangzhou 510631,P.R.China
5 Institute for Developmental Biology,University of Cologne,Cologne 50674,Germany
Abstract The mutant efficiency and hatching ratio are two key factors that significantly affect the construction of genome-modified mutant insects. In the construction of CRISPR/Cas9-mediated dsLmRNase2-/- mutant locusts,we found that the tanned eggs which experienced a 20-min contact with the oocyst exhibited a higher success rate compared to fresh newlylaid eggs that were less tanned. However,the heritable efficiency of the dsLmRNase2 deletion to the next generation G1 progeny was similar between adults derived from the tanned or less tanned engineered eggs. Further,the similar effective mutant ratios in the normally developed eggs and G0 adults of tanned and less tanned eggs also indicated that tanning did not reduce the absolute mutation efficiency induced by CRISPR/Cas9. Moreover,we found that the syncytial division period,which was longer than the time for tanning,conferred a window period for microinjection treatment with efficient mutation in both tanned and less tanned eggs. We further found that tanned eggs exhibited a higher hatching rate due to a reduced infection rate following microinjection. Both the anti-pressure and ultrastructure analyses indicated that the tanned eggs contained compressed eggshells to withstand increased external pressure. In summary,tanned eggs possess stronger defense responses and higher efficiency of genome editing,providing an improved model for developing Cas9-mediated gene editing procedures in locusts.
Keywords:brown tanned eggs,CRISPR/Cas9,mutant locusts,microinjection,defense ability
1.lntroduction
Genome editing has been widely used in the functional analysis of genes and identified as a powerful technology for gene therapy in human diseases,as well as pest control applications in the agricultural and forestry fields (Barrangouet al.2007;Jineket al.2012;Conget al.2013). Efficiently generating mutant individuals (including insects) is the critical step for the application of all the various genome editing technologies (Liet al.2016;Khalil 2020).
Successful construction of mutant animals depends on high efficiency of genome editing and hatching. The CRISPR/Cas9 system can specifically target the locus of interest based on the interactions of single guide RNA (sgRNA) and the Cas9 protein (Jianget al.2013),and has been widely used in life science research (Liuet al.2015). Further,a simple efficient system containing Cas9 protein and sgRNAs transcribedin vitroto produce functional CRISPR ribonucleoproteins (RNPs) can also be used to transfer the CRISPR/Cas9 system into fertilized eggs or the gonads (ovaries or testes) of parent animals to generate mutant individuals (Sanoet al.2018). Microinjection is the most widely applied method in this type of study. As for insects,this is especially true as many genetically engineered lines have been generated in this way,includingDrosophila melanogaster(Gratzet al.2014),Aedes aegypti(Kistleret al.2015),Bombyx mori(Maet al.2014a),Papilio xuthus(Liet al.2015),Gryllus bimaculatus(Awataet al.2015),Plutella xylostella(Huanget al.2016) andLocusta migratoria(Liet al.2016). Given this,the selection of fertilized eggs suitable for microinjection of RNPs is the fundamental process to guarantee the mutant efficiency and the hatching rate,finally ensuring the construction rate in these applications.
Insect eggs are often tanned and sclerotized after oviposition through a series of multistage chemical reactions that take place on the surface of eggshells (Eisneret al.1966;Andersen 2012),providing the chance to improve the construction efficiency of producing mutant insects.Locusta migratoriais not only an important agricultural pest (Enserink 2004) but also acts as an ideal research model for various fields of insect biology (Leoet al.2005),including aggregation (Guo X Jet al.2020b),phase transition (Houet al.2019;Guo Wet al.2020a),cuticle metabolism (Yuet al.2016),gut development (Zhanget al.2020) and pest control (Zhanget al.2021). Effective generation of gene-edited locusts could be achieved by microinjection of CRISPR/Cas9 into the fresh eggs (Liet al.2016). Newly oviposited“yellow”locust eggs are surrounded by a liquid substance,which quickly forms a loose rod-shaped oothecal cover and darkens the eggs,finally resulting in their distinctive brown color as the tanned eggs. Although the fresh eggs of locusts are easy to isolate due to this liquid ootheca,it is not easy to obtain the CRISPR/Cas9-mediated genome edited locusts with a very high efficiency (Liet al.2016).
In this study,we first demonstrated that the brown locust eggs tanned in the ootheca had a higher success rate in the construction of CRISPR/Cas9-mediatedLmdsRNase2knock-out mutant locusts than the less tanned yellow eggs. We then confirmed that mutations of G0individuals could inherit to the G1generation and genome editing efficiencies were similar between the tanned and less tanned eggs because the syncytial division happened three hours after tanning. Finally,we proved that the tanned eggshells improved the construction efficiency of mutant locusts by increasing the hatching rate after microinjection. Our analysis revealed that higher efficiency of generating mutant insects may be achieved by using the tanned eggs for microinjection,which provides a higher hatching rate benefiting from the stronger eggshell ultrastructure to defend against infection.
2.Materials and methods
2.1.lnsect culture
The locusts used in this experiment were cultured in cages of 25 cm×25 cm×25 cm at (28±2)°C with 14 h of light and 40% relative humidity in the Research Institute of Applied Biology of Shanxi University,Taiyuan,China. The locusts were fed with 7-15 cm of fresh wheat seedlings which were cultured at 24°C.
2.2.Design and synthesis of sgRNA for LmdsRNase2 in the CRlSPR/Cas9 system
LmdsRNase2is the main enzyme for dsRNA degradation in the locust gut,but it does not affect the survival rate from nymph to nymph stage in locusts after dsRNA microinjection (Songet al.2017),which makes it an excellent candidate gene for optimizing the Cas9-based genome editing procedure in locusts. The genomic DNA sequence ofLmdsRNase2was blasted at the website (http://159.226.67.243/viroblast/viroblast.php) using its cDNA sequence as the query data (Appendix A). The website (http://crispr.mit.edu/) was used to screen sgRNA targets online in the first exon ofLmdsRNase2(including all ORF regions). The sgRNAs for two target sites,namely sgRNA685 and sgRNA726,were selected based on the principle of high scores,fewer off-target sites,and high GC in the six bases upstream of the PAM. The sgRNA685 and sgRNA726 were synthesized using the GeneArt™ gRNA Synthesis Kit (A29377,Invitrogen,USA)in vitro. Briefly,the DNA template of sgRNA was amplified through PCR using mixed primers of Tracr Fragment,T7 primer and the specific target primers (Appendix B). The DNA template was transcribed into the corresponding sgRNA using the TranscriptAid™ Enzyme Mix and finally purified with the gRNA Clean Up Kit according to the manufacturer’s instructions. The synthesized sgRNA685 and sgRNA726 were stored at -20°C for later use. For one sgRNA microinjection,300 ng sgRNA726 and 300 ng Cas9 protein from Invitrogen TrueCut Cas9 Protein v2 (A36498,Invitrogen,USA) were mixed into 10 μL to form the RNP mixture in RNase-free water. For double sgRNAs microinjection,300 ng sgRNA685,300 ng sgRNA726 and 300 ng Cas9 protein were mixed into 10 μL to form the RNP mixture.
2.3.Observation of tanned and less tanned eggs
The female locust lays a rod-shaped egg sac consisting of about 40-80 fertilized eggs in sandy soils. To observe the process of egg tanning,we collected the egg sacs in the sand basins that were previously placed in the cages as soon as the ootheca was extruded into the basin. The eggs isolated from the oocysts were separated into two groups within 5-10 min after oviposition. One group was washed with water to remove the oocysts,and in the other group the eggs were still kept in touch with the oocysts.Eggs of the first group remained yellow and were named as the“less tanned eggs”,while 20 min after oviposition,the eggs of the second group turned brown and were named as the“tanned eggs”. Both less tanned yellow and tanned brown eggs were rinsed in distilled water three times to ensure that there was no residual sand on the surface,and then imaged with camera TruceChrome II on an OPTEC microscope.
2.4.Microinjection
The cleaned eggs (tanned or less tanned) were placed on a glass plate with a baffle to keep the surface moist (Appendix A). The microinjector (FemtoJet 4X,Eppendorf,Germany) was used to inject the RNP through a glass needle into the eggs near the micropyle with the parameters of 300 pounds per square inch (psi) of injection pressure,30-40 ms of injection time,and 20 psi of anti-pressure. The capillary glass needles (G-100,Narishige,Japan) with a diameter of 1 mm were prepared using a needle puller (MODEL P-97,Sutter,USA) with the parameters of Heat-600,Pull-55,Velocity-80,and Time-10. The eggs were lined up on the glass plate and moved into the capillary needle in sequence as shown in Appendix A. The injected eggs were placed in disposable Petri dishes covered with moist filter paper and cultured in an incubator at 30°C for 14 days. The total time for microinjection was completed within 2 h after the oviposition.
2.5.Screening and verification of the mutant locusts
To detect the hatching rate after RNP microinjection,40 tanned or 40 less tanned eggs,which were injected with either single or double sgRNA,were cultured in the disposable Petri dishes covered with moist filter paper. The hatching rate of each group was calculated as the number of hatched eggs compared to the number of injected eggs. In order to screen mutant locusts,a 382-bp fragment containing the target gene sequence was amplified by nested PCR (Appendix A) using an alkali-treated antenna sample as the DNA template and sequenced. The first 25 μL nested PCR reaction contained 23 μL of Gold Mix (TSE101,TSINGKE,China),1 μL of the alkali-treated antenna template and 1 μL of 0.4 μmol L-1long forward and reverse primer (Appendix C). The second 50 μL nested PCR reaction contained 47 μL of Gold Mix,1 μL of template of the PCR products from the first nested PCR and 2 μL of 0.4 μmol L-1short forward and reverse primer (Appendix C). Both amplifications were carried out at 95°C for 5 min,followed by 28 cycles of 95°C for 30 s,55°C for 30 s and 72°C for 40 s,and then followed with another 72°C for 10 min. The mutant sequence was identified by comparing the sequencing results (sequences and peak patterns) with the wild type sequence. The number of mutant individuals from the above group was used to calculate the efficiency of mutant individuals generated from all injected eggs.
To further compare the genome editing efficiencies of normally developed eggs and individuals from tanned and less tanned eggs,five health developed eggs and four individuals in each group were selected to detect the mutation by PCR and sequencing. To further evaluate the genetic outcomes of using tanned eggs,we assessed the inheritance patterns of engineered locusts in the G1generation. The obtained G0mutant individuals (chimeras) were crossed with the wild type to obtain the G1generation. Groups of 5-6 eggs in a single oocyst of the G1generation were randomly selected for lysis and amplification to estimate the mutation of whole oocyst and ensure whether it would be suitable to culture further. The inheritance efficiency of mutant target in the G1progeny was calculated by the number of mutations in every three oocysts in the G1progeny.
2.6.Fixation and nucleus staining of eggs
In order to confirm the time of syncytial division in the fertilized locust egg,we fixed the eggs at different developmental stages as described in Zhanget al.(2018).We first selected the developing fertilized eggs at 0,2,4,8,24 and 36 h,perforated the two poles of the egg with pins more than three times,placed them in a 2-mL Eppendorf tube,and then added 4% paraformaldehyde to fix them for more than 24 h. The fixed eggs were washed with PBS and then stained with SYTOX®Green to label the nucleus. Stained eggs were imaged with a fluorescence microscope (X51,Olympus,Japan).
2.7.Comparison of the eggshell ultrastructure by transmission electron microscopy
To compare the ultrastructure of the tanned and less tanned eggshells,a transmission electron microscopy (TEM) technique was used as previously described (Zhanget al.2018). Briefly,5 μm paraffin sections of the microinjected eggs at 5-days-old (E5) were prepared and fixed with 3% glutaraldehyde in 0.2 mol L-1PBS (pH 7.2) solution at 4°C for 48 h. The slides were washed three times with PBS,and fixed with 1% tetroxide at 4°C for 3 h. The fixed samples were washed twice for 10 min each,and then dehydrated with an increasing concentration gradient of acetone (50,70,80,90 and 100%). The tissues were embedded in Epon 812 for 2 h at room temperature. The samples were trimmed,after which ultrathin sections were prepared and fixed on a copper mesh. Then,the images of the ultrathin sections of embryo eggshells were observed with a JEM-1200EX transmission electron microscope (TEM,JEOL,Japan). The thicknesses of the eggshell and its inside granule gray scales were circled and measured in Photoshop 6.0 Software.
2.8.Measurement of the eggshell hardness with a texture analyzer
To acquire more information about the characteristics of the eggshells,the texture analyzer was used to measure the difference of hardness between tanned and less tanned eggshells. The tanned and less tanned eggs after day 5 of development (E5) were cleaned with PBS to remove impurities and keep moist. The cleaned eggs were placed on the stage and the hardness was tested in the texture analyzer (Universal TA,Shanghai Tengba,China). The operating parameters are as follows:experimental test speed=20 mm min-1,starting force=0.100 N and puncture distance=1.0 mm. Each test group included 13 measured eggs.
2.9.Statistical analysis
The experimental data were analyzed using the oneway analysis of variance between two groups. The data between two groups were statistically analyzed by Student’st-test for independent sample. Asterisks indicate significance (*,P<0.05;**,P<0.01). All the experiments were repeated at least three times.
3.Results
3.1.lnjection of tanned eggs increased the construction efficiency of CRlSPR/Cas9-mediated LmdsRNase2 knock-out locusts
The freshly laid locust eggs had two fates:One in which the oocytes will turn brown because of the tanning effect of the oocysts within a short period of about 20 min to become the tanned eggs,and the other in which eggs isolated from newly laid oocysts immediately will become the less tanned eggs due to their insufficient contact with the oocysts (Fig.1-A). The outcomes of genome editing on the tanned and less tanned eggs were used to evaluate the efficiency of producing knockout locusts. Analysis of the genome structure and amino acid sequence of target geneLmdsRNase2showed that the nuclease domain is in exon1 (Appendix A). Thus,we selected targets at positions 726 and 685 within theLmdsRNase2and designed sgRNAs to act as efficient indicators for genome editing mediated by CRISPR/Cas9 RNPs (Appendix A). RNPs with single or double sgRNA formats were injected into tanned or less tanned eggs within 2 h after oviposition. The results show that the hatching rate of tanned eggs was 30% more than the less tanned eggs in both single and double sgRNA injection groups (Fig.1-B). Further,the efficiency of producing mutant individuals in all injected tanned eggs was about 15% more than that in less tanned eggs in the single sgRNA injection group,while this difference reached up to 21% in the double sgRNA injection group (Fig.1-C). The sequence data of individuals from single sgRNA injection of both tanned and less tanned eggs exhibited multiple peaks at 3 bp before the protospacer-adjacent motif (PAM) and a similar mutation was also detected in the double sgRNA injection group (Appendix D). This standard mutation type is common to non-homologous ending joining,and indicates that the CRISPR/Cas9 RNPs had been able to efficiently cleave their target by microinjection within 2 h after oviposition in both tanned and less tanned eggs. Similar patterns of improved efficiency of mutant individuals in tanned eggs were also observed forLmDicer1andLmDicer2amutant locusts (Appendix E).
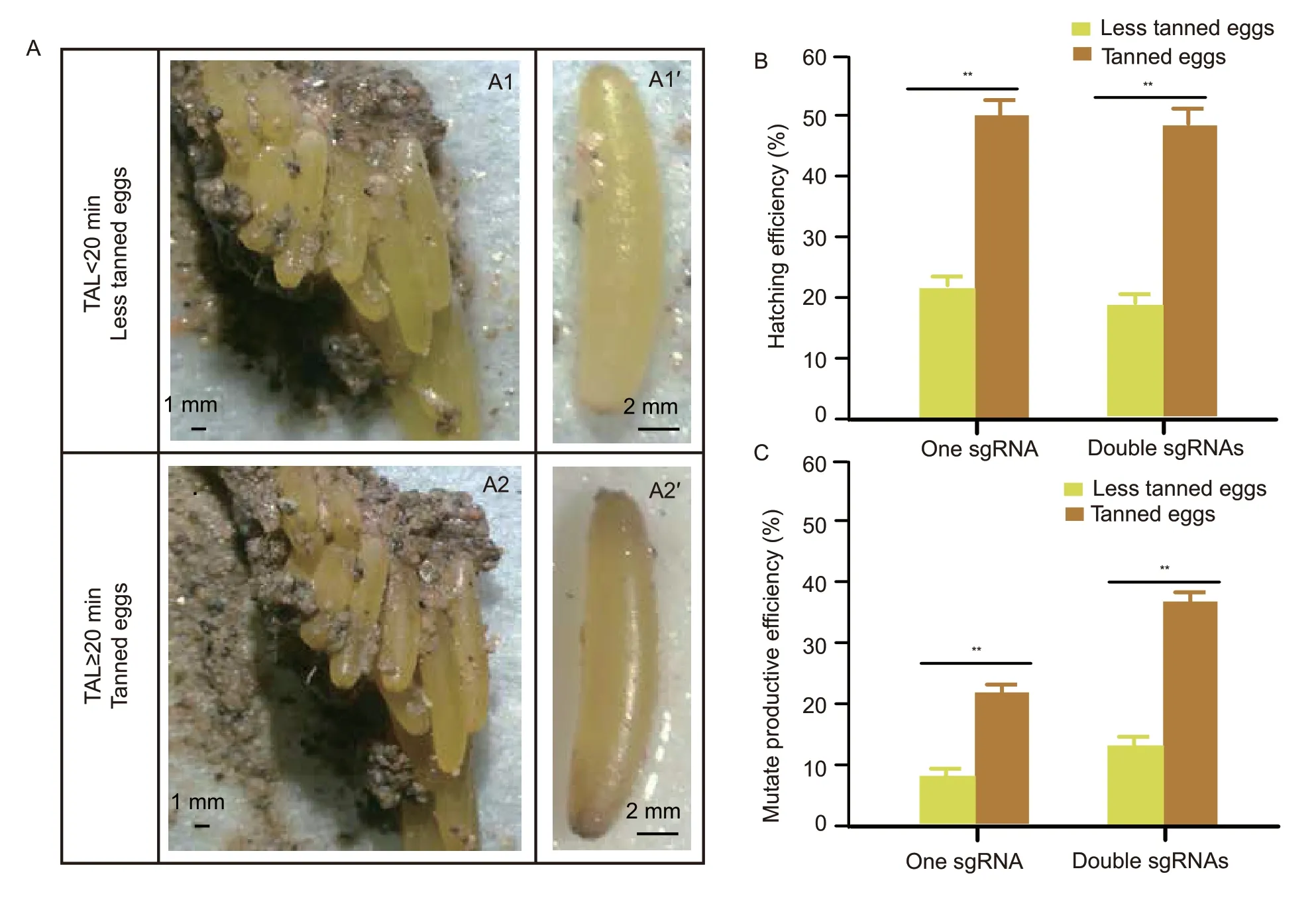
Fig.1 Egg tanning increased the efficiency of producing mutant individuals in the eggs injected with CRISPR/Cas9. A,egg tanning of locust. The freshly laid eggs are compressed with oocysts (A1) and 20 min later they will turn brown due to the tanning by the oocysts (A2 and A2´). The fresh eggs removed from the egg pod within 20 min after lying are still yellow (A1´) because of incomplete tanning. B,comparison of the hatching efficiency between tanned and less tanned eggs. C,comparison of construction efficiency between tanned and less tanned eggs. The tanned and non-tanned eggs used were injected with CRISPR/Cas9 system consisting of single or double sgRNAs. Each group contained 40 eggs and experiments were repeated three times.
3.2.G1 insects could inherit the mutation from individuals from both tanned and less tanned eggs
In the inherited mutation detection assay,the less tanned and tanned eggs injected with RNPs including single sgRNA were all shown to maintain about 11-22% mutation inheritance rates in G1progeny without a significant difference (Fig.2-A). For groups injected by RNPs of double sgRNA,they had about 33-44% mutation rates,higher than the single sgRNA groups,but also without a significant difference between tanned and less tanned eggs (Fig.2-C). In addition,the sequencing results of the G1insects,for all groups,produced an identical set of patterns similar to the G0samples,indicating that allLmdsRNase2mutations were inherited (Appendix D). Finally,TA cloning and sequencing of the target region demonstrated that both tanned and less tanned eggs produced similar random indels in the G1generation in both the single and double sgRNA groups (Fig.2-B and D). These results suggested that the mutations at site 726 inLmdsRNase2produced with CRISPR/Cas9 can be inherited by the next generation without experiencing any unwanted changes (Appendix D).
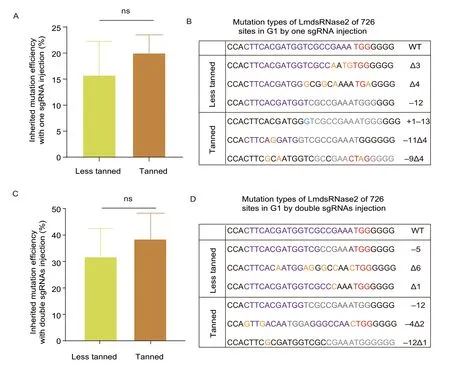
Fig.2 G1 progeny inherited mutations from the G0 locusts which were hatched from the injected eggs. A,comparison of inherited mutation efficiency of single sgRNA. The inherited mutation efficiency was calculated by the number of mutant oocysts divided the number of all oocysts in G1 progeny. Each group contains three oocysts and was repeated three times (n=3). There were no significant differences between the two groups. B,indels in G1 progeny of single sgRNA. C,comparison of inherited mutation efficiency of double sgRNAs. The inherited mutation efficiency of 685/726 double sgRNAs-mediated gene alterations was calculated by the number of mutant oocysts divided by the number of all oocysts in G1 progeny. Each group contains three oocysts and was repeated three times (n=3). There were no significant differences between the two groups.D,indels in G1 progeny of double sgRNAs.
3.3.The tanning of locust eggs happens before syncytial division and does not significantly affect the genome editing efficiency of the CRlSPR/Cas9 system
The mutation rate in healthy developed eggs was about 66% in both tanned and less tanned egg groups on E5 after RNP injection (Fig.3-A). The mutation rate in live adults was also about 91% in tanned eggs and about 80% in less tanned eggs (Fig.3-A). The equivalent mutation rates in these tested embryos and adults between tanned and less tanned eggs indicated that tanned eggs retained the efficient absolute mutation efficiency. The SYTOX®Green staining on new fresh eggs showed that new individual green cells around the egg emerged after 4 h,which indicated the end of the syncytial division stage (Fig.3-B and Appendix F). These nuclei in the syncytial division stage are all in one cell,so they can be edited through a single injection and then all repaired before the nuclei are moved to their own cells,allowing for a significantly more even distribution of any gene editing events. Further,we could not detect any green cell signals at 0 or 4 h,suggesting that syncytial division in the fertilized locust eggs occurs more than 4 h after laying (Fig.3-B). Therefore,the window for genome editing in locusts is narrowed to the first 4 h following oviposition,during which all preparative and microinjection procedures must be completed. Considering that the eggshell tanning caused by the ootheca takes about 20 min (Fig.1-A),this still allows more than 3 h for microinjection manipulation before the syncytial division,which explains why the mutation rate in tanned eggs was similar to that in the less tanned eggs.
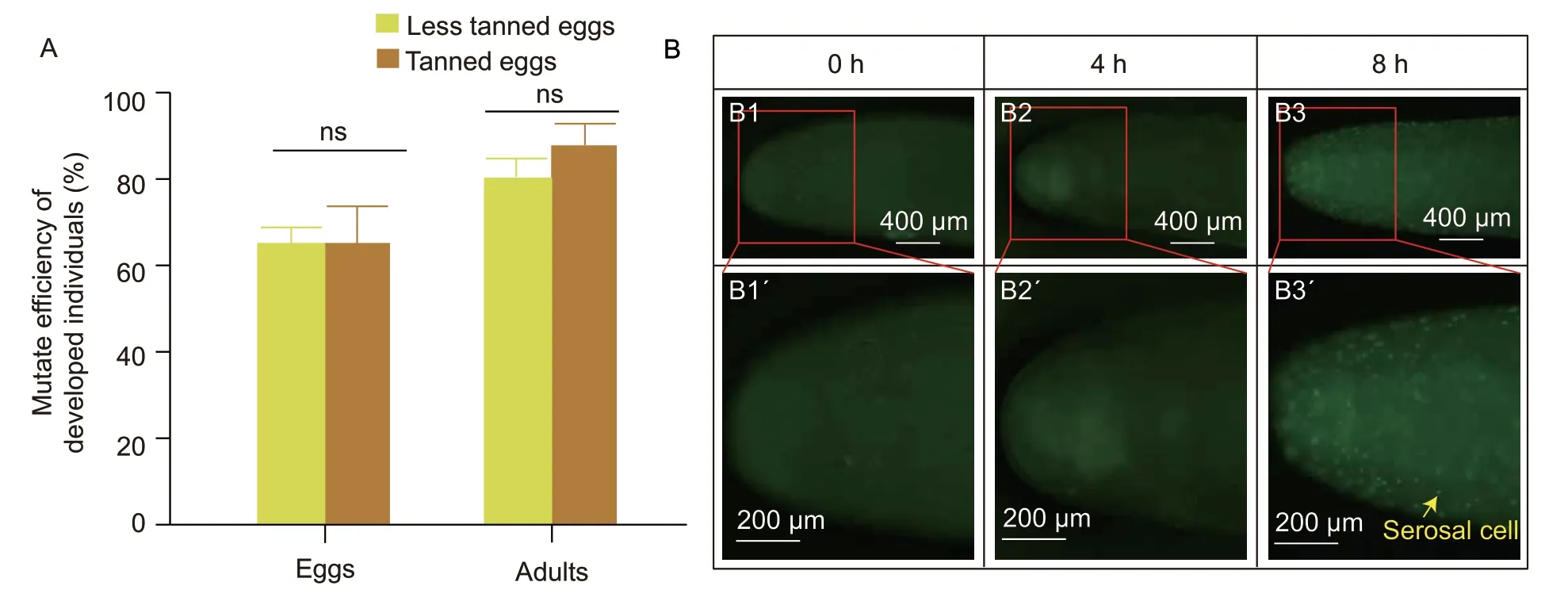
Fig.3 Egg tanning could not affect the mutation efficiency mediated by CRISPR/Cas9. A,comparison of gene editing efficiency in developed eggs and adults. The health developed eggs after microinjection (n=5) and live adults in G0 animals (n=4) between less tanned and tanned eggs were used to analyze the mutation efficiency. Bar is SD. ns indicates no significance. Each group was repeated three times. ns means no significance. B,the syncytial division of locust eggs. The syncytial division in the embryos lasts for 4 h,which is longer than egg tanning. The SYTOX® stains the nucleus green. The staining of the migratory locust eggs at 0 h (B1 and B1´),4 h (B2 and B2´) and 8 h (B3 and B3´),indicated that the differentiation of fertilized migratory locust eggs occurred within the first 4-h window (n=3).
3.4.Tanned eggs increased the hatching rate by reducing the infection ratio
During the five days of culture after microinjection,the less tanned eggs often have a bulging pustule accompanied by yolk coagulation in the injected wound,while the tanned eggs remained intact and developed egg structures similar to the un-injected eggs (Fig.4-A).The less tanned eggs after microinjection showed about a 37% infection rate while the tanned eggs had about a 13% infection rate (Fig.4-B). However,the un-injected less tanned eggs also had a higher infection rate than the tanned eggs (Fig.4-B),further suggesting that the less tanned eggs had lower anti-infection abilities. The ultrastructural analysis of these eggs showed that many fungi and bacteria colonized the outside of the less tanned (yellow) eggshells,while tanned eggs exhibited a significantly smaller number of bacteria on their surfaces (Fig.4-C). This suggested that the tanned eggs were more resistant to invasion following microinjection,and so the tanned eggs may be a better choice for generating mutant locusts using CRISPR/Cas9 system.
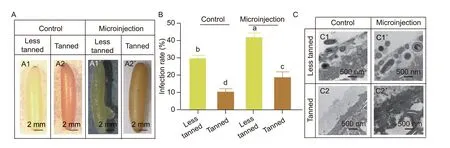
Fig.4 Egg tanning significantly reduced the infection rate after microinjection. A,the phenotypes of tanned and non-tanned eggs after microinjection. The less tanned eggs (A1) exhibited an infection phenotype with purulent wounds (A1´) after microinjection,while the tanned eggs exhibited intact and brown eggshell before (A2) and after (A2´) microinjection. B,comparison of infection rates. The infection rates for less tanned and tanned eggs with or without the microinjection during the embryogenesis were calculated. Each group contained 15 eggs and was repeated three times. Bar is SD (n=3). Different letters indicate the significant difference (P<0.05) among different groups. C,electron microscopic analysis of the external ultrastructure of eggs. The eggshell of less tanned eggs (C1 and C1´) is surrounded by many more microorganisms than the tanned eggs (C2 and C2´) (n=3).
3.5.Tanned eggs had a compressed structure to improve their defense ability
Tanned eggs had about 3 N of hardness detected by the texture analyzer (Fig.5-A) and demonstrated increased hardness compared to the less tanned eggs at E5,suggesting that tanned eggs developed an improved resistance to external pressure,which may help to explain why they experienced fewer infections than the less tanned eggs (Fig.5-B). Further,the TEM analysis showed that locust eggshells were made up of irregularly shaped electron-dense granules (Fig.5-C),and revealed that the tanned eggshells were thinner than the less tanned eggshells. The analysis of eggshell thickness showed that the eggshells of tanned eggs were about 30% thinner than the eggshells of less tanned eggs (Fig.5-D and Appendix G),suggesting that these thinner eggshells may support the increased pressure resistance in tanned eggs.Tanned eggs also had approximately 1 700 electrondense granules per square micron,which was about twice as many as the less tanned eggs evaluated (Fig.5-E and Appendix G). This suggested that tanned eggs had a high density of electron-dense granules per unit area resulting in its high rate of compression,and producing a thinner and stronger pressure-resistant eggshell.
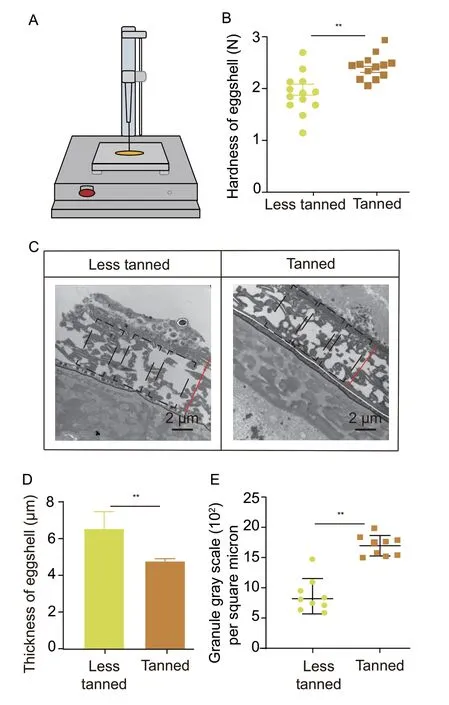
Fig.5 Tanned eggs exhibited enhanced defense ability due to its much stronger eggshell than the less tanned eggs. A,the principle and instrumentation of the egg hardness test. B,the hardness of eggshell analysis of less tanned and tanned eggs at day 5 of development (n=13). C,electron microscopic analysis of the ultrastructure of less tanned eggs and tanned eggs (n=3).D,eggshell thickness in less tanned eggs and tanned eggs. Bars are SD (n=3). E,comparison of eggshell density between less tanned eggs and tanned eggs. Tanned eggs have a denser shell than the less tanned eggs (n=9). ** indicates significant difference between two groups (P<0.05).
4.Discussion
4.1.Efficient mutation and high hatching rate after microinjection are both essential for increasing the production of mutant insects
The successful construction of mutant insects was based on the efficient mutation ratio caused by genome editing tools in conjunction with the high hatching rate after microinjection. The microinjection of ribonucleases into the fertilized eggs is still the most widely used method for constructing mutant insects,with successful application in several different types of insects. The reported mutation efficiency in injected eggs was 27% inD.melanogaster(Gratzet al.2014),24.57% inA.aegypti(Kistleret al.2015),16.8-30.3% inB.mori(Maet al.2014b),18.33-90.85% inP.xuthus(Liet al.2015),33.3% inG.bimaculatus(Awataet al.2015),35% inP.xylostella(Huanget al.2016) and 33% inL.migratoria(Liet al.2016). Meanwhile,effective CRISPR/Cas9 tools have undergone the development of several formats,including vectors containing Cas9 and sgRNA expression cassettes,Cas9 mRNA and sgRNA mixtures and the recent advent of a simple efficient system to produce RNP complexes,which have improved the mutation efficiency in various kinds of animals. In locusts,injection of RNPs consisting of one or two sgRNAs could generate mutant individuals with more than 21% mutation rate in tanned eggs but only about 10-15% mutation rate in less tanned eggs (Fig.1),indicating the RNPs injected into the locust eggs could introduce double-stranded breaks at the target site effectively in both tanned and less tanned eggs.
Improving the hatching rate after microinjection is an essential step for increasing the efficiency of mutant insect constructions. In mosquito vectors,higher genome editing efficiencies were only achieved when both microinjection parameters and culture conditions of the injected eggs were optimized (Kumar and Puttaraju 2012).While in Firebrat,Thermobia domestica,the pretreatment of eggs in dry conditions improved their hatching rate (Ohdeet al.2020). In locusts,we found that changing the microinjection parameters,optimizing the concentration of the CRISPR/Cas9 mixture,and improving the culture conditions (data not shown) as well as using tanned eggs significantly improved the hatching rate of treated locusts (Fig.1).
In this article,we found that microinjecting the RNP into tanned eggs improved the efficiency of producing mutantLmdsRNase2locusts no matter whether one or two sgRNA target sites were used. We found that tanned eggs increased both the hatching rate and mutation efficiency based on the total number of injected eggs,and finally increased the construction efficiency of CRISPR/Cas9-mediatedLmdsRNase2knock-out locusts. Meanwhile,the improvement of the mutant rate in tanned eggs was also found during constructions of other mutant locusts,such asLmDicer1andLmDicer2a(Appendix E),indicating that the improved hatching rate benefits from tanning but not the function of the genes. Taken together,these results indicate that tanned eggs could be used in CRISPR/Cas9-mediated genome editing projects to improve mutant locust production,with the use of tanned eggs producing an increase in hatching rate of more than 20%.
4.2.Eggshell tanning occurs before syncytial division,ensuring efficient and inheritable genome editing in locust
Rapid eggshell tanning provides enough time between tanning and syncytial division to allow for efficient genome editing in locust eggs. The genome editing at the single-cell stage is critical to the successful production of mutant animals,in which the genetic material from parental donors are fused and then later divided into two or more cells (Cooperet al.2018). Gene editing should be completed before and at least during the syncytial stage,where all nuclei are found in a single fertilized cell,allowing the free passage of information (Hoet al.1997;Gilbert 2000) and producing mutations that can be inherited by multiple cell lineages to ensure generational transfer. In insects,including the locust,the first 13 nuclear divisions are completed in the absence of cytokinesis,producing an embryo of approximately 6 000 nuclei in a single common cytoplasm in the syncytial stage (Hoet al.1997;Gilbert 2000). The cell nucleus stain assay showed that the syncytial division of locust eggs occurs at some point between 0 and 4 h after laying (Fig.3-B and Appendix F),which is much longer than the 20 min required for tanning. Based on our protocol,the microinjection of 100 fertilized migratory locust eggs takes about 40 min. If we combine these procedures,we can complete the tanning and microinjection of the fertilized eggs within 2 h,leaving at least 2 h for Cas9-mediated cleavage and repair of the target genes. The success of this approach can be seen in the presence of various mutations in theLmdsRNase2gene in the G1generation (Fig.2). Our data showed that eggshell darkening occurs before syncytial division,leaving sufficient time for CRISPR/Cas9-mediated mutation.
4.3.Tanned locust eggs improve hatching rate via improved invasion resistance
Tanning is a multistage chemical reaction that happens on the surface of cuticle and eggshell to make them pigmented and sclerotized (Eisneret al.1966;Andersen 2012). It is reported that knocking down the genes of the tanning pathway could cause the tanning deficiency in both cuticle and eggshell,accompanied by decreasing the resistance to pathogens (Duet al.2017;Luet al.2019;Sterkelet al.2019),which indicates that tanning of the cuticle or eggshell improves the ability to defend against infection. In our research,brown tanned eggs demonstrated a reduced infection rate following microinjection when compared to less tanned eggs (Fig.4). Ultrastructural analysis revealed significantly more fungi and bacteria around the outside of the less tanned eggshells,suggesting that the tanned eggshells were somehow more resistant to infection (Fig.4).Therefore,it was hypothesized that the density of the electron-dense granules inside the eggshell was a critical parameter in determining both the hardness and pressure resistance of these shells. In addition,the brown eggs exhibited improved pressure resistance,likely as the result of minor changes in the compression and density of these eggshells (Fig.5). Taken together these results suggested that the mechanical wound caused by microinjection was more easily repaired in tanned eggs because of their compressed eggshell structure,and that these cells had an improved immune response to infection,which improved their overall hatching rate.
5.Conclusion
Egg tanning is completed in less than 4 h which is needed for the syncytial division,which not only improved the stronger defensive responses of injected eggs,but also maintained the efficient genome editing mediated by CRISPR/Cas9,and ultimately increased the ratio of construction mutant individuals inL.migratoria.Therefore,microinjection of CRISPR/Cas9 into tanned eggs provides an improved model for developing Cas9-mediated gene editing procedures in locusts.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32070502,31730074,32072419 and 31501905). We thank Professor Bernard Moussian in Université Côte d’Azur from France for polishing the language of this article.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
杂志排行
Journal of Integrative Agriculture的其它文章
- lmpacts of climate change on drought risk of winter wheat in the North China Plain
- Triple bottom-line consideration of sustainable plant disease management:From economic,sociological and ecological perspectives
- A rice geranylgeranyl reductase is essential for chloroplast development
- Rapid determination of leaf water content for monitoring waterlogging in winter wheat based on hyperspectral parameters
- Does nitrogen application rate affect the moisture content of corn grains?
- Effects of drought stress on root morphology and spatial distribution of soybean and adzuki bean
