Identification and Analysis of Chemical Components in Zhideke Granules by UPLC-Q-Orbitrap HRMS
2021-10-21JieLIANGGuangqiangHUANGZhengyiSUNTanfangXIEYaohuaLIJingQIYueFENGJianliLIANGHuaZHULichunZHAO
Jie LIANG, Guangqiang HUANG, Zhengyi SUN, Tanfang XIE, Yaohua LI, Jing QI, Yue FENG, Jianli LIANG, Hua ZHU,4*, Lichun ZHAO*
1. Guangxi University of Chinese Medicine, Nanning 530200, China; 2. Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning 530011, China; 3. Guangxi Zhuang Yao Medicine Center of Engineering and Technology, Nanning 530200, China; 4. Guangxi Key Laboratory of Zhuang and Yao Ethnic Medicine, Nanning 530200, China
Abstract [Objectives] To identify and analyze chemical components in Zhideke Granules by ultra performance liquid chromatography-quadrupole/electrostatic field orbitrap high resolution mass spectrometry (UPLC-Q-Orbitrap HRMS). [Methods] Zhideke Granules were isolated on a Thermo Hypersil Gold C18 column (2.1 mm×100 mm, 1.9 μm). The mobile phase was 0.1% formic acid acetonitrile and 0.1% formic acid aqueous solution (containing 10 mmol ammonium acetate) with gradient elution. Chemical components in Zhideke Granules were rapidly isolated and identified by HRMS in the positive and negative ion mode with full scan data-dependent two stage threshold-triggered mass modes (Full MS/dd-MS2). [Results] The secondary fragment ion information of the target compound was selected and compared with the compound reported in the databases and related literatures for further confirmation. In total, 30 chemical compounds were identified, including 12 flavonoids and glycosides, 9 organic acids, 3 nitrogen-containing compounds, and 6 other components. [Conclusions] In this study, the UPLC-Q-Orbitrap HRMS was used for the first time to analyze the chemical components in Zhideke Granules. It is intended to provide a reference for the quality evaluation and further study of pharmacodynamic materials of Zhideke Granules.
Key words Zhideke Granules, UPLC-Q-Orbitrap HRMS, Chemical components
1 Introduction
Zhideke Granules consist of Scutellariae Radix, Belamcandae Rhizoma, Menthae Herba, Schizonepetae Herba, Bupleuri Radix, Platycodonis Radix, Eriobotryae Folium, Cynanchi Stauntonii Rhizoma et Radix, Herba Nervilia Plicatae, and Sauropi Folium. Long-term clinical practice has proved that Zhideke Granules have the effects of clearing away heat and detoxifying, relieving cough and resolving phlegm, especially in the treatment of colds, coughs and bronchial asthma[1]. At present, however, there are few studies on the quality control and chemical components of Zhideke Granules both at home and abroad. In the quality of Zhideke Granules, studies are limited to the analysis of individual components in Cynanchi Stauntonii Rhizoma et Radix, Belamcandae Rhizoma and Scutellariae Radix. The chemical components in other remaining medicines such as Platycodonis Radix and Sauropi Folium are not included in the quality control system of this prescription[2]. In terms of chemical components, there have been reports on the chemical components of the single-medicine in the prescription, the chemical components of Zhideke Granules have not been systematically studied[3-6]. Besides, because the chemical components contained in the traditional Chinese medicine compound prescription are affected by various factors such as medicinal materials and preparation technology, the quality of the traditional Chinese medicine compound prescription is varied[7]. In view of this, using the ultra performance liquid chromatography-quadrupole/electrostatic field orbitrap high resolution mass spectrometry (UPLC-Q-Orbitrap HRMS), we rapidly identified and analyzed chemical components in Zhideke Gradules, to make clear the chemical components in Zhideke Granules, and provide a reference for the study of the medicinal effects of Zhideke Granules, provide references for establishing a safer, more effective and controllable quality control system.
2 Instruments and reagents
2.1 InstrumentsU3000 Ultra Performance Liquid Chromatography System, Q-Exactive Mass Spectrometer, and TraceFinder Data Analytical Software (Thermo Fisher Scientific, USA); KQ5200DB Ultrasonic Cleaner (Kunshan Ultrasonic Instruments Co., Ltd., China); Milli-Q Synergy Ultra-pure Water Purifier (Millipore, USA); XS205DU Electronic Analytical Balance (Mettler-Toledo, Swiss); 5430R Centrifuge (Eppendorf Shanghai, China).
2.2 ReagentsMethanol, formic acid (chromatographically pure, Thermo Fisher Scientific, USA); acetonitrile (chromatographically pure, Merck Company, Germany); ammonium acetate (chromatographically pure, Shanghai Sixin Biological Technology Co., Ltd., China). Zhideke Granules (Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, batch No.20190701, specification: 15 g/bag).
3 Methods
3.1 Preparation of test sample solutionFirst, took 1.0 g sample of Zhideke Granules, added 50 mL of 50% methanol. Then, performed the ultrasonic extraction for 30 min, centrifuged, filtered, took the additional filtrate, and injected the sample for analysis.
3.2 Analytical conditions
3.2.1Chromatographic conditions. The chromatographic column adopted a Thermo Hypersil Gold C18column (2.1 mm×100 mm, 1.9 μm); the mobile phase was 0.1% formic acid acetonitrile (A)-0.1% formic acid water (B) (containing 10 mmol ammonium acetate); the flow rate was 0.3 mL/min; gradient elution (0-2.0 min, 5% A; 2.0-42.0 min, 5%-95% A; 42.0-47.0 min, 95% A; 47-50 min, 95%-5% A); column temperature was 35 ℃; the injection volume was 1 μL.
3.2.2Mass spectrometry conditions. The ion source adopted heated ESI (HESI) source in positive and negative ion detection mode; auxiliary gas flow rate: 10 μL/min; sheath gas volume flow rate: 30 μL/min; ion spray voltage: 3.5 kV(+), 3.2 kV(-); auxiliary gas temperature: 300 ℃. Ion transfer tube temperature: 320 ℃. The scanning mode was FullMS/dd-MS2mode. The primary Full MS selected a resolution of 70 000 FWHM, the dd-MS2secondary scan selected a resolution of 17 500 FWHM, and the positive and negative ion scanm/zrange: 100-1 500, the specific ion scan mode was "off", the apex was excited for 2-8 s, the collision gas was high-purity nitrogen, and the spray gas was nitrogen.
3.3 Data processingWith the aid of the TraceFinder software, we obtained the molecular formula and the precise relative molecular mass of the primary mass spectrum by fitting, and compared the results with the existing database, and made a preliminary estimation of the chromatographic peaks. Using the primary fragment ion and secondary fragment ion information of the target compound, we compared with references and databases such as Chemspider and massbank to further identify and determine unknown chemical components.
4 Results and analysis
In the positive and negative ion detection mode, we obtained the total ion chromatogram of Zhideke Granules (Fig.1), and identified a total of 30 chemical components, including 12 flavonoids and their glycoside compounds, 9 organic acid compounds, and 3 nitrogen-containing compound and 6 other compounds. The specific information of identified chemical compounds are listed in Table 1.
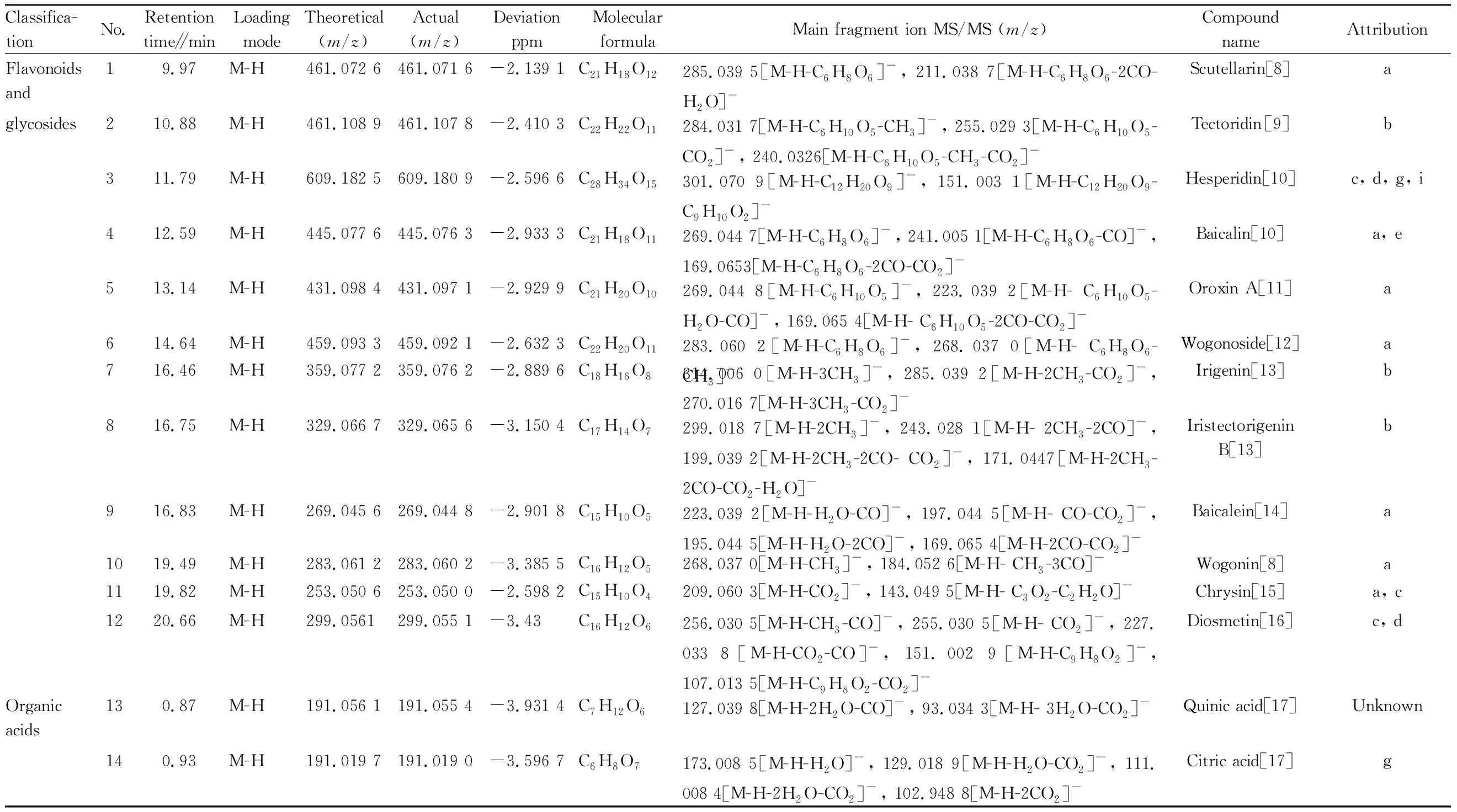
Table 1 Chemical components in Zhideke Granules identified by UPLC-Q-Orbitrap HRMS
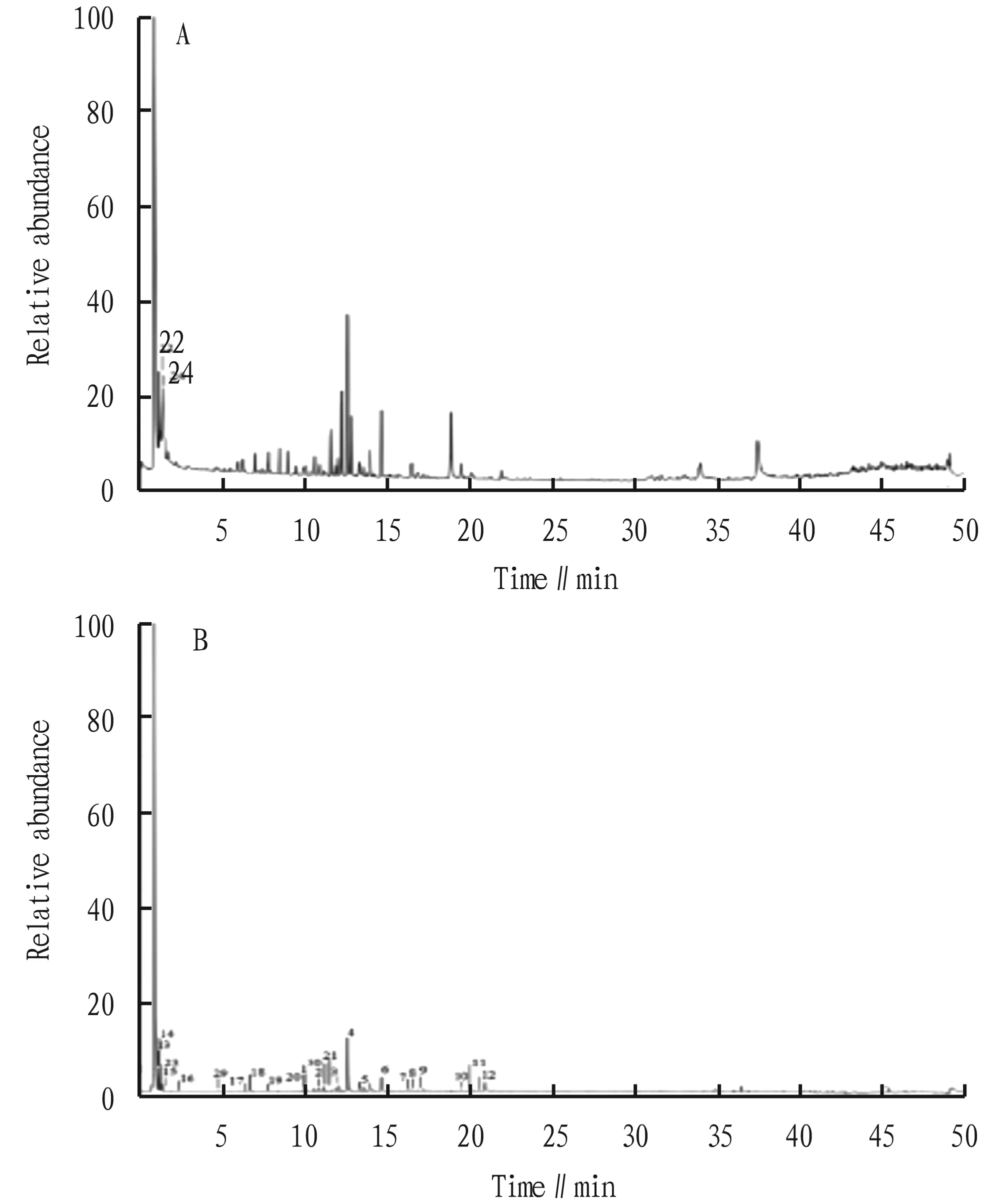
Fig.1 Total ion chromatogram of Zhideke Granules in positive (A) and negative mode (B)

Classifica-tionNo.Retentiontime∥minLoadingmodeTheoretical(m/z)Actual(m/z)DeviationppmMolecularformulaMain fragment ion MS/MS (m/z) CompoundnameAttribution151.54M-H169.014 2169.013 6-3.601 1C7H6O5125.023 9[M-H-CO2]-, 97.029 2[M-H- C2O3]-, 69.034 4[M-H-C3O4]-Gallic acid[16]162.26M-H197.045 6197.044 8-3.729 8C9H10O5179.034 3[M-H-H2O]-, 151.039 9[M-H-H2O-CO]-, 135.036 9[M-H-H2O-CO2]-, 72.992 8[M-H-C7H8O2]-Salvianic acid A(Danshensu)[18]Unknown176.35M-H353.087 8353.086 7-3.056C16H18O9191.055 4[M-H-C9H6O3]-, 179.034 3[M-H- C7H10O5]-, 173.044 8[M-H-C9H6O3-H2O]-Cryptochlorogenicacid[17]Unknown186.79M-H179.035 0179.034 3-3.958 8C9H8O4135.044 7[M-H-CO]-, 107.049 8[M-H-CO2-CO]-, 89.039 5[M-H-CO2-CO-H2O]-Caffeic acid[17]c, d, f198.76M-H163.040 1163.039 6-3.184 6C9H8O3119.049 8[M-H-CO2]-, 91.018 5[M-H-CO2-CO]-p-hydroxy-cinnamic acid[19]Unknown209.97M-H537.103 8537.102 7-2.213 5C27H22O12313.071 0[M-H-C9H8O4]-, 295.060 1[M-H-CO2-C9H10O5]-, 109.029 1[M-H-CO2-C9H10O5-C11H6O3]-Lithospermicacid[20]c2111.76M-H187.097 6187.096 9-3.670 2C9H16O4143.107 0[M-H-CO2]-, 125.096 7[M-H- CO2-H2O]-, 97.065 4[M-H-CO2-H2O-C2H4]-, 69.034 2[M-H-CO2-H2O-C4H8]-Azelaic acid[17]h Nitrogen-containing 221.13M+H182.081 2182.080 3-4.572 0C9H11NO3147.043 4[M+H-NH3-H2O]+, 136.075 1 L-[M+H-HCOOH]+, 119.048 8[M+H-COOH-NH3]+, 91.054 3[M+H-OH-NH3-C2H3O2]+L-tyrosine[10]ccompounds231.14M-H243.062 3243.061 5-3.093 1C9H12N2O6111.019 8[M-H-C5H8O4]-, 110.024 5[M-H- C5H9O4]-, 82.029 6[M-H-C5H9O4-CO]-Uridine[21]e, j241.27M+H123.055 3123.054 8-3.842 2C6H6N2O96.044 4[M+H-CHN]+, 95.060 6[M+H-CO]+, 80.049 7[M+H-CONH]+, 78.034 1[M+H-NH3-CO]+Nicotinamide[22]jOthers250.84M-H181.071 8181.071 1-3.780 6C6H14O6163.067 0[M-H-H2O]-, 101.024 0[M-H- H2O-C2H6O2]-, 89.024 1[M-H-H2O-C3H6O2]-, 71.013 5[M-H-2H2O-C3H6O2]-, 59.013 6[M-H-H2O-C4H8O3]-Mannitol[23]Unknown260.84M-H341.108 9341.107 8-3.347 7C12H22O1189.024 1[M-H-C9H16O8]-, 71.013 6[M-H- C9H16O8-H2O]-Sucrose[24]h270.84M+HCO2549.167 2549.166 0-2.340 7C18H32O1671.013 8[M-H-C6H10O5-C9H16O8-H2O]-Raffinose[25]Unknown280.85M+H127.039 0127.038 4-4.256 5C6H6O381.033 7[M+H-H2O-CO]+, 71.049 5[M+H-2CO]+, 53.039 2[M+H-H2O-2CO]+5-hydroxymethy-lfurfural[26]b, h294.74M-H137.024 4137.023 8-4.274 9C7H6O3109.021 3[M-H-CO]-, 108.021 3[M-H- CHO]-, 93.034 2[M-H-CO2]-, 81.034 3[M-H-2CO]-Protocatechual-dehyde[27]c, d3011.14M-H623.198 1623.196 6-2.480 4C29H36O15461.165 8[M-H-C9H6O3]-, 179.034 3[M-H-C20H28O11]-, 161.023 8[M-H-C20H28O11-H2O]-Isoacteoside[28]h
4.1 FlavonoidsFlavonoids are organic compounds with C6-C3-C6nucleus formed by connecting two benzene rings through three carbon atoms. In flavonoids, hydroxyl, methyl and methoxy are very common, so their mass spectroscopy fragmentation rules are mainly losing CO, H2O, CO2and side chain substituents[10]. Taking compound 7 as an example, from the primary mass spectrum, it can be known that its quasi-molecular ion peak ism/z359.076 2[M-H]-, and the molecular formula is C18H16O8. The quasi-molecular ion peak was fragmented, and three CH3groups were lost at the same time, then a fragment ion ofm/z314.006 0[M-H-3CH3]-was obtained. Later, the fragment ion ofm/z314.006 0 was fragmented, a neutral CO2molecule was lost, and the fragment ion ofm/z270.016 7[M-H-3CH3-CO2]-was obtained. In addition, the quasi-molecular ion peak could lose two CH3fragments and one neutral CO2molecule, generating the fragment ion ofm/z285.039 2[M-H-2CH3-CO2]-. Combined with the mass spectrometry information stated in the literature[13], it can be inferred that the compound 7 is irigenin.
For the compound 12, from the primary mass spectrum, it can be known that its quasi-molecular ion peak ism/z299.055 1[M-H]-, and its molecular formula is C16H12O6. The quasi-molecular ion peak was fragmented, and one CH3and one neutral CO molecule were lost successively, then a fragment ion ofm/z256.030 5[M-H-CH3-CO]-was obtained. At the same time, the quasi-molecular ion peak could also lose a neutral CO2molecule, then a fragment ion ofm/z255.030 5[M-H-CO2]-was obtained. Later, the fragment ion ofm/z255.030 5 was fragmented, a neutral CO molecule was lost, and the fragment ion ofm/z227.033 8[M-H-CO2-CO]-was obtained. The fragment ions ofm/z151.002 9[M-HC9H8O2]-were generated by the retro Diels-Alder reaction (rDA) of the quasi-molecular ion peakm/z299.055 1, which loses the fragment group of C9H8O2. Later, the fragment ion ofm/z151.002 9 was fragmented, a neutral CO2molecule was lost, and the fragment ion ofm/z107.013 5[M-H-C9H8O2-CO2]-was obtained. Therefore, it can be inferred that compound 12 is diosmetin[16], and the possible fragmentation pathway is shown in Fig.2.

Fig.2 Possible fragmentation pathway of diosmetin
According to the fragmentation rules of the above-mentioned flavonoids, it is inferred that compounds 8, 9, 10 and 11 are iristectorigenin B, baicalein, wogonin, and chrysin, respectively. Most of the flavonoids in nature exist in the form of glycosides. Therefore, the fragmentation of glycosidic bonds is the most important fragmentation pathway of flavonoid glycosides, and the fragmentation pathway of its aglycon is consistent with the fragmentation rules of flavonoids. Compound 2: from the primary mass spectrum, it can be known that its quasi-molecular ion peak ism/z461.107 8[M-H]-, and its molecular formula is C22H22O11. The glycosidic bond of the quasi-molecular ion peak was fragmented and the fragment group of C6H10O5was lost, generatingm/z299.107 8[M-H-C6H10O5]-flavonoid aglycone fragmention. The aglycone fragment ion continued to fragment, losing a CH3group and a neutral CO2molecule, respectively, generatingm/z284.031 7[M-H-C6H10O5-CH3]-and 255.029 3[M-H-C6H10O5-CO2]-characteristic fragment ions. Later, the fragment ion ofm/z284.031 7 was fragmented and lost a neutral CO2molecule, then a fragment ion ofm/z240.032 6[MH-C6H10O5-CH3-CO2]-was obtained. Therefore, it can be inferred that the compound 2 is tectoridin[9], and its possible fragmentation pathway is shown in Fig.3.
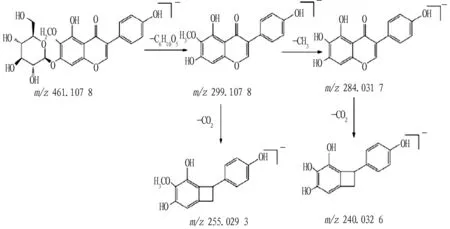
Fig.3 Possible fragmentation pathway of tectoridin
Compound 6: from the primary mass spectrum, it can be known that its quasi-molecular ion peak ism/z459.092 1[M-H]-, and its molecular formula is C22H20O11. The quasi-molecular ion peak glycosidic bond broke, and one molecule of glucuronic acid C6H8O6fragments was lost, then the fragment ion ofm/z283.060 2[M-H-C6H8O6]-was obtained. Later, the quasi-molecular ion peak continued to fragment and one CH3fragment was lost, generating the fragment ion ofm/z268.037 0[M-H-C6H8O6-CH3]-. Combined with the mass spectrometry information stated in the literature[12], it can be inferred that the compound 6 is wogonoside. According to the fragmentation rules of the components of the above-mentioned flavonoid glycosides, it can be inferred that compounds 1, 3, 4 and 5 are scutellarin, hesperidin, baicalin, and oroxin A, respectively.
4.2 Organic acidsThe main components of organic acids in Zhideke Granules exist in Eriobotryae Folium, Platycodonis Radix, and Menthae Herba. According to the difference in groups connected to the carboxyl group, organic acids are divided into fatty acids and aromatic acids. Fatty organic acid is the compound formed by connecting carboxyl group and hydrocarbon group. Its fragmentation rules usually shows that it first loses one molecule of H2O, CO and CO2, and then loses fragment ions such as hydrocarbon group. Compound 13: from the primary mass spectrum, it can be known that its quasi-molecular ion peak ism/z191.055 4[M-H]-, and its molecular formula is C7H12O6. The quasi-molecular ion peak was fragmented, and two H2O and one neutral CO molecule were lost successively, then a fragment ion ofm/z127.039 8[M-H-2H2O-CO]-was obtained. In addition, the quasi-molecular ion peak could lose three H2O molecules and one neutral CO2molecule, generating the fragment ion ofm/z93.034 3[M-H-3H2O-CO2]-. Therefore, it can be inferred that the compound 13 is quinic acid[17].
Compound 14: from the primary mass spectrum, it can be known that its quasi-molecular ion peak ism/z191.019[M-H]-, and its molecular formula is C6H8O7. The quasi-molecular ion peak was fragmented, and one H2O molecule was lost, generating the fragment ion ofm/z173.008 5[M-H-H2O]-, which was further fragmented, losing one H2O molecule and one neutral CO2molecule, then the fragment ion ofm/z111.008 4[M-H-2H2O-CO2]-was obtained. At the same time, the fragment ion ofm/z173.008 5 could directly lose one neutral CO2molecule, generating the fragment ion ofm/z129.018 9[M-H-H2O-CO2]-. In addition, the quasi-molecular ion peak could lose one neutral CO2molecule, generating the fragment ion ofm/z102.948 8[M-H-2CO2]-. Therefore, it can be inferred that the compound 14 is citric acid[17].
Compound 21: from the primary mass, it can be known that its quasi-molecular ion peak ism/z187.096 9[M-H]-, and its molecular formula is C9H16O4. The quasi-molecular ion peak was fragmented, and one neutral CO2molecule was lost, generating the fragment ion ofm/z143.107 0[M-H-CO2]-. This fragment ion continued to fragment, lose one H2O, generating the fragment ion ofm/z125.096 7[M-H-CO2-H2O]-, which further fragmented, losing the fragments of C2H4and C4H8, generating the fragment ions ofm/z97.065 4[M-H-CO2-H2O-C2H4]-andm/z69.034 2[M-H-CO2-H2O-C4H8]-. Therefore, it can be inferred that the compound 21 is azelaic acid[17], and its possible fragmentation pathway is shown in Fig.4.
Aromatic acids generally refer to compounds containing both a benzene ring and a carboxyl group in the molecular structure. Such compounds tend to lose aromatic side chain groups during mass spectroscopy fragmentation, and subsequently lose neutral molecules such as CO2, H2O, and CO. Compound 16: from the primary mass spectrum, it can be known that its quasi-molecular ion peak ism/z197.044 8[M-H]-, and its molecular formula is C9H10O5. The quasi-molecular ion was fragmented, and one H2O molecule was lost, generating the fragment ion ofm/z179.034 3[M-HH2O]-, which was further fragmented, losing one neutral CO2molecule and one neutral CO2molecule, then generating the fragment ions ofm/z151.039 9[M-H-H2O-CO]-andm/z135.036 9[M-H-H2O-CO2]-at the same time. Besides, the benzyl carbon in the quasi-molecular ion peak could also be fragmented, losing the fragment group of C7H8O2, and generating 72.992 8[M-HC7H8O2]-fragment ion. Therefore, it can be inferred that the compound 16 is Danshensu[18], and its possible fragmentation pathway is shown in Fig.5.
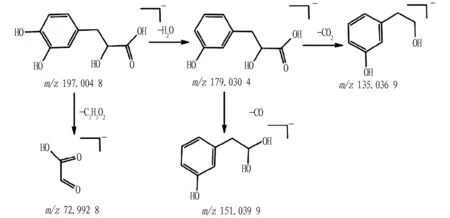
Fig.5 Possible fragmentation pathway of Danshensu
Compound 17: from the primary mass spectrum, it can be known that its quasi-molecular ion peak ism/z353.086 7[M-H]-, and its molecular formula is C16H18O9. The quasi-molecular ions were fragmented, losing the fragment groups of C7H10O5and C9H6O3, generating fragment ions ofm/z179.034 3[M-H-C7H10O5]-andm/z191.055 4[M-H-C9H6O3]-. Later, the fragment ion ofm/z191.055 4[M-H-C9H6O3]-continued to fragment, and one molecule of H2O was lost, generating the fragment ion ofm/z173.044 8[M-H-C9H6O3-H2O]-. Therefore, it can be inferred that the compound 17 is cryptochlorogenic acid[17], and its possible fragmentation pathway is shown in Fig.6.
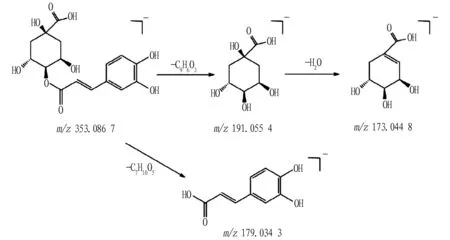
Fig.6 Possible fragmentation pathway of cryptochlorogenic acid
Compound 20: from the primary mass spectrum, it can be known that its quasi-molecular ion peak ism/z537.102 7[M-H]-, and its molecular formula is C27H22O12. The fragment ion ofm/z493.102 7[M-H-CO2]-was generated by the loss of a CO2molecule from the quasi-molecular ion peak. Later, the fragment ion continued to fragment, lose the fragment ions of C9H10O5and C9H8O4, generating the fragment ions ofm/z295.060 1[M-H-CO2-C9H10O5]-and /z 313.071 0[M-HC9H8O4]-. The obtained fragment ion ofm/z295.060 1 continued to fragment, losing fragment group of C11H6O3, and generating fragment ion ofm/z109.029 1[M-H-CO2-C9H10O5-C11H6O3]-. Therefore, it can be inferred that the compound 20 is lithospermic acid[20]. According to the above-mentioned fragmentation rules of aromatic acids, it can be inferred that compounds 15, 18 and 19 are gallic acid, caffeic acid and p-hydroxy-cinnamic acid, respectively.
4.3 Nitrogen containing compoundsNitrogen-containing compounds are mainly organic compounds containing nitrogen in the molecular structure, mainly including nucleosides, amino acids and nicotinamide. The nitrogen-containing compounds in Zhideke Granules are mainly derived from Menthae Herba and Bupleuri Radix. The mass spectrometry fragmentation rule of nitrogen-containing compounds is mainly manifested in the loss of fragment groups such as H2O, CO2, NH3, COOH, and OH. At the same time, the nucleoside bonds between nucleoside compounds are prone to breakage. Compound 22: from the primary mass spectrum, it can be known that its quasi-molecular ion peak ism/z182.080 3[M+H]+and its molecular formula is C9H11O3. The quasi-molecular ion peak was fragmented, and a COOH fragment group was lost, generating a fragment ion ofm/z136.075 1[M+H-HCOOH]+, which continued to fragment and lose a CH3fragment, generating the fragment ion ofm/z119.048 8[M+ H-COOH-NH3]+. In addition, the quasi-molecular ion peak may be fragmented in another pathway, losing one OH group and one NH3molecule, and generating a fragment ion ofm/z147.043 4[M+H-H2O-NH3]-. The fragment ion ofm/z147.043 4 lost the fragment ion of C2H3O2to obtain the fragment ion ofm/z91.054 3[M+H-OH-NH3-C2H3O2]+. Therefore, it can be inferred that the compound 22 is L-tyrosine[10], and its possible fragmentation pathway is shown in Fig.7.
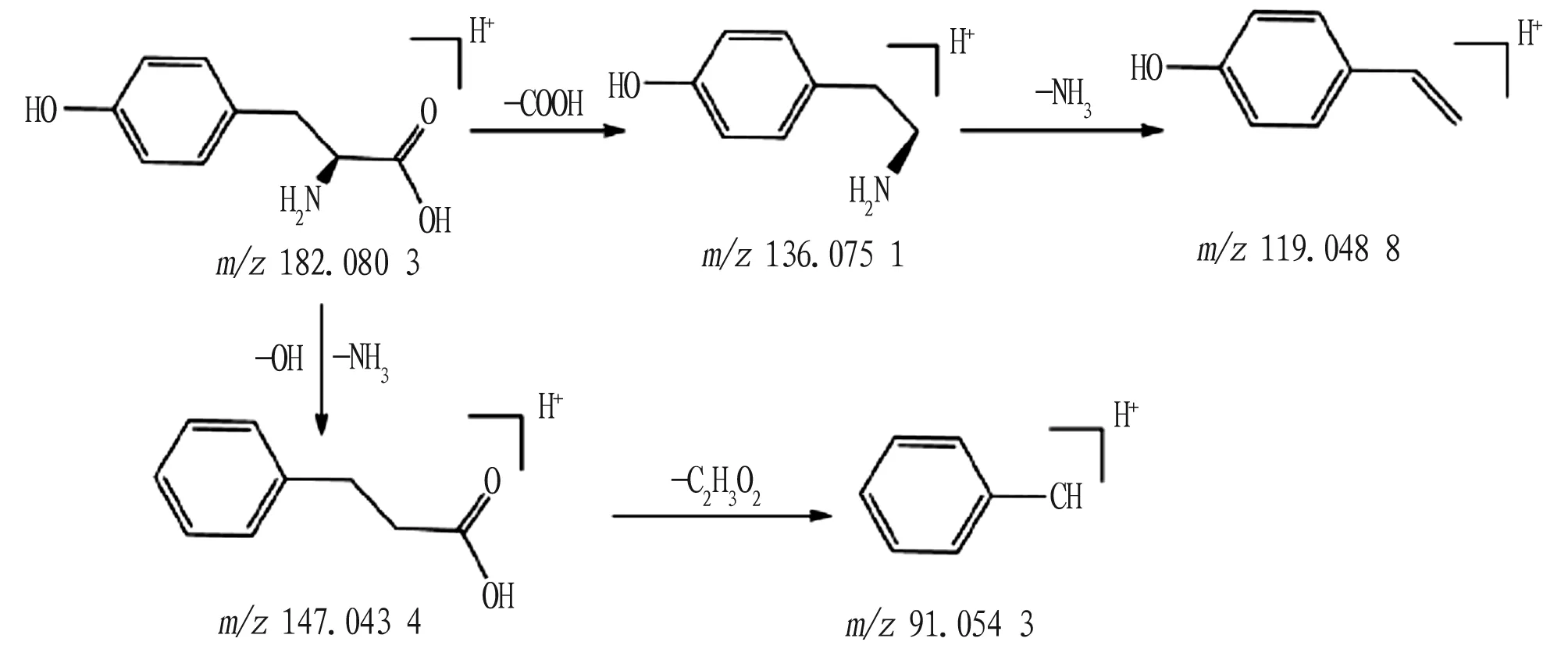
Fig.7 Possible fragmentation pathway of L-tyrosine
Compound 23: quasi-molecular ion peak obtained in the negative ion mode ism/z243.061 5[M-H]-, and its molecular formula is C9H12N2O6. The quasi-molecular ion peak was fragmented, and the groups of C5H8O4and C5H9O4were lost, then the fragment ions ofm/z111.019 8[M-H-C5H8O4]-andm/z110.024 5[M-H-C4H7NO4]-. Later, the fragment ion ofm/z110.024 5 continued to fragment, lose one neutral CO molecule, generating the fragment ion ofm/z82.029 6[M-H-C5H9O4-CO]-. It can be inferred that the compound 23 is uridine[21], and its possible fragmentation pathway is shown in Fig.8.
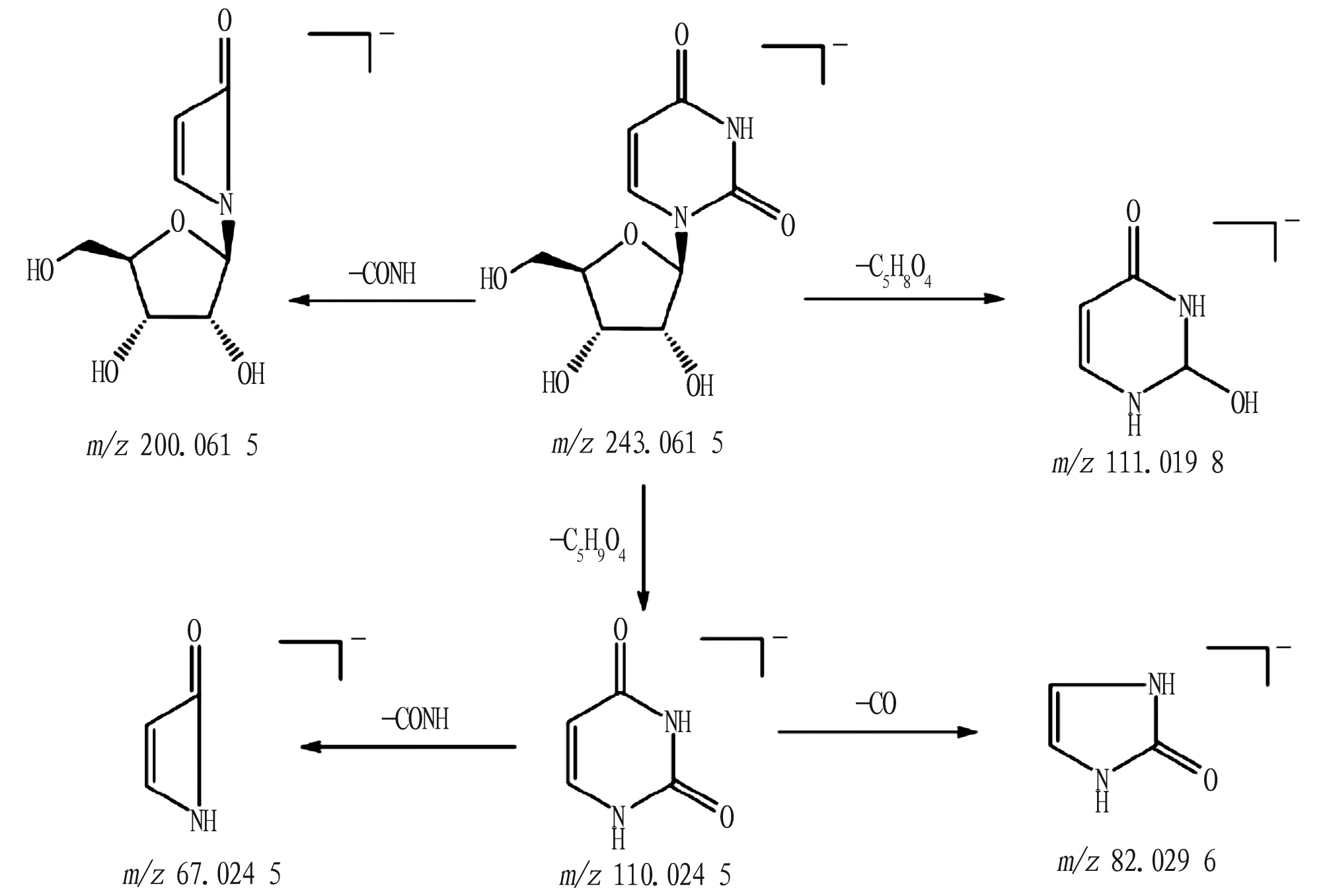
Fig.8 Possible fragmentation pathway of uridine
Compound 24: from the primary mass, it can be known that its quasi-molecular ion peak ism/z123.054 8[M+H]+and its molecular formula is C6H6N2O. The quasi-molecular ion peak was fragmented, losing a neutral CO molecule, and generating the frag-
ment ion ofm/z95.060 6[M+H-CO]+. In addition, the quasi-molecular ion peak was fragmented, and one NH3molecule and one neutral CO molecule were lost successively, then a fragment ion ofm/z78.034 1[M +H-NH3-CO]+. At the same time, the C-C bond between the benzene ring and the carbonyl group and the pyridine ring can also be fragmented, losing the CHN and CONH fragment groups, respectively, generating fragment ions ofm/z96.044 4[M+H-CHN]+andm/z80.049 7[M+H-CONH]+. It can be inferred that the compound 24 is nicotinamide[22].
4.4 Other compoundsIn addition to the above-mentioned chemical components, there are some other types of compounds in Zhideke Granules, including sugars, phenols and some volatile components. Compound 25: from the primary mass spectrum, it can be known that its quasi-molecular ion peak ism/z181.071 1[M-H]-and its molecular formula is C6H14O6. The molecular ion peak was fragmented, and one H2O molecule was lost, generating a fragment ion ofm/z163.067 0[M-H-H2O]-, which continued to fragment and lose fragment groups of C2H6O2, C4H8O3, and C3H6O2, then the fragment ions ofm/z101.024 0[M-HH2O-C2H6O2]-,m/z59.013 6[M-H-H2O-C4H8O3]-andm/z89.024 1[M-H-H2O-C3H6O2]-. Later, the fragment ion ofm/z89.024 1 continued to fragment, lose one H2O molecule, generating the fragment ion ofm/z71.013 5[M-H-2H2O-C3H6O2]-. It can be inferred that the compound 25 is mannitol[23], and its possible fragmentation pathway is shown in Fig.9.

Fig.9 Possible fragmentation pathway of mannitol
Compound 26: from the primary mass, it can be known that its quasi-molecular ion peak ism/z341.107 8[M-H]-and its molecular formula is C12H22O11. The quasi-molecular ion peak was fragmented, and C9H16O8fragment group was lost, generating the fragment ion ofm/z89.024 1[M-H-C9H16O8]-, which was further fragmented, losing one H2O molecule and generating the fragment ion ofm/z71.013 6[M-H-C9H16O8-H2O]-, which was basically consistent with the mass spectrometry data reported in the literature[24]. Thus, it can be inferred that the compound 26 is sucrose. Similarly, combined with the literature[25], it can be inferred that the compound 27 is raffinose.
Compound 28: the quasi-molecular ion peak in the positive ion mode ism/z127.038 4[M+H]+and its molecular formula is C6H6O3. The quasi-molecular ionm/zwas fragmented, and one H2O molecule and one neutral CO molecule were lost, generating the fragment ion ofm/z81.033 7[M+ H-H2OCO]+, which was further fragmented, losing one neutral CO molecule, and generating the fragment ion ofm/z53.039 2[M+H-H2O-2CO]+. In addition, the quasi-molecular ion peak could lose two neutral CO molecules, generating the fragment ion ofm/z71.049 5[M+H-COCO]+. Therefore, it can be inferred that the compound 28 is 5-hydroxymethylfurfural[26].
Compound 29: from the primary mass spectrum, it can be known that its quasi-molecular ion peak ism/z137.023 8[M-H]-, and its molecular formula is C7H6O3. The quasi-molecular ion peak was fragmented, losing one neutral CO molecule, one neutral CO2molecule, and one CHO fragment group, and generating fragment ions ofm/z109.021 3[M-H-CO]-,m/z93.034 2[M-H-CO2]-, andm/z108.021 3[M-H-CHO]-, respectively. The obtained fragment ion ofm/z109.021 3 continued to fragment, losing one neutral CO molecule, and generating the fragment ion ofm/z81.034 3[M-H-2CO]-. Combined with the mass spectrometry in literature[27], it can be inferred that the compound 29 is protocatechual-dehyde.
Compound 30: from the primary mass spectrum, it can be known that its quasi-molecular ion peak ism/z623.196 6[M-H]-, and its molecular formula is C29H36O15. The quasi-molecular ion peak was fragmented and the caffeoyl C9H6O3was lost, generating the fragment ion ofm/z461.165 8[M-H-C9H6O3]-. At the same time, the quasi-molecular ion could also lose the fragment group of C20H28O11, generating the fragment ion ofm/z179.034 3[M-H-C20H28O11]-, which was further fragmented, losing one H2O molecule, and generating the fragment ion ofm/z161.023 8[M-H-C20H28O11-H2O]-. Combined with the mass spectrometry in literature[28], it can be inferred that the compound 30 is isoacteoside.
5 Discussions
In this study, using PLC-Q-Orbitrap HRMS, we identified and analyzed 30 compounds in Zhideke Granules. The results show that the main components of Zhideke Granules are flavonoids and organic acids. Flavonoids have antibacterial, antiviral, anti-inflammatory, antipyretic and antitussive pharmacological activities[29], while most organic acids have cough and asthma relief, anti-inflammatory, antibacterial and antiviral effects[30-31]. These are basically consistent with the clinical application of Zhideke Granules for colds and coughs, relieving coughs and resolving phlegm. It is expected to provide a scientific basis for the better clinical application of this prescription. However, there are few studies about Zhuang medicines such as Herba Nervilia Plicatae and Sauropi Folium. Most of their chemical components are still not clear. Therefore, it is impossible to attribute the sources of some chemical components. In addition, due to the limitation of existing database and literature, there is still difficulty in identification of compound structure simply from the mass spectrometry. Thus, future study should compare with reference substance, combine preparation of liquid phase and macroporous adsorption resin to separate and purify the chemical components in Zhideke Granules, and combine hydrogen spectrum, carbon spectrum, IR spectrum methods to confirm the structure of the separated compounds, so as to make clear the chemical substance basis of Zhideke Granules, provide references for the further quality control of the prescription and the study of material basis of the efficacy.
杂志排行
Medicinal Plant的其它文章
- Research Progress on Pharmacological Action of 5-O-methylvisammioside
- Review of Processing Methods of Jujube
- Advances in Application of PCR Amplification in Etiologic Diagnosis
- Research Progress of Traditional Chinese Medicine in the Treatment of Acute Respiratory Distress Syndrome
- Advances in Research of Resources and Cultivation Techniques of Chinese Medicinal Material Radix Bupleuri
- Advances in Research of Pharmacological Effects of Peimine
