Recent advances in nanotherapeutics for the treatment and prevention of acute kidney injury
2021-10-18
Institute of Pharmaceutics, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, China
ABSTRACT Acute kidney injury (AKI) is a serious kidney disease without specific medications currently except for expensive dialysis treatment.Some potential drugs are limited due to their high hydrophobicity,poor in vivo stability,low bioavailability and possible adverse effects.Besides,kidney-targeted drugs are not common and small molecules are cleared too quickly to achieve effective drug concentrations in injured kidneys.These problems limit the development of pharmacological therapy for AKI.Nanotherapeutics based on nanotechnology have been proved to be an emerging and promising treatment strategy for AKI,which may solve the pharmacological therapy dilemma.More and more nanotherapeutics with different physicochemical properties are developed to efficiently deliver drugs,increase accumulation and control release of drugs in injury kidneys and also directly as effective antioxidants.Here,we discuss the recent nanotherapeutics applied in the treatment and prevention of AKI with improved effectiveness and few side effects.
Keywords:Nanotherapeutic Acute kidney injury Drug delivery Target Controlled release Inorganic nanoparticles
1.Introduction
Acute kidney injury (AKI) is a severe kidney disease characterized by a sharp decline in renal function [1],accompanied by high morbidity,medical costs and mortality[2],and is also an important risk factor for chronic kidney disease (CKD) [3] .Among hospitalized patients,AKI is one of the most serious and common complications [4],resulting in approximately 2 million deaths worldwide each year [5] .Currently,there is no reliable drug therapy and clinicians can only rely on expensive generalized supportive care [6,7] .Therefore,it is urgent to develop drugs that are effective for AKI with minimal side effects).
AKI is associated with various etiologies and pathophysiological mechanisms [8],such as ischemia/reperfusion [9,10],sepsis [11,12] and drug toxicity [13,14],as shown in Table 1,and most forms of AKI are characterized by endothelial dysfunction,microcirculation changes,renal tubular damage,and intrarenal inflammation [15] .Oxidative stress is known to be a critical pathogenic factor involved in the initiation,development,and progression of most kidney diseases [16,17] .Specifically,renal hypoxia and ischemia promote the excessive production of reactive oxygen species(ROS),which would oxidize and destroy biomolecules and various cell membranes,affect the function of organelle and cause inflammation,renal tubule cell damage,and vascular dysfunction [18] .
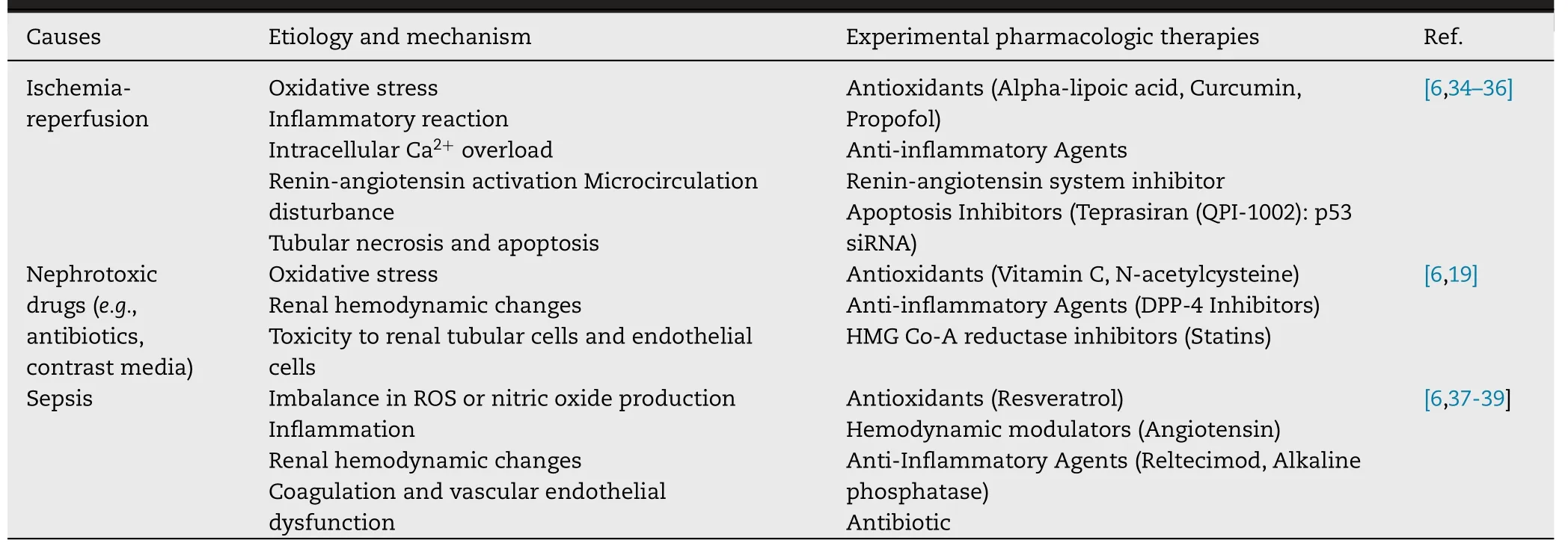
Table 1–Experimental pharmacologic therapies according to the etiology and mechanism of AKI.
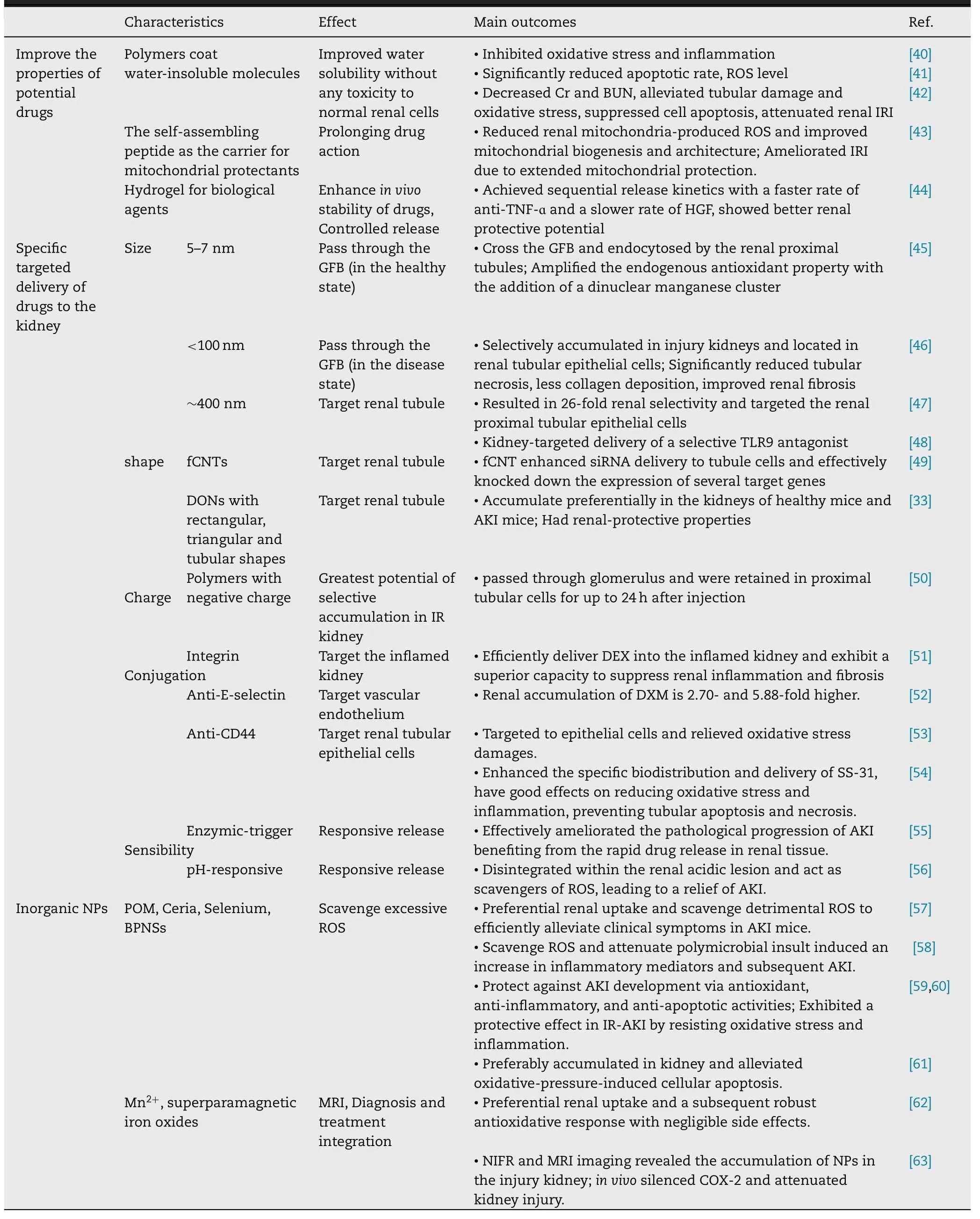
Table 2–Characteristics of nanotherapeutic agents for acute kidney injury.
With the development of mechanistic research,several chemical and biological agents have been proved to have beneficial effects in AKI models [19,20] .However,some potential drugs are highly hydrophobic or easily degradablein
vivo
,resulting in poor bioavailability,which limits their application in AKI therapy.Besides,kidney-targeted drugs are not common,and most small molecules are easily cleared quickly and can not achieve effective concentrations in injured kidneys.As a result,high doses of drugs are needed,which may cause adverse consequences.Therefore,the current strategy of alleviating AKI is to develop efficient drug delivery systems that can specifically deliver drugs to renal targets and achieve effective concentration with few adverse effects.Fortunately,the development of nanotechnologybased nanotherapeutics makes this strategy possible.Nanotherapeutics can help improve the bioavailability of drugs,increase the targeting delivery efficiency,and control the drug release [21–25] .The reported nanotherapeutic agents include micelles,liposomes,polymer nanoparticles(NPs),solid lipid NPs,dendrimers,metal NPs,etc.,which have been applied in the diagnosis and treatment of various diseases,such as cancer [26,27],Alzheimer’s disease,cardiovascular disease and various inflammations[28] .Nanotherapeutics is also expected to solve the AKI treatment dilemma [29–33] .This review focuses on recently developed nanotherapeutics for AKI prevention and treatment.We discuss nanocarriers that can enhance the bioavailability of potential drugs and renal-target delivery systems that can deliver various drugs specifically into the kidney through passive and active targeting,responsively release cargoes,and lodged in the kidney to exert effects,and certain inorganic NPs studied as therapeutic agents for AKI therapy.Last but not least the potential nephrotoxicity of these nanotherapeutic agents is discussed.
2.Nanotherapeutics for acute kidney injury
2.1.Improve the properties of potential drugs
Lots of chemical agents,biological agents and immunotherapy agents,including antioxidants,cytokines,growth factors and microRNAs,have been shown to prevent or alleviate AKI in animal experiments or clinical studies[63–66] .However,due to deficiencies such as poor solubility,in vivo
instability,low bioavailability rapid clearance and possible adverse effects,some agents have limited their application.Therefore,researchers have tried to overcome these problems of agents with nanotechnology and the use of nanomaterials,including lipids,modified biomacromolecules,polymers,to enhance the properties of potential drugs have been proved to be promising [67–69] .Here are some representative examples.2.1.1.Improved
water
solubility
Because of the important role of oxidative stress and inflammation in the development of AKI,anti-oxidation and anti-inflammatory have received great attention during the treatment [70] .Flavonoids including eupafolin,curcumin,kaempferol,myricetin,apigenin and luteolin,have excellent anti-oxidant and anti-inflammatory effects [71,72] .However,due to the low water solubility and permeability,flavonoids are extremely limited in the treatment of AKI.Nanocarriers can improve the availability and efficacy of flavonoids [73] .Zhang and colleagues prepared water-soluble eupafolin NPs using polyvinyl alcohol (PVA) and Eudragit E100,which can resolve the physicochemical drawbacks of the original eupafolin,and improve the water solubility by reducing the particle size,with no toxicity to normal renal cells.Eupafolin NPs could inhibit LPS-induced kidney damage with better anti-oxidant and anti-inflammatory activities[40] .Resveratrol is a non-flavonoid polyphenol compound with strong antioxidant capacity but also poor water solubility.Researchers have prepared resveratrol-loaded NPs using polymers,such as poly(N-vinylpyrrolidone)-bpoly(epsilon-caprolactone) polymer and PVP-b-PCL diblock
copolymer,which significantly improved the water solubility of resveratrol and could prevent ischemia/reperfusion renal injury in rats [41,42] .
2.1.2.Prolonging
drug
action
Mitochondrial dysfunction is important in the occurrence and development of AKI [74,75],the biogenesis of mitochondria is critical in the recovery of renal function [76] .Mito-2,2,6,6-tetramethylpiperidine-n-oxy (tempo) (MT) is an antioxidant against mitochondria that has shown benefits to AKI.However,it has short-term half-life and side effect.To resolve the deficiencies,Zhao et al.developed a carrier based on the self-assembling peptide (SAP) to slowly release MT for longer-time protective effect on AKI.Since MT-loaded SAP has extended mitochondrial protection,it shows a better effect of ameliorating ischemia/reperfusion induced-AKI compared to free MT [43] .
2.1.3.Enhance
in
vivo
stability
of
drugs
Numerous biological agents have been studied in AKI therapy,such as growth factor and antibody.Nanotechnology can be used to achieve controlled release and protect these sensitive cargoes from degradation [77] .In one study,self-assembling peptide/heparin (SAP/Hep) hydrogel was employed to codeliver hepatocyte growth factor (HGF) and TNF-α neutralizing antibody (anti-TNF-α),which could control the release of two drugs,allowed for a faster release of anti-TNF-α with a sustained release of HGF,prevented two drugs from rapidly degradingin
vivo
and preserved their bioactivities [44] .This nanoscale delivery platform could achieve effective delivery of two easily degradable drugs and reduce inflammatory and promote tissue repair in the ischemia/reperfusion induced-AKI mice.2.2.Specific targeted delivery of drugs to the kidney
The need for kidney-targeted drugs is based on the relatively poor pharmacokinetic characteristics,including shorter renal retention time,lower disease site accumulation,and systemic off-target effects [78] .Designing drugs-loaded NPs that can specifically accumulate in the kidneys is a promising strategy to alleviate AKI [79] and the NP-based methods to specifically deliver molecules and increase the retention in the kidney are starting to be explored in animal models.
Due to the strict selectivity of the kidney,only limited nanomaterials and macromolecular are used for kidneytargeted drug delivery,such as poly (vinyl pyrrolidone dimethyl maleic acid copolymer (PVD),polyglutamate,low molecular weight chitosan (LMWC),low molecular weight proteins (LMWP),liposomes,biopolymers,NPs,prodrugs and specific antibodies [30,73,80,81] .The applications of kidney-targeted drug delivery system for AKI therapy will be discussed in detail from two aspects:passive or active targeting in the following section.
2.2.1.Passive
target:
size,
shape
and
charge
Size
:Glomerular filtration membrane has strict size selectivity and NPs with a diameter less than 5–7 nm can easily pass through the glomerular filtration barrier (GFB).Therefore,one strategy is to design small enough NPs to pass through the GFB and then be absorbed by epithelial cells [82] .Uchida and group reported a very small protein-based nanoparticle,a DNAbinding protein (Dps),which is a hollow dodecameric protein cage with putative cytoprotective properties.The diameter of shell is 9 nm and the inner diameter is 5 nm.Dps is easily filtered and could pass through the GFB to reach and accumulate in the proximal tubules [45] .When AKI occurs,the particle size selection of GFB seems to be different.During the occurrence and development of AKI,the glomerular endothelial cell lining and podocyte structure are destroyed.Significant changes in structure and permeability of GFB may allow much larger substances passing through,and these characteristics can be used for the transport of NPs to various renal cells [83–85] .An excellent study has shown that 100 nm poly (lacticco-glycolic acid) (PLGA) NPs can specifically accumulate in injury kidneys of mice,which is related to the renal injury degree and administration time at the initial stage of ischemia/reperfusion-induced AKI (IR-AKI).NPs were internalized by the epithelial cells of renal tubules with strict particle-size selection.Specifically,100 nm PLGA NPs accumulate significantly in injured kidneys,while 200 nm and 300 nm PLGA NPs hardly accumulate.Thus 100 nm PLGA oltipraz NPs can selectively target the injured kidneys during the early stage of IR-AKI and continuously release oltipraz to effectively treat AKI [46] (Fig.1).
Interestingly,the accumulation of drugs in peritubular capillaries seems to have little to do with size,showing distinct absorption characteristics in different parts of the nephron [86] .Unlike the glomerular filtration membrane,which is a physical barrier to NPs,proximal tubules act as a chemical barrier due to their active endocytosis mechanism.A study indicated that NPs with a diameter of 400 nm preferentially localize in the kidney and specifically accumulate in the proximal renal tubules.Since such large NPs are difficult to pass through the GFB,the mechanism may be that NPs are endocytosed by the endothelial cells of the peritubular capillaries,and thereby accumulate in the proximal tubules for a long time [87] .Based on this mechanism,Sang JunHan and colleagues developed a mesoscale nanoparticle-mediated Toll-like receptor 9(TLR9) antagonist kidney-targeted drug delivery system for ischemia/reperfusion injury (IRI).After intravenous injection,these polymer-based mesoscale NPs with a diameter of 300–400 nm could be localized in the renal tubules to effectively alleviate AKI [48] .
Shape
:Besides suitable particle size,NPs with special shapes may also be able to pass through the GFB.Ammoniumfunctionalized single-walled carbon nanotubes (fCNTs) have a very favorable renal glomerular filtration and clearance characteristics [88] and part of filtered fCNTs are reabsorbed at the renal proximal tubular cell brush border and endocytosed[89] .In the cisplatin-induced AKI model,fCNTs with 5-nmdiameter were used to deliver drugs to the renal tubules.Compared with the control group,fCNT-mediated delivery increased the siRNA renal accumulation by 10 times,and the portion of the dose that was not delivered to the kidney was quickly eliminated (<
1 h) [49] (Fig.2).In addition to fCNTs,DNA origami nanostructures (DONs) with tubular,triangular and rectangular shapes have also been proved to preferentially accumulate in kidneys of rhabdomyolysisinduced AKI mice [33] .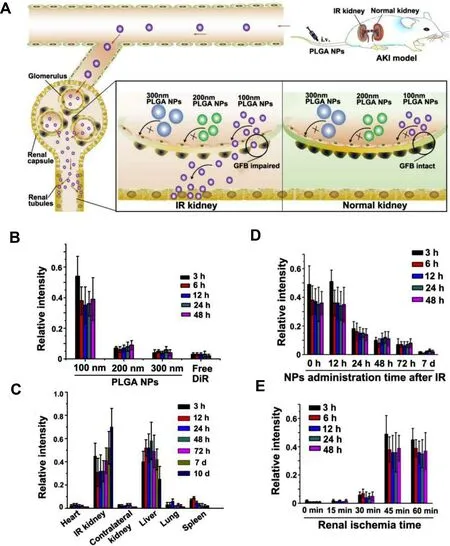
Fig.1–In vivo distribution of PLGA-oltipraz nanoparticles.(A) Application of PLGA-oltipraz NPs for effective treatment of IR-induced renal injury (RIRI).(B) Distribution of PLGA NPs with different particle sizes in IR kidneys after intravenous injection immediately after surgery.(C) Distribution of 100 nm PLGA NPs in the main organs after injection in RIRI mice.(D) Distribution of 100 nm PLGA NPs in IR kidneys after injection with 100 nm PLGA NPs at different time points after surgery.(E) Distribution of 100 nm PLGA NPs in IR kidneys after injection with different renal ischemia time immediately after surgery.(n=3).(Reproduced with permission from [46] (Fig.1).Copyright 2019 Elsevier).
Charge
:In addition to size selectivity,glomerular filtration membrane also has charge selectivity.Endothelial cell glycocalyx,glomerular filtration membrane and podocyte glycocalyx are negatively charged,allowing positively charged NPs to pass through faster than neutral NPs,followed by negatively charged NPs.In one study,a series of fluorescentlabeled polymers with different molecular weights and charges were synthesized to investigate the relationship between renal filtration efficiency and the charge of NPs.The results showed that the polymers with a negative charge and neutral had the greatest potential of selective accumulation in IRI kidney.These polymers pass through the glomeruli and stay in the proximal tubular cells for up to 24 h after injection [50] .However,some studies contradict the general understanding of charge -dependent glomerular filtration,which reported highly negatively charged NPs are cleared faster than less negatively charged NPs [90] .Therefore,further research is required to draw a more general conclusion.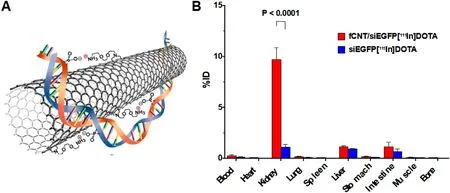
Fig.2–(A) An illustration of the noncovalent bonding interactions between an fCNT and a siRNA (n.b.not to scale).(B) Biodistribution of fCNTs in a panel of tissues expressed as%ID.(n=5) (Reproduced with permission from [49] (Fig.2).Copyright 2016 Elsevier).
2.2.2.Active
targeting:
conjugation
and
sensibility
Active targeted drug delivery systems have gained widespread attention,and researchers try to improve the targeting efficiency by screening for suitable targeting molecules and designing nano-systems with lesion microenvironment responsiveness [90-92] .We will discuss the application of active targeting in AKI therapy from these two aspects.
Conjugation
:Compared to NPs with physiochemical properties such as suitable size and charge,NPs conjugated with targeted ligands have higher specificity [32],which is desirable to achieve targeted therapy of diseased cells,and minimize off-target toxicity [93–95] .Megalin and Cubilin,located in the proximal renal tubules,are the major glycoproteins for reabsorption and are also the most concerned receptors for targeting the renal tubule system.So far,multiple targeting ligands have been exploited,including peptides,aptamers,small organic molecules and antibodies.There have been several studies of widely used kidneytargeted peptides,including Epsilon poly-l-lysine-derivatives(ε
PLL),galectin-3 carbohydrate recognition domain (G3-C12),elastin-like polypeptides (ELPs) and the carrier peptide(KKEEE) 3 K [96,97] .In the AKI pathology,a variety of cells,such as inflammation-related cells (e.g.macrophages) and vascular endothelial cells,are activated and undergo a series of changes,which characterized by the expression of specific receptors in most cases.Here,we focus on cases of using these specific receptors to achieve efficient targeted drug delivery.
Microbubble (MV) is a kind of small vesicle that falls off the cell membrane after cell activation,injury or apoptosis.Researchers incubated raw macrophage with dexamethasone(DEX) and then separated MV-DEX from the supernatant by centrifugation.There is an obvious integrin expression pattern on the surface of MV-DEX,in which integrin α 4 β 1(val-4) and α L β 2 (LFA-1) enable them to target the kidney with inflammation.Therefore,MVs derived from RAW 264.7 macrophage cells can be used as DEX carriers targeting the inflamed kidney efficiently [51] .Compared with conventional DEX treatment,MV-DEX can significantly reduce renal injury,and has enhanced therapeutic effect on nephritis and fibrosis in LPS-induced nephrotic rat model,and significantly reduce side effects of chronic glucocorticoid treatment.
In IR-AKI,E-selectin overexpression on the surface of vascular endothelial cells could be exploited as a promising target for specific-target drug delivery [99] .Hu and colleagues have reported that solid lipid NPs with sialic acid (SA-NPs)as targeting-vascular endothelium carriers for IR-AKI.Cell uptake results indicated that SA-NPs were specifically uptake by inflamed vascular endothelial cells (HO-stimulated human umbilical vein endothelial cells),which is related to the specific binding of SA and E-selectin receptors expressed on the inflamed vascular endothelial cells [52] .Furthermore,the biodistribution results showed that SA-NPs increased the accumulation of DXM in kidneys of AKI mice.After 6 h of intravenous injection of SA-NPs,the content of DXM in the kidneys of mice was 5.88 and 2.70 times higher than those injected with NPs and free DXM,respectively (Fig.3).
During renal IRI,CD44,a transmembrane proteoglycan,is upregulated in injured renal tubular epithelial cells,which is the most important hyaluronic acid (HA) receptor on the cell surface.Based on the inherent characteristics of HA targeting CD44 receptor,Hu et al.developed a polymer prodrug(HA-curcumin,HA-cur) for targeting epithelial cells and the bio-accumulation of HA-cur in the kidney increased by 13.9 times compared to the free curcumin.It is a novel and effective drug delivery system for the application of HA-based polymer prodrugs and has great potential in the treatment of kidney diseases [53] .In another study,Liu and colleagues designed kidney-targeted nanopolyplexes also based on HA targeting CD44 receptor to effectively deliver mitochondrial antioxidant,D-Arg-dimethylTyr-Lys-Phe-NH 2 (SS-31).These nanopolyplexes are electrostatically complexed using anionic hyaluronic acid and cationic chitosan as materials,which could be uptake by CD44-overexpressed cells and accumulate in injured areas [54] .
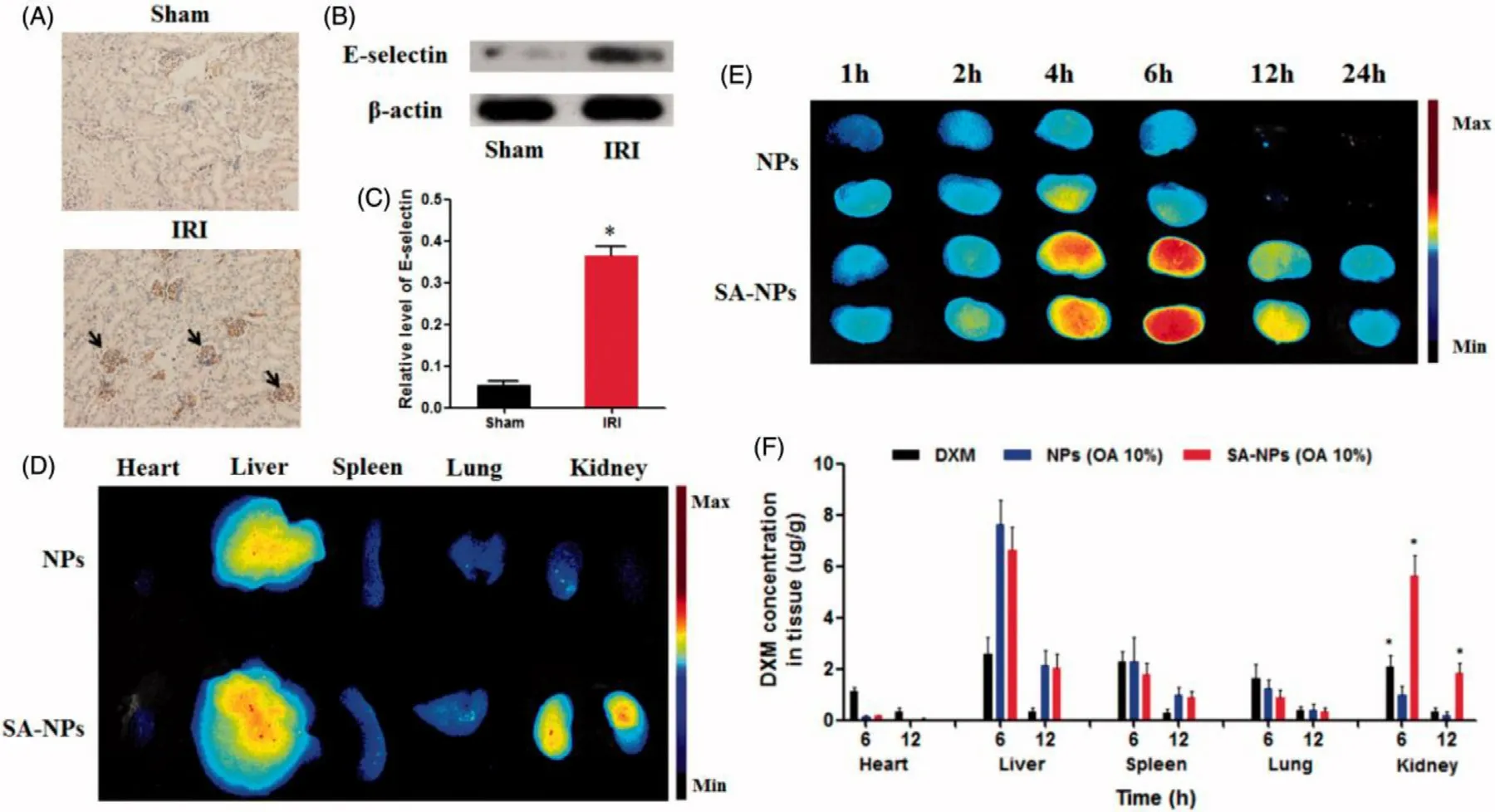
Fig.3–The overexpression of E-selectin in IR-induced injured kidney and the renal targeted distribution of NPs with SA modification.(A) Representative immunohistochemical images of E-selectin in kidneys of healthy and IRI mice.(B and C)Western blot analysis of E-selectin protein expression in kidneys of healthy and IRI mice.(D) The fluorescent signal image of major organs of IRI mice treated with ICG-labeled NPs and SA-NPs at 6 h after injection.(E) The fluorescent signal image of kidneys of IRI mice treated with ICG-labeled NPs and SA-NPs at different times.(F) The distribution of SA-NPs,NPs and DXM in IRI mice at 6 and 12 h after injection at a dose of 1.2 mg/kg DXM,respectively.(n=3) (Reproduced with permission from [52] .Copyright 2017 Elsevier).
Sensibility
:Changes of kidney microenvironment,such as excessive ROS,increased enzymatic activity,or lower pH,are expected to be used for drug active targeted delivery.By being endowed with environmental responsiveness,drug delivery systems can gather more intelligently at the lesion site and release drug specificity.In fact,any design of multifunctional drug nanocarriers should include the function of making the carriers release drugs in the target area and keep stable in the non-target area to ensure treatment efficiency and reduce the systemic side effects [12] .Nano-drug delivery systems or prodrugs that can selectively target kidneys usually release drugs through the action of renal enzymes or the microenvironment changes at the pathological site.For example,based on the overproduction of matrix metalloproteinase-2 (MMP-2) in kidneys during AKI,Hu and his colleagues developed an MMP-2 enzyme-triggered polymerized prodrug.The use of the MMP-2 reactive peptide PVGLIG gives the polymerization prodrug the ability to release curcumin quickly after reaching the target and increases the drug concentration in the target tissue [55] .Besides,since AKI is often associated with acidosis,Yoshitomi et al.designed a pH-responsive nitroxide radical-containing NPs,which could disintegrate within the renal acidic lesion and scavenge excessive ROS to relieve AKI[56] .
2.3.Inorganic nanoparticles as antioxidant and contrast agent
In addition to being drug carriers,NPs are also one of the research hotspots as directly effective and low-toxicity drugs.Inorganic NPs have the advantages of excellent thermal stability and biological stability,versatility,easy preparation,and adjustable activity [99],which therefore have been widely used in chemical,biological,and pharmaceutical fields.In recent years,certain inorganic NPs have been applied to various ROS related-diseases,including AKI because of their unique capacity of scavenging ROS,including platinum [100],ceria [101–102],and so on.
Molybdenum can scavenge harmful ROS due to the volatile state of molybdenum ions (between Moand Mo).Dalong Ni and colleagues reported molybdenum-based polyoxometalate (POM) nanoclusters as a new type of nanoantioxidants to protect kidney with preferential renal uptake.The results indicated that with excellent compatibility,these ultrasmall POM nanoclusters (average diameter about 1 nm)had high renal uptake capacity and could efficiently alleviate kidney injury in AKI mice,as confirmed by dynamic PET imaging,renal biomarkers detection,serum tests and kidney tissue staining [57] .
Ceria NPs could remove ROSvia
superoxide dismutase(SOD)-mimetics and catalase (CAT)-mimetics and have regenerated ROS-elimination ability.Manne et al.have investigated the therapeutic efficacy of ceria NPs in the treatment of peritonitis-induced AKI by polymicrobial insult.The results showed that ceria NPs with size between 10–40 nm,could reduce the damage of glomeruli and renal tubules caused by peritonitis,through reaction with excessive ROS in the kidneyin
vivo
[58] .Ceria NPs were also proved to exhibit a protective effect on cisplatin-Induced nephrotoxicity and further research is needed on the potential use of ceria NPs in AKI induced by other factors including sepsis [103] .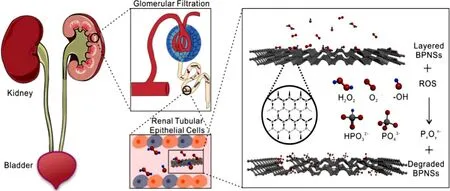
Fig.4–Black phosphorus nanosheets (BPNSs) are used as ROS scavengers to cure AKI in mice.(Reproduced with permission from [61] .Copyright 2020 Elsevier).
Selenium is a natural trace element and plays a key role in the activity of several enzymes and proteins,including glutathione peroxidase.In glycerol-induce AKI mice model,selenium NPs could reduce the biochemical,molecular,and histological changes,which suggested that it could prevent the development of AKI through antiinflammatory,antioxidant and anti-apoptotic activities [59] .In another study,porous Se@SiO 2 nanospheres synthesized by nanotechnology could also directly or indirectly scavenge free radicals and ROS in cells to inhibit oxidative stress [60] .
Black phosphorus nanosheets (BPNSs) is a new type of two-dimensional material with strong chemical reactivity towards ROS.A research group has designed a new BPNSsbased drug delivery platform for AKI therapy.BPNSs has kidney-targeted drug delivery ability because of the same geometrical framework as flake-like DNA and has the capacity of scavenging excessive ROS.Moreover,BPNSs will degrade into phosphorous oxides without any cytotoxicity after treatment.With these advantages simultaneously,BPNSs is promising in AKI therapy [61] (Fig.4).
In addition to application as antioxidants,inorganic NPs may be more clinically valuable to be used in diagnosis.Early diagnosis of AKI is important for treatment,but it is often difficult.Currently,the diagnosis of AKI is mostly based on the measurement of blood urea nitrogen and creatinine levels,which has numerous problems,including non-specificity and delay rise of these elements [104] .Fortunately,the specific nano-biological interactions andin
vivo
transport of inorganic NPs that cleared by the kidney provide an opportunity for the detection of early renal diseases,especially,the early detection of dysfunctional renal tubules [105–108] .Nanoparticle-based magnetic resonance imaging (MRI) has been used as a strategy to detect early changes that may be consistent with AKI.One study found that in combination with MRI,dextran-coated iron oxide NPs (HD=20 nm) can be used for early diagnose of kidney injury while conventional biomarkers,such as creatinine,fail to report [109]At present,integrated therapy of diagnosis and treatment is more attractive and also under exploration.Tuanwei Sun and colleagues synthesized ultrasmall Mn+chelated melanin (MMP) NPs through simple coordination and selfassembly strategies,which were further combined with polyethylene glycol (PEG).The final NPs (MMPP) have ultra-small size,good stability,high R1 relaxation,stable radiolabeling performance,and great antioxidative ability.In the mouse AKI model,T1-weighted magnetic resonance(MR) and positron emission tomography (PET) imaging could revealin
vivo
circulation of MMPP NPs,and MMPP NPs could efficiently scavenge ROS to alleviate AKI [62] (Fig.5).In another study,a theranostic nanoparticle for siRNA delivery and multimodal imaging was developed.The core was formed by encapsulation of superparamagnetic iron oxides and indocyanine green in a PLGA matrix and the surface was coated with polyethyleneimine (PEI) for siRNA delivery [63] .3.Nephrotoxicity of nanoparticles
The use of NPs provides promising results for AKI pharmacologic therapy.However,the nephrotoxicity of NPs is often a limit of drug delivery,which has not been extensively investigated [104] .Generally,the nephrotoxicity of NPs seems to be closely related to the chemical composition,size,surface charge and functionalization of NPs [110] .And there are disputes over the nephrotoxicity of nanomaterials because different studies may use different types of cellsin vitro
toxicity tests,routes of exposure and so on.Previous studies have shown that nanomaterials that exhibit excellent renal clearance could avoid the toxicity problems that may result from the long-term accumulation of nanomaterials in the body [89] .Although some studies highlight some of the properties of NPs and how they may affect the kidney,there are noin
vivo
human studies that particularly investigate the nephrotoxicity of these biological NPs.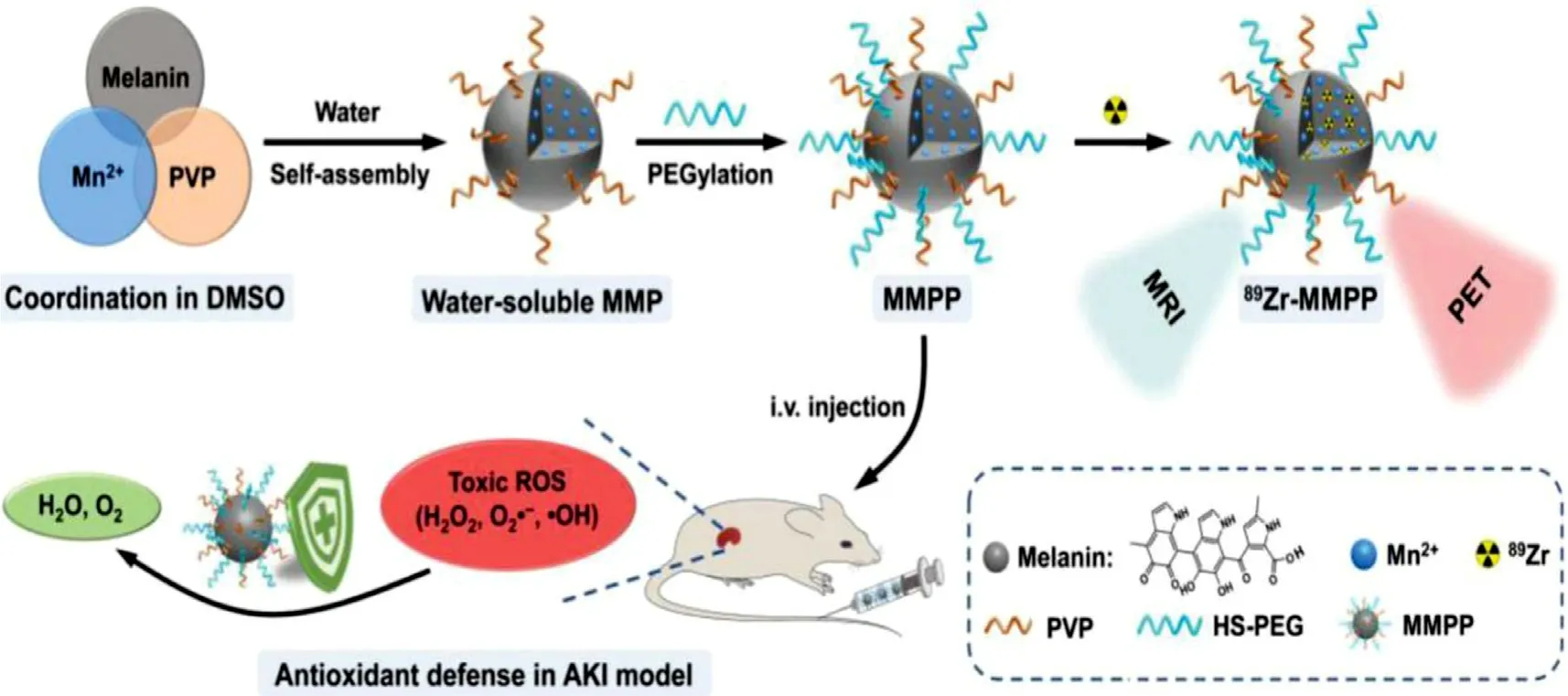
Fig.5–The ultrasmall Mn 2+ chelated melanin (MMP) NPs can simultaneously achieve MRI and effectively scavenge ROS to alleviate AKI.(Reproduced with permission from [62] .Copyright 2019 Elsevier).
4.Conclusions and prospects
In recent years,researchers have made remarkable achievements in the treatment of various diseases based on nanotechnology and increasing preclinical work has also shown its potential in AKI therapy.At present,no specific drugs have been employed to relieve AKI in the clinic,and some potential drugs have been greatly hindered from clinical application by several shortcomings,including low bioavailability rapid clearance and severe systemic side effects.The nanocarriers under research are excellent in improving potential drug properties,increasing bioavailability,selective accumulation,controlling drug release,and reducing systemic side effects,which have shown to significantly improve the efficacy of potential drugs in alleviation AKI in experimental models.
There have been some reviews summarizing the application of nanotechnology in kidney-targeting drug delivery systems,but rarely specifically related to diseased kidney.AKI involves a large number of signal molecules,multiple pathways and different cell types,and its pathological mechanism has not been fully understood so far.Since the pathological mechanism of AKI is very complicated,it is necessary to take the differences compared with healthy kidney into consideration,including changes in filtration,renal tubular reabsorption capacity,and microenvironment.Therefore,we outline the recent nanotherapeutics in AKI,including encapsulation and delivery of water-insoluble molecules and easily degradable agents,employing the filtering characteristics of kidneys in AKI and modification with ligands target to molecules highly expressed under inflammatory condition,such as CD44 to achieve passive and active targeting,as well as inorganic NPs as directly excellent antioxidants and application in the integration of diagnosis and treatment.
Currently,there are no clinically approved kidneyspecific nanotherapeutics.But with the further study of the pathogenesis of AKI and the understanding of the mechanism of NPs interaction and transport clearance in the body.We expect to see more and more applications of nanotherapeutics in AKI.
Conflict of interest
The authors report no conflicts of interest.
Acknowledgments
This work was supported by New Century 151 Talent Project of Zhejiang Province;Joint Institute of Lishui Hospital and Zhejiang University for nanomaterials and nanotechnology.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- An understanding of mitochondria and its role in targeting nanocarriers for diagnosis and treatment of cancer
- An insight into the in vivo imaging potential of curcumin analogues as fluorescence probes
- Size,shape,charge and“stealthy”surface:Carrier properties affect the drug circulation time in vivo
- Macrophage membrane-mediated targeted drug delivery for treatment of spinal cord injury regardless of the macrophage polarization states
- The effect of ethanol on the habit and in vitro aerodynamic results of dry powder inhalation formulations containing ciprofloxacin hydrochloride
- Targeting the resolution pathway of inflammation using Ac2–26 peptide-loaded PEGylated lipid nanoparticles for the remission of rheumatoid arthritis