Biscaesalmins A and B from Caesalpinia minax,highly oxidized dimeric cassane diterpenoids as interleukin-1β inhibitors
2021-10-14YunshoXuTinZhngLuFengZhelingFengQingwenZhngYngYeLisheGnLigenLin
Yunsho Xu,Tin Zhng,Lu Feng,Zheling Feng,Qingwen Zhng,Yng Ye,c,Lishe Gn*,Ligen Lin,*
a State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Avenida da Universidade, Taipa,Macau 999078, China
b State Key Laboratory of Drug Research, & Natural Products Chemistry Department, Shanghai Institute of Materia Medica, Chinese Academy of Sciences,Shanghai 201203, China
c School of Life Science and Technology, Shanghai Tech University, Shanghai 201203, China
d School of Biotechnology and Health Sciences, Wuyi University, Jiangmen 529020, China
1 These authors contributed equally to this work.
ABSTRACT To search naturally occurring interleukin-1β(IL-1β)inhibitors,biscaesalmins A(1)and B(2),two highly oxidized dimeric cassane diterpenoids with a newly formed alicyclic skeleton, have been isolated from the traditional Chinese medicine Kushilian(Caesalpinia minax).Their full structures were determined by comprehensive spectroscopic analysis and quantum chemical TD-DFT (time-dependent density functional theory) calculation.Biosynthetically, 1 and 2 were formed via an intermolecular [4+2]Diels-Alder cycloaddition of two monomers,affording an additional six-membered carbon ring linkage.Compounds 1 and 2 inhibited nitric oxide production on lipopolysaccharide-stimulated THP-1 macrophages, with IC50 values being at 1.20±0.23 and 2.30±0.15 μmol/L, respectively.Furthermore,compound 1 inhibited NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) inflammasomemediated IL-1β production and blocked the migration of macrophages towards adipocyte conditioned medium.Biscaesalmins A and B might be candidates for treating inflammation-related metabolic diseases.
Keywords:Caesalpinia minax IL-1β Dimeric cassane diterpenoids NLRP3 TD-DFT
Interleukin-1β (IL-1β) is a major pro-inflammatory cytokine produced mostly by macrophages; biologically active IL-1β is formed through cleavage of pro-IL-1β by NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) inflammasome-activated caspase-1 [1].IL-1β has been considered as the culprit of the inflammatory crosstalk between macrophages and adipocytes in obese objects [2].Exogenous stimuli, such as lipopolysaccharide(LPS)and excessive lipids,promote the production and secretion of IL-1β in macrophages,which in turn induces local inflammation in adipocytes, resulting in systematic inflammation and insulin resistance.Thus, inhibition of IL-1β production and secretion might put a brake on the vicious cycle of macrophage infiltration and escalate inflammatory response in adipose tissue, thereby improving insulin sensitivity and metabolic disorders.
Naturally occurring diterpenoid dimers are a rare group of complex metabolites usually featuring an ester bond, ether bond,C--C bond or a ring linkage between two monomers[3,4].Due to their intriguing architectures and broad bioactivities,isolation and synthesis of diterpenoid dimers have been the hot spots in organic chemistry communities.Cassane diterpenoids are tricyclic diterpenoids with one or two carbon side chain on the C ring,existing as the characteristic constituents of many medical plants in the Caesalpinia,Erythrophleum and Cylicodiscus genera(Fabaceae),and possess a wide range of bioactivities,including anti-inflammatory,antitumor and anti-viral properties [5-7].Up to now, over 450 cassane diterpenoids have been identified and only one dimer with an additional six-membered carbon ring linkage, cyclodione, was reported [8].
Caesalpinia minax is widely distributed throughout tropical and subtropical areas, and many parts of this plant, including seeds,leaves and roots,are used for ethnomedicinal purposes[9-11].The seeds of C.minax, known as Kushilian in traditional Chinese medicine, are frequently applied for treating anemopyretic colds,dysentery, and skin itching and sores.Chemical investigations on this species have resulted in the identification of more than 150 cassane diterpenoids[5].In our ongoing efforts in the search for IL-1β inhibitors, the seeds of C.minax were thoroughly investigated,which led to the discovery of two highly oxidized dimeric cassane diterpenoids with a newly formed alicyclic skeleton,biscaesalmin A(1)and B(2).The diterpenoid dimers possess an additional sixmembered carbon ring with C-17-C-15′and C-15-C-16′linkage through a[4+2]Diels-Alder cycloaddition.Herein,we describe the isolation and full structural elucidation of these compounds, and their inhibitory effect of IL-1β production on LPS-induced THP-1 macrophages.
The air-dried seeds of C.minax (15 kg) were ground and extracted with 95% ethanol thrice (7 days each).The pooled extracts were concentrated under reduced pressure to yield a residue (1.58 kg), which was then suspended in water (5 L) and extracted with petroleum ether (PE), chloroform, and EtOAc,successively.The chloroform fraction (86 g) was further purified with MCI gel and preparative HPLC to give compounds 1(4.9 mg)and 2 (11.6 mg) (Fig.1).
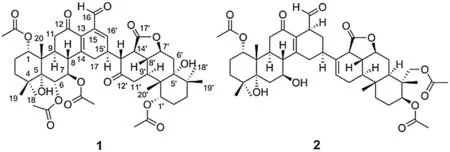
Fig.1.Structures of biscaesalmins A (1) and B (2).
Biscaesalmin A(1)was obtained as a white amorphous powder.Its molecular formula,C48H62O15,was deduced by the ion peak at m/z 879.4151[M+H]+in the HR-ESIMS,which indicated 18 degrees of unsaturation.The IR spectrum suggested the existences of hyroxy group (3573 cm-1) and carbonyl groups (1742 and 1677 cm-1).The13C and DEPT NMR spectra of 1 showed 48 resonances,ascribed to 10 methyls,8 methylenes,14 methines(twelve sp3and two sp2),and 16 quaternary carbons(six sp3and ten sp2)(Table S1 in Supporting information).The1H NMR spectrum showed signals for one aldehydic proton[δH9.69(s,H-16)],one olefinic proton[δH6.42(d,J=3.5 Hz,H-16′)],five oxygenated methine protons[δH5.54(d,J=8.2 Hz,H-6),5.41(m,H-7),4.84(t,J =2.9 Hz,H-1),4.80(m,H-1′),and 4.57(td,J=10.9,4.9 Hz,H-7′)],and ten tertiary methyls[δH2.18(s,OCOCH3-1′),2.12(s,OCOCH3-1),2.07(s,OCOCH3-6),2.01(s,OCOCH3-7),1.28(s,CH3-20),1.17(s,H3-20′),1.12(s,6H,H3-18/19),1.11(s,H3-19′),and 1.07(s,H3-18′)](Table S1).The1H and13C NMR data of 1 indicated the presence of one aldehyde group,two ketone groups,two double bonds,and four acetyl groups in the structure of 1.All the above evidence suggested that 1 might contain two units of cassane diterpenoids.
The linkage of the two diterpenoid units was deduced by detailed analysis of the1H-1H COSY, HSQC, and HMBC spectra(Fig.2).In the first unit, two moieties were constructed by the1H-1H COSY correlations,H-1-H2-2-H2-3 and H-6-H-7-H-8-H-9-H2-11.The HMBC correlations from H-9 to C-1,C-5 and C-20,H3-18/H3-19 to C-3, C-4 and C-5, and H3-20 to C-1, C-5, C-9 and C-10 connected the two moieties to form a typical tricyclic ring system in cassane diterpenoid.Three acetyl groups were assigned at C-1,C-6 and C-7, respectively, based on the corresponding HMBC correlations and low-field proton signals at δH4.84(H-1),5.54(H-6), and 5.41 (H-7).The ketone carbonyl carbon at δC195.1 was inferred as C-12 by the HMBC correlations from H-9 and H2-11 to this carbon.The above signals quite resembled those of caesalpin B[10].In the second cassane unit,a small moiety,H-1′-H2-2′-H2-3′,and a big moiety, H-6′-H-7′-H-8′(-H-9′-H2-11′)-H-14′-H-13′-H-15′(-H2-17)-H-16′,were constructed by1H-1H COSY correlations.The fourth acetyl group was assigned at C-1′according to the HMBC correlations and low-field proton signal at δH4.80 (H-1′).The HMBC correlations from H-8′,H-13′and H-14′to the carbonyl carbon at δC174.7, as well as from H2-11′, H-13′and H-14′to the ketone carbonyl carbon at δC207.4, allowed the assignment of C-17′and C-12′, respectively.The aforementioned functionalities occupy 16 of the 18 degrees of unsaturation in 1,the remaining two degrees of unsaturation might be ascribed to two ring moieties.
The HMBC correlations from H-9 to C-14,from H2-17 to C-8 and C-13, from H-15′to C-14, and from H-16′to C-13, C-14 and C-15,constructed a 1,3-cyclohexadiene ring.The aldehyde group was inferred to link at C-15 by the HMBC correlations from the aldehyde proton to C-13, C-15′and C-16′, and from H-16′to the aldehyde carbon.The remaining ring was deduced as a γ-lactone between C-7′and C-17′due to the downfield chemical shift of H-7′and C-7′,and the molecular weight of 1.Thus,the gross structure of biscaesalmin A was determined.
The relative configuration of 1 was established through analysis of ROESY spectrum (Fig.2).The strong NOE correlations between H3-20 and H-1, H-6 and H-8, as well as between H3-20′and H-1′and H-8′,revealed that the acetyl groups at C-1,C-6 and C-1′are αoriented, and H-8, H3-20, H-8′and H3-20′are β-oriented, and confirmed the same relative configuration of the two monomers from biogenesis point of view.The NOE correlations of H-7/H-9,H-7′/H-9′,and H-9′/H-14′suggested that the acetyl group at C-7 and the γ-lactone ring between C-7′and C-14′as being β-oriented,as well as H-9 and H-9′as being α-oriented.The NOE correlations between the active proton OH-5 and H-7 and H3-18, as well as between OH-5′and H-7′and H3-18′,supported the α-orientation of OH-5 and OH-5′.Furthermore, the NOE correlations of H-9/H-15′and H-8′/H-13′supported the α-orientation of H-15′and βorientation of H-13′, respectively.A potential energy surface scan on the dihedral angle of C-12′-C-13′-C-15′-C-17 was further carried out to confirm the free rotation of the C-13′-C-15′linkage bond between the two monomer parts by modredundant optimization at the semi-empirical AM1 level in Gaussian 09 program [12].The energy barrier ΔG (relative Gibbs energy) is lower than 20 Kcal/mol(Fig.S2 in Supporting information),which implies no atropisomers exist in 1 [13].In order to further determine the absolute configuration of 1, the theoretical electronic circular dichroism (ECD) was calculated and compared with the corresponding experimental data (for details of the calculation, see Supporting information).In the 200-400 nm region (Fig.3), both the experimental and theoretical ECD curves showed small negative first Cotton effects around 330 nm,positive second Cotton effects around 295 nm, and negative last Cotton effects.The theoretical ECD spectrum of the enantiomer of 1 showed opposite values comparing with the experimental one.Therefore, qualitative analysis of the above data allowed the assignment of the absolute configuration of 1 as shown.

Fig.2.1H-1H COSY, key HMBC, and ROESY correlations of compounds 1 and 2.
Biscaesalmin (2) was isolated as a white amorphous powder.The ion peak at m/z 807.4318 [M+H]+in the HR-ESIMS indicated that 2 has a molecular formula of C46H62O12, with 16 degrees of unsaturation.The1H and13C NMR spectra of 2,together with the IR spectrum,showed the existence of one aldehyde group,one ketone carbonyl group, tow double bonds, three acetyl groups, and six singlet methyls in the structure of 2 (Table S2 in Supporting information).The detailed analysis of1H-1H COSY, HSQC, and HMBC data allowed full construction of the core skeleton of compound 2,which was the same as 1(Fig.2).Three acetyl groups were determined at C-1, C-3′and C-18′, respectively, according to the corresponding HMBC correlations and low-field proton signals at δH4.85(t,J=2.9 Hz,H-1),4.76(dd,J=11.5,4.5 Hz,H-3′),and 3.86/3.75 (d, J =12.0 Hz, H-18′).Next, a ROESY experiment was used to determine the relative configuration of 2 (Fig.2).The NOE correlations between H3-20 and H-1 and H-8 indicated the acetyl group at C-1 is α-oriented.The β-orientation of hydroxyl at C-7 and acetyl group at C-3′was inferred by the NOE correlations of H-7/H-9, and H-3′/H-5′and H-3′/H2-18′, respectively.The NOE correlations of H-5′/H-7′, H-7′/H-9′and H-9′/H-14′suggested the γlactone ring between C-7′and C-14′as being β-oriented.Additionally, the NOE correlations of H-7/H-15 and H-15/H-15′indicated the three protons face to the same direction and the formyl group at C-15 is β-oriented.Similarly, the PES (potential energy surface)scan and theoretical ECD calculation again allowed the assignment of the absolute configuration of 2(Fig.3).Thus,the structure of compound 2 was determined.
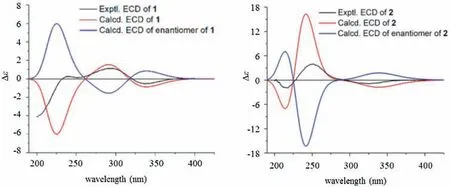
Fig.3.Theoretical (red lines, blue lines for their enantiomer's) and experimental (black lines) ECD spectra of compounds 1 and 2.
A plausible biogenetic pathway for compound 1 is proposed from two known compounds, caesalmins B and C, which were obtained in this plant in previous studies (Fig.S8 in Supporting information)[14,15].The furan ring in caesalmins B and C might be oxidized and opened to form the intermediator I and II,respectively.The mono-substituted alkene might participate in a[4+2]cycloaddition with diene to form a cyclohexene ring,which could be further oxidized to produce compound 1.When the two monomers were caesalmin M and caesalpinin ME[11,16],the same biogenetic pathway could result in the formation of compound 2.
Macrophage inflammation and infiltration into adipose tissue play a vital role in metabolic disorders[17].Many molecules have been reported to alleviate adipose tissue inflammation [18].The MTT assay indicated that compounds 1 and 2 did not show obvious cytotoxicity on THP-1 macrophages up to 10 μmol/L (Fig.S9 in Supporting information ).LPS significantly enhanced nitric oxide(NO) production in THP-1 cells, and compounds 1 and 2 concentration dependently inhibited NO production, with IC50values of 1.20±0.23 and 2.30±0.15 μmol/L,respectively(Fig.4a).As the more potent one,compound 1 was chosen in the following studies.The Western blotting results suggested that compound 1 reversed LPS-induced increase of inducible nitric oxide synthase(iNOS)expression in a concentration-dependent manner(Fig.4b).The nuclear factor-κB(NF-κB)signaling pathway is a key regulator of inflammatory response[19,20].LPS increased the phosphorylation of IκBα(inhibitor of NF-κB α),IKKα/β(IκB kinase α/β),and p65 remarkably, which was reversed by compound 1 (Fig.4b).The immunofluorescent staining results revealed that greater proportion of p65 translocated into the nuclei in LPS-stimulated THP-1 cells,while pre-treatment of compound 1 suppressed the nuclear accumulation of p65 (Fig.4c).
THP-1 macrophages were treated with or without compound 1,and then stimulated with LPS plus ATP to induce IL-1β secretion.Intriguingly, compound 1 dose-dependently alleviated LPS plus ATP-induced increase of IL-1β production and release,assessed by immunofluorescence staining (Fig.4d) and ELISA (Fig.4e),respectively.The NLRP3 inflammasome in macrophages modulates the maturation and release of IL-1β [1].As compound 1 inhibited the production of IL-1β,the monitoring of compound 1 on NLRP3 inflammasome activation was taken into consideration.As expected,compound 1 dose-dependently inhibited NLRP3 expression and caspase-1 activation in LPS plus ATP-induced THP-1 macrophages(Fig.4f).Furthermore,the migration capacity of THP-1 macrophages towards 3T3-L1 adipocytes conditioned medium(CM)was evaluated using a Transwell chemotaxis assay.Pretreatment of 1 to the macrophages prevented their migration towards adipocyte CM (Fig.4g).
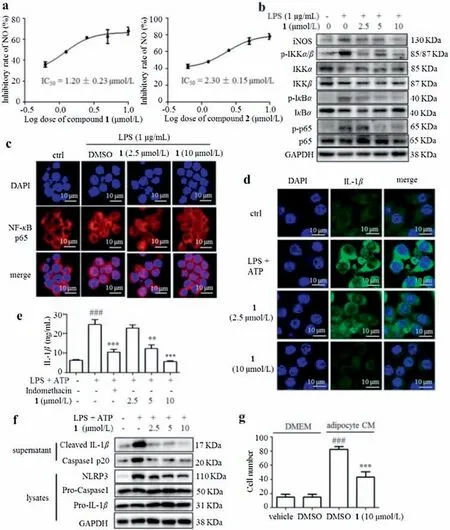
Fig.4.Compound 1 inhibited IL-1β production in THP-1 macrophages.(a)NO inhibitory rates for compounds 1 and 2.(b)The protein levels of iNOS,p-IKKα/β,IKKα,IKKβ,p-IκBα, IκBα, p-p65 and p65 were detected by Western blot analysis.GAPDH (glyceraldehyde 3-phosphate dehydrogenase) was used as an internal loading control.(c) The nuclear translocation of p65 was detected by immunostaining.Nuclei were stained with DAPI(4′,6-diamidino-2-phenylindole).Scale bar=10 μm.(d)Immunofluorescence staining of IL-1β was performed.Scale bar=10 μm.(e)The protein level of IL-1β in the culture medium from THP-1 cells was determined by ELISA.Data are expressed as means±SEM(n=6).###P <0.001 LPS+ATP vs.control,**P <0.01 and***P <0.001, 1 vs.LPS+ATP.(f)Cleaved IL-1β,NLRP3 and Caspase 1 in the supernatant or lysates of THP-1 cells were detected by western blotting.GAPDH was used as an internal loading control.(g)Migrated THP-1 cells towards adipocyte CM were quantified.Data are expressed as means±SEM(n=6).###P <0.001,DMEM(Dulbecco's modified Eagle's medium)+DMSO(dimethyl sulfoxide)vs.adipocyte CM+DMSO;***P <0.001,adipocyte CM+ 1 vs.adipocyte CM+DMSO.
Biscaesalmins A and B are highly oxidized dimeric cassane diterpenoids with a newly formed alicyclic skeleton by a cyclohexene ring through intermolecular[4+2] Diels-Alder cycloaddition, which established these heptane-ring system.The cyclohexene ring, constructed by concomitant formation of a mono-substituted alkene(C-15′-C-16′)of one cassane moiety and a diene(C-15-C-13-C14-C17)of the other,represents a new way to connect two diterpene halves.Furthermore, both compounds suppress NO secretion on macrophages,and compound 1 inhibits NLRP3 inflammasome-mediated IL-1β production on macrophages and attenuates the migration capacity of macrophages towards adipocytes.IL-1β plays a key role in the inflammatory crosstalk between macrophages and adipocytes; thus, compound 1 might alleviate adipose tissue inflammation through preventing macrophage accumulation.Taken together, our findings expand the linkage types of dimeric diterpenoids, offer new targets for synthetic chemists,and supply new candidates for treating adipose tissue inflammation and its related metabolic disorders.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
We are thankful for the financial support of the Science and Technology Development Fund,Macau SAR(No.FDCT 0031/2019/A1), the National Natural Science Foundation of China (Nos.81872754 and 81872756), and University of Macau, Macau SAR(Nos.MYRG2017-00109-ICMS and MYRG2018-00037-ICMS).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.09.048.
杂志排行
Chinese Chemical Letters的其它文章
- D-A-D structured selenadiazolesbenzothiadiazole-based near-infrared dye for enhanced photoacoustic imaging and photothermal cancer therapy
- Synthesis and biological evaluation of a lipopeptide-based methamphetamine vaccine
- Nucleic acids induced peptide-based AIE nanoparticles for fast cell imaging
- Titanate nanofibers reduce Kruppel-like factor 2(KLF2)-eNOS pathway in endothelial monolayer: A transcriptomic study
- Drug-induced hierarchical self-assembly of poly(amino acid) for efficient intracellular drug delivery
- Co-delivery of anticancer drugs and cell penetrating peptides for improved cancer therapy
