An AIE singlet oxygen generation system based on supramolecular strategy
2021-10-14MinznZuoWeiruiQinMinHoKiyWngXioYuHuLeyongWng
Minzn Zuo,Weirui Qin,Min Ho,Kiy Wng,Xio-Yu Hu,*,Leyong Wng
a College of Material Science and Technology, Nanjing University of Aeronautics and Astronautics, Nanjing 211106, China
b Key Laboratory of Mesoscopic Chemistry of MOE, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China
ABSTRACT The design of supramolecular systems with efficient singlet oxygen generation has attracted considerable interests.Herein,an AIE-based singlet oxygen generation system with chemiluminescence properties is reported in aqueous media based on supramolecular host-guest assembly between a water-soluble pillar[5]arene (WP5) and an AIE photosensitizer (TPEDM).The formed supramolecular nanoparticles exhibit significant singlet oxygen generation ability as well as enhanced fluorescence.In addition, by introducing catalase, this H2O2-responsive supramolecular system shows increased 1O2 generation efficiency compared with the blank nanoparticles.An efficient chemiluminescence system can also be achieved by entrapping an energy donor adamantane derivative(AMPPD).Moreover,the present system can function as nanoreactors to perform the photooxidation of dopamine to form polydopamine with visible light irradiation.This work provides a new strategy for the construction of 1O2 generation system based on supramolecular nanomaterials, which has potential applications in the fields such as chemiluminescence imaging and controlled photocatalysis.
Keywords:Aggregation-induced emission Singlet oxygen Self-assembly Chemiluminescence Photocatalysis
Singlet oxygen (1O2), a kind of reactive oxygen species (ROS),has attracted a lot of attention because of its broad applications in photodynamic therapy,sewage treatment, catalytic synthesis and so on [1-5].1O2generation is related to photosensitizer, light source, and molecular oxygen.Upon being irradiated with light,the photosensitizer is excited to the singlet state (S1), which can further switch to the excited triplet state (T1) through the gap crossing.Consequently, the energy is transferred to the triplet oxygen, generating reactive oxygen radicals [6-8].The common photosensitizers include BODIPY derivatives, porphyrin derivatives, and chlorin derivatives [9,10].Although various photosensitizers have been explored so far,most of them have a severe aggregation-induced quenching effect (ACQ).Due to their rigid planar structures,these dyes tend to aggregate in aqueous solution,resulting the energy consumption in a non-radiative manner[11-13].Thus designing of novel photosensitizers that can avoid these shortcomings in aqueous phase is urgently needed.
Photosensitizers with aggregation induced emission (AIE)effect have offered a good opportunity to address the aforementioned issues[14,15].Due to the prohibition of energy dissipation and restriction of intramolecular motion,AIE photosensitizers can avoid the ACQ effect and show highly bright fluorescence in the aggregated state.What is more, by incorporating strong donor/acceptor units into a photosensitizer, the HOMO and LUMO distribution can be separated to decrease the ΔEST(singlet-triplet energy gap).Therefore,the AIE photosensitizers can show efficient1O2generation in the aggregate state [16-18].Currently, most of the AIE photosensitizer systems are focused on the fluorescence emission.However, the requirement of an external light source hinders their application of deep tissue imaging and treatment[19,20].Therefore, a highly efficient chemiluminescent system without the requirement for an external light source remains to be improved.Meanwhile, considering that the photobleaching of photosensitizers can make them undergo various side reactions,photostability of the photosensitizers is also a thorny issue to be overcome.
Herein, an AIE-based singlet oxygen system constructed by supramolecular assembly has been reported (Scheme 1).The AIE photosensitizer (TPEDM) was used as the guest.TPEDM has a typical donor-acceptor(D-A)structure,therefore,it shows efficient1O2generation property.Considering TPEDM has a quaternary ammonium terminal group, water-soluble pillar[5]arene (WP5)was used as a more suitable host molecule.In this case,the strong binding affinity between WP5 and the quaternary ammonium salt can induce the formation of stable amphiphilic host-guest complex, which can further self-assemble into supramolecular self-assemblies in aqueous solution.Consequently, supramolecular nanoparticles with efficient1O2generation and high fluorescence were achieved.The obtained nanoparticles exhibited excellent photostability.By encapsulation of catalase, the supramolecular system could show increased1O2generation efficiency in response to H2O2.Upon encapsulating adamantane derivative(AMPPD)as an energy donor,a highly efficient chemiluminescence system could be achieved, where the AIE photosensitizer can be excited chemically.Such water-soluble self-luminescent polymeric nanoparticles exhibited high brightness with long-lasting time that could be observed by naked eyes.Moreover, the supramolecular system can function as nanoreactors to perform the photooxidation of dopamine to form polydopamine with visible light irradiation.
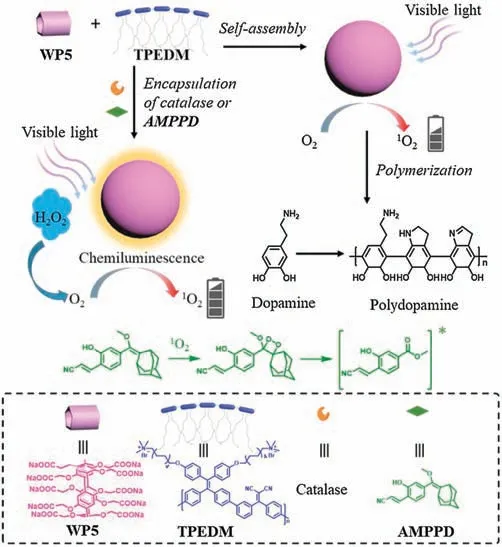
Scheme 1.Schematic illustration of the supramolecular AIE chemiluminescent singlet oxygen generation system.
As we know, WP5 can combine with the alkyl quaternary ammonium salts through hydrophobic and electrostatic interaction to form a stable supramolecular complex [21], the selfassembly behavior of WP5⊃TPEDM amphiphiles and the formed supramolecular nanostructures were further explored.Firstly,the aggregation behavior of free TPEDM was investigated.As shown in Fig.S14 (Supporting information), no Tyndall effect could be observed, suggesting that free TPEDM can be well dispersed in water.However, when WP5 was added into the TPEDM solution,notable opalescence as well as obvious Tyndall effect could be clearly observed,indicating that WP5 can induce the self-assembly behavior of WP5⊃TPEDM complex to form highly dispersive nanoparticles [22].The obtained nanoparticles were quite stable under physiological condition, and the average diameters of nanoparticles did not show obvious change within one week(Fig.S21 in Supporting information).Subsequently,the best molar ratio between WP5 and TPEDM for constructing supramolecular nanoparticles was determined by optical transmittance tests.As shown in Fig.S15 (Supporting information), upon gradually increasing the concentration of WP5, the transmittance at 600 nm first underwent a rapid decrease to a minimum and thereafter an inverse increase.Thus, the best molar ratio for the formation of supramolecular aggregates was deemed to be 7:1([TPEDM]/[WP5]) at the inflection point.Based on the obtained molar ratio, the critical aggregation concentration (CAC) for WP5⊃TPEDM complex was determined to be 1.8×10-6mol/L(Fig.S16 in Supporting information).ζ-Potential assay was also conducted to examine the stability of the WP5⊃TPEDM nanoparticles at the best molar ratio.The results showed a negative ζ-potential(-38.5 mV,Fig.S17a in Supporting information)for the formed nanoparticles, indicating that the electrostatic repulsive force is able to prevent nanoparticle agglomeration and improve their stability.
Next,the size distribution and morphology of the WP5⊃TPEDM nanoaggregates were determined by dynamic laser scattering(DLS)and transmission electron microscopy(TEM)measurements.DLS data revealed that the WP5⊃TPEDM nanoaggregates displayed a narrow size distribution with an average diameter of 185 nm(Fig.1a).The TEM image(Fig.1c)indicated the formation of a number of shrunk nanoparticles with diameters around 150 nm.

Fig.1.DLS results of the nanoaggregates formed by WP5⊃TPEDM(a)and AMPPDWP5⊃TPEDM (b); TEM images of the WP5⊃TPEDM nanoaggregates (c) and AMPPD-WP5⊃TPEDM nanoaggregates (d).Scale bar: 200 nm.
Given that TPEDM is an AIE-based photosensitizer with typical donor-acceptor structure, it may exhibit excellent1O2generation efficiency.Thus the1O2generation efficiency of the systems was explored.Herein,visible light was used to excite TPEDM.As shown in Fig.2a, nearly 63% 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABDA), a commercially available1O2indicator, was consumed for the free TPEDM in water at a decomposition rate of 38.7 μmol/min within 100 s of irradiation.When WP5 was introduced to the system,the decomposition rate of WP5⊃TPEDM nanoparticles was determined to be 28.9 μmol/min within 100 s of irradiation.The above phenomenon confirms that both TPEDM and WP5⊃TPEDM nanoparticles have high1O2generation efficiency under visible light irradiation.Moreover, the slightly different1O2generation efficiency between free TPEDM and WP5⊃TPEDM nanoparticles may be due to the fact that WP5 with electron rich cavity can strongly bind with the TPEDM guest,which further influences the donor-acceptor structure of the TPEDM,resulting the changes of ΔESTand1O2generation efficiency[23,24].The1O2quantum yield of the WP5⊃TPEDM nanoparticles was calculated to be 0.89,using Rose Bengal(1O2quantum yield:0.75)as a reference photosensitizer(Fig.S20 in Supporting information).
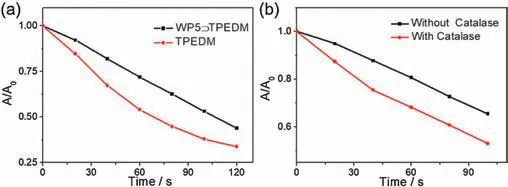
Fig.2.(a) Singlet oxygen generation efficiency of free TPEDM and WP5⊃TPEDM nanoparticles ([WP5]=0.5×10-4 mol/L, [TPEDM] = 0.08 mg/mL, 90 W/m2 visible light);(b)Singlet oxygen generation efficiency of WP5⊃TPEDM nanoparticles with and without the presence of catalase, respectively ([WP5]=0.5×10-4 mol/L,[TPEDM] = 0.08 mg/mL, 70 W/m2 visible light).
In addition,the absorption band at 420-500 nm of the formed nanoparticles has no obvious change after prolonged irradiation(Fig.S18a in Supporting information), indicating that the nanoparticles have good stability and will not undergo photobleaching[25].To further improve the1O2generation efficiency,catalase was used for fabricating H2O2-responsive nanoparticles.Catalase can catalyze the decomposition of hydrogen peroxide to produce oxygen,which can be used as the source of oxygen for the system and increase the generation efficiency of1O2.As was expected,the1O2generation of the catalase-WP5⊃TPEDM system was significantly improved,and the ABDA decomposition rate was 1.5 times higher than that of the WP5⊃TPEDM nanoparticles within 100 s of irradiation (Fig.2b).
Chemiluminescence has many advantages over fluorescence in imaging, due to its unnecessity of external light sources and high sensitivity.Thus, in order to construct a supramolecular chemiluminescent nanosystem,we further encapsulated a suitable energy donor, adamantane derivative, in the empty nanoparticles.The loaded AMPPD can react with singlet oxygen to produce extremely unstable intermediate, which tend to slowly decompose and release energy at room temperature to achieve chemiluminescence [26,27].Therefore, when the AMPPD-loaded nanoparticles were irradiated with visible light, effective chemiluminescence could be observed by naked eye.The size distribution and morphology of the AMPPD-WP5⊃TPEDM nanoaggregates were also investigated.As shown in Fig.1b, AMPPD-WP5⊃TPEDM nanoaggregates displayed a narrow size distribution with an average diameter of 241 nm.The TEM image further demonstrated the formation of circular nanoparticles with sizes around 200 nm(Fig.1d).Next,the fluorescence characteristics of the above system were investigated.Since the free TPEDM present dispersed state,the molecules can vibrate freely and the energy is dissipated in a non-radiative manner,therefore,no obvious fluorescence could be observed (Fig.S14).However, when WP5 was added, the WP5⊃TPEDM in aggregation state could prohibit the nonradiative transition and aggregation-induced intersystem crossing, leading to the enhanced fluorescence intensity.Moreover, when AMPPD was encapsulated, significant fluorescence could be found for the AMPPD-WP5⊃TPEDM system.This may contribute to Förster resonant energy transfer (FRET) from AMPPD to TPEDM.
We further conducted the study on its UV and fluorescence properties in order to verify the above speculation.The results showed (Fig.3a) that the fluorescence emission band of AMPPD(from 400 nm to 600 nm) is partially overlapped with the UV absorption band of WP5⊃TPEDM (from 300 nm to 600 nm).Besides,since AMPPD was encapsulated into the nanoparticles,the confined distance between AMPPD and the WP5⊃TPEDM complex is close enough to ensure the efficient FRET process.Moreover,compared with the WP5⊃TPEDM system,the emission band of AMPPD at 490 nm in the AMPPD-WP5⊃TPEDM system is relatively weak, while the emission band of TPEDM at 590 nm is greatly enhanced, indicating that the energy of AMPPD may be transferred to TPEDM (Fig.3b).This phenomenon further indicated the FRET process from AMPPD to TPEDM, resulting in a significant increase in the fluorescence.When the nanoparticles were irradiated with visible light for 5 min, significant chemiluminescence could be observed by naked eye, and the chemiluminescence fluorescence could be kept for several minutes.
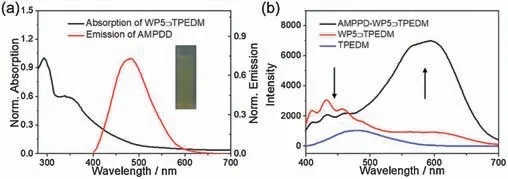
Fig.3.(a) UV absorption spectra of WP5⊃TPEDM nanoparticles and fluorescence spectra AMPPD.Inset: Chemiluminescent image of AMPPD-WP5⊃TPEDM nanoparticles after the irradiation with xenon lamp for 5 min ([WP5]=0.5×10-4 mol/L, [TPEDM] = 0.08 mg/mL, [AMPPD] = 0.02 mg/mL); (b) Fluorescence spectra TPEDM, WP5⊃TPEDM nanoparticles, and AMPPD-WP5⊃TPEDM nanoparticles([WP5]=0.5×10-4 mol/L, [TPEDM] = 0.08 mg/mL, [AMPPD] = 0.02 mg/mL).
Considering the excellent1O2generation efficiency of the nanoassemblies, we further investigated the possibility of the nanoplatform as nanoreactors.It was dated that the1O2can oxidize dopamine to polydopamine, a multifunctional polymer which has strong adhesive property and outstanding photothermal conversion efficiency[28,29].Therefore,dopamine was chosen as the substrate to demonstrate the photooxidation properties of the nanoassemblies (Fig.4a).Since the WP5⊃TPEDM nanoparticles have larger surface area compared with free TPEDM solution,they can adsorb dopamine to promote the catalytic effect.Therefore,WP5⊃TPEDM nanoparticles were chosen as nanoreactors for the photooxidation process.The results depicted in Fig.4b showed that absorbance of the polydopamine solution only increased upon the light irradiation (For details, see Fig.S19 in Supporting information),while the absorbance showed no significant changes without light irradiation.This phenomenon may contribute to the short half-life of1O2, which is convenient for controlling the polymerization process by regulating the “On/Off” mode of the light irradiation.Because the formed polydopamine may adhere to the surface of the nanoparticles, this smart1O2generation system would provide a new insight into fabrication of novel photothermal/photodynamic system.

Fig.4.(a) Schematic illustration of dopamine photooxidation catalyzed by WP5⊃TPEDM nanoparticles upon light irradiation.(b) Absorbance changes at 480 nm of the dopamine solution in the presence of WP5⊃TPEDM nanoparticles upon light irradiation as a function of time ([WP5]=0.5×10-4 mol/L, [TPEDM]=0.08 mg/mL, 90 W/m2 visible light).
In summary, an AIE-based singlet oxygen system has been successfully exploited based on the supramolecular self-assembly between WP5 and TPEDM.In this system, the tetrastyrene conjugated polymer with a donor-acceptor structure exhibited excellent photostability as well as the ability to produce singlet oxygen under visible light irradiation.The fabricated WP5⊃TPEDM nanoparticles showed similar singlet oxygen generation efficiency with enhanced fluorescence intensity.By introducing catalase, the self-oxygen supply environment can be provided and a significant increase in the singlet oxygen yield can be achieved compared with the blank nanoparticles.Moreover,an efficient chemiluminescent supramolecular system was constructed by entrapping a suitable energy donor AMPPD, which leads to the FRET induced fluorescence and chemiluminescence.Additionally, the present nanoparticles can function as nanoreactors to promote the photooxidation of dopamine to form polydopamine by regulating the “On/Off” mode of the light irradiation.This work provides a new strategy for the construction of1O2generation system based on supramolecular nanomaterials,as well as potential application value for chemiluminescence imaging and controlled photocatalysis.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.21871136), the Natural Science Foundation of Jiangsu Province (No.BK20180055), and the Fundamental Research Funds for the Central Universities (No.NE2019002).
Appendix A.Supplementary data
Supplementary material related to this article can be found, in the online version,at doi:https://doi.org/10.1016/j.cclet.2020.09.033.
杂志排行
Chinese Chemical Letters的其它文章
- D-A-D structured selenadiazolesbenzothiadiazole-based near-infrared dye for enhanced photoacoustic imaging and photothermal cancer therapy
- Synthesis and biological evaluation of a lipopeptide-based methamphetamine vaccine
- Nucleic acids induced peptide-based AIE nanoparticles for fast cell imaging
- Titanate nanofibers reduce Kruppel-like factor 2(KLF2)-eNOS pathway in endothelial monolayer: A transcriptomic study
- Drug-induced hierarchical self-assembly of poly(amino acid) for efficient intracellular drug delivery
- Co-delivery of anticancer drugs and cell penetrating peptides for improved cancer therapy
