深绿木霉HB20111产挥发性物质及其功能分析
2021-10-12扈进冬隋丽娜李玲陈凯李纪顺
扈进冬 隋丽娜 李玲 陈凯 李纪顺


摘要 为了解深绿木霉HB20111产挥發性物质的成分以及潜在的生物学功能,采用顶空气相色谱-质谱法测定了深绿木霉HB20111产挥发性物质的主要成分,并进行了成分分析;采用对扣法测定了深绿木霉HB20111产挥发性物质对5种植物病原菌的抑菌率;同时测定了深绿木霉HB20111产挥发性物质对小麦幼苗生长的影响。结果表明,深绿木霉HB20111产挥发性物质烯类的相对含量最高,为49.68%、醇类为21.78%,酮类为8.56%,其余类别相对含量较低;这些挥发性物质对5种植物病原菌均有一定的抑制效果,其中对立枯丝核菌抑菌率最高,为5062%;挥发性物质还可以促进小麦幼苗生长,与对照相比,对小麦幼苗的株高有显著的促进作用(P<0.05)。这些结果为深绿木霉HB20111的开发应用提供了理论基础。
关键词 深绿木霉; 挥发性物质; 气质联用; 吡喃酮
中图分类号: S 476
文献标识码: A
DOI: 10.16688/j.zwbh.2020655
Identification and functional analysis of volatile organic compounds from Trichoderma atroviride HB20111
HU Jindong1, SUI Lina2, LI Ling1, CHEN Kai1, LI Jishun1*
(1. Shandong Provincial Key Laboratory of Applied Microbiology, Institute of Ecology, Qilu University of Technology
(Shandong Academy of Sciences), Jinan 250103, China; 2. Institute of Biological Engineering, Qilu University of
Technology (Shandong Academy of Sciences), Jinan 250353, China)
Abstract
In order to determine the components and potential biological functions of volatile organic compounds (VOCs) produced by Trichoderma atroviride HB20111, the headspace solid phase microextraction-gas chromatography-mass spectrometry (HS-SPME-GC-MS) was used to analyze the VOCs from T.atroviride HB20111. The inhibition rate of VOCs against five plant pathogenic fungi was measured by using plate-to-plate method, and the promoting effect of the VOCs on the growth of wheat seedlings was explored. The results showed that there were three major classes of VOCs, including alkenes,alcohols and ketones, accounting for 4968%, 21.78% and 8.56% of the total amount, respectively. These VOCs had certain inhibitory effects on the five plant pathogens, and the highest inhibition rate was 50.62% against Rhizoctonia solani. The VOCs also promoted the growth of wheat seedlings and significantly promoted the plant height of wheat seedlings (P<005) compared with the control. These results provide a basis for further application of T.atroviride HB20111.
Key words
Trichoderma atroviride; volatile organic compounds (VOCs); gas chromatography-mass spectrometry; 6-pentyl-2H-pyran-2-one (6PP)
近年来,木霉Trichoderma spp.作为生物防治菌剂防治植物病害已逐渐被人们所熟知[1-2]。同时,已有多种木霉被登记为生物肥料、生物农药、植物刺激剂等产品,广泛用于农业生产。在世界范围内,木霉是最重要的生物农药制剂来源之一,如在印度有大约250种木霉及其衍生物的生物杀菌剂产品[3];我国也有18个已在农业部正式登记的木霉生物农药产品。木霉除具备优良生防功能外,还可以促进植物侧根和根毛生长、提高植物生物量、高度、叶片数量、分蘖、分枝、果实产量等[4-5];对植物光合作用、气孔导度、气体交换、营养物质吸收同化等生理过程具有正向调控作用[6-7];可以分泌植酸酶和有机酸类物质促进植物根系对养分的溶解和吸收[8];通过提高植物氧化应激等方面的作用改善植物在胁迫条件下的生长和繁殖[9-10]。因此,在当前提倡绿色生态健康发展环境下,木霉作为化学杀菌剂的有效替代品具备大规模应用的潜力。
深绿木霉Trichoderma atroviride HB20111是本实验室筛选获得的一株优良生防菌株,已发现通过小麦拌种可以促进小麦的根系发育和冬前分蘖,对小麦茎基腐病也有较好的防治效果,还发现其在生长过程中会产生浓郁的椰香味挥发性物质。有研究发现这种真菌产生的挥发性物质有些可以通过激活植物的防御反应抑制植物病原菌的增殖[11]。为了解深绿木霉HB20111所产挥发性物质的成分以及潜在的生物学功能,本文通过顶空气相色谱-质谱法对其产生的挥发性有机物进行了测定和成分分析,研究了木霉挥发性物质的种类和含量及其对不同植物病原真菌抑制效果和对植物的促生作用,以期为该菌的开发利用提供理论依据和参考。
1 材料与方法
1.1 菌株及培养条件
深绿木霉 Trichoderma atroviride HB20111, 假禾谷镰孢Fusarium pseudograminearum、尖镰孢F.oxysporum、层出镰孢F.proliferatum、麦根腐平脐蠕孢Bipolaris sorokiniana、立枯丝核菌Rhizoctonia solani由实验室分离保存。培养基采用马铃薯葡萄糖琼脂培养基(PDA),115℃,30 min灭菌后备用。上述菌株至少在60 mm直径的PDA平板上活化两次,培养温度(28±0.5)℃。
1.2 深绿木霉HB20111产生的挥发性物质鉴定
为进行气相色谱离子迁移谱(GC-MS)分析,将预培养活跃生长的菌落边缘一个直径5 mm的琼脂块倒置接种在20 mL顶空瓶中,瓶底装有5 mL PDA。瓶子用特氟龙螺旋盖封闭,并加金属盖。放置于(28±0.5)℃培养箱中培养7 d。固相微萃取头在氦气流中于240℃老化20 min,然后将经过老化的萃取头插入顶空瓶中,于50℃条件下吸附45 min,吸附完成后迅速插入色谱仪进样口,开始GC-MS检测分析[12]。
气相色谱-质谱联用条件[13]:采用安捷伦DB-624弱极性毛细管柱分离挥发性物质,载气为氮气,流速1 mL/min,进样口温度为250℃;进样方式为手动;分流比5∶1;升温程序:50℃维持2 min,再以10℃/min升温至200℃,离子源温度:230℃;电离电压:70 eV;质谱采用全扫描模式,速率3.5 scans/s;质谱范围29~500 amu。
数据处理:深绿木霉HB20111样品经过固相萃取后进行GC-MS分析鉴定,所得色谱和质谱信息采用安捷伦公司的质谱分析软件进行分析,在NIST05/Wiley275标准谱图库进行检索,选取化合物质谱数据库中最小匹配度均大于80的成分,同时配合人工检索、对照和解析,确认匹配化合物。利用面积归一法计算各成分的相对百分含量。
1.3 深绿木霉HB20111产挥发性物质对病原菌的抑菌率
采用对扣法测定木霉产挥发性物质对病原菌的抑菌率,用5 mm灭菌打孔器分别打取已在PDA平板上活化两次的深绿木霉HB20111和5株病原菌菌饼,用灭菌牙签将其转接于90 mm的PDA平板中间。将接有深绿木霉HB20111的PDA平板与接有病原真菌的PDA平板对扣起来,接有拮抗菌的一面朝上,用封口膜密封。同时设置对照组,只接病原真菌,不接拮抗菌。将上述平板放置于培养箱中,在(28±0.5)℃下培养。每个处理设3个重复。培养5 d后采用十字交叉法测量测试病原菌菌落直径,计算抑菌率[14]。
抑菌率=
对照病原菌菌落直径-处理病原菌菌落直径对照病原菌菌落直径-5×100%。
1.4 小麦幼苗形态指标测定
将小麦种子分为两组,每组30粒置于发芽袋中萌发。发芽袋放入密闭的塑料盒中,其中一组塑料盒内放入培养5 d的开盖的HB20111平板;对照组放置仅含培养基的空白平板,小麦萌发后7 d测定幼苗根长、株高和鲜重。用刻度尺测量30株小麦根长和株高,每株幼苗测量3根最长的根,计算各组的平均根长和株高;千分之一电子天平称量每株鲜重;根冠比根据根系鲜重与地上部分鲜重的比值计算获得。
2 结果与分析
2.1 气相色谱-质谱联用鉴定深绿木霉HB20111有机挥发物质
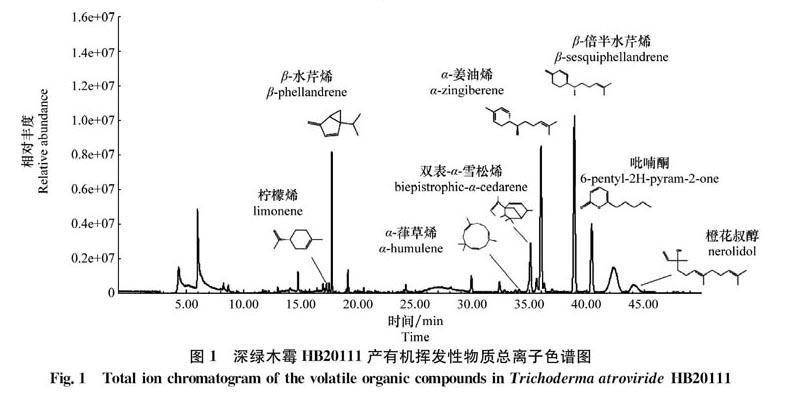
深绿木霉HB20111样品经GC-MS分析,从挥发性物质中共分离出56个离子峰,从中鉴定出53种化合物,用面积归一化法确定了各挥发性物质的相对百分含量。鉴定的挥发性物质结果见表1,总离子流图见图1。
通过质谱分析,深绿木霉HB20111共鉴定53种挥发性物质。在这些挥发性物质中烷烃类5种,酯类4种,烯类17种,醇类5种,酮类4种,醛类2种,其他16种。其中烯类相对含量最高,为4968%、醇类为21.78%,酮类为8.56%,其余类别相对含量较低。在烯类中主要的挥发性物质有β-倍半水芹烯、α-姜油烯、β-水芹烯、双表-α-雪松烯,其中β-倍半水芹烯和α-姜油烯分别为18.34%和13.53%,相对含量较高。除此之外,吡喃酮含量也相对较高,为784%,猜测这些成分应该是深绿木霉HB20111主要的功能性揮发物质。
2.2 深绿木霉HB20111产挥发性物质对病原菌的拮抗效果
由图2可以看出,深绿木霉HB20111产生的挥发性物质对5种病原菌均有明显的抑菌作用,但对不同病原菌的抑菌率不同(表2)。其中,对立枯丝核菌抑菌率最高,为50.62%;对假禾谷镰孢的抑菌率最低,为16.00%。对层出镰孢、尖镰孢、麦根腐平脐蠕孢的抑菌率分别为32.73%、43.65%和44.00%。
2.3 深绿木霉HB20111产挥发性物质对小麦幼苗的促生作用
由图3可以看出,深绿木霉HB20111产生的挥发性物质对小麦幼苗生长具有明显的促生作用,处理小麦幼苗7 d后根长、株高、鲜重和根冠比分别为6.63 mm、5.60 mm、55.29 mg和0.72,与对照相比均有一定的促进作用,其中对小麦幼苗株高有显著的促进作用(表3)。
3 討论
目前已从木霉中鉴定出至少480种VOCs,其中吡喃酮(6-戊基-2H-吡喃-2-酮,6PP)是从该真菌属最早分离出来的挥发物之一[14-16]。木霉释放的这些VOCs与其拮抗活性有关,有研究表明,它们可以抑制植物病原真菌的菌丝生长[17]。绿木霉T.virens和拟康氏木霉T.pseudokoningii所产挥发性有机物可以促进拟南芥侧根的形成和生长[12,17-19];深绿木霉Tatroviride P1菌株除合成吡喃酮外还产生烷烃类、醇类、酮类、内酯、呋喃、单萜、倍半萜烯和C8化合物,其中1-辛-3-醇、3-辛醇、3-辛酮等是脂肪酸代谢的终产物[19],认为可能是调控真菌发育和菌落间通讯的信号分子。
本研究中深绿木霉HB20111产挥发性物质中除吡喃酮外,还有大量的烯萜类物质,与已报道到的木霉产挥发性物质组成差异较大,其中β-倍半水芹烯、α-姜油烯、β-水芹烯、双表-α-雪松烯均具有一定的生物学功能,如β-倍半水芹烯被认为具有与姜黄素相当的抗癌潜力[20],在β-倍半水芹烯氧化酶和脱氢酶作用下可以生成β-姜黄酮,其具有抗脂质过氧化作用[21];β-水芹烯在低浓度时能增加细胞膜通透性,造成细胞质膜的不可逆损伤,进而导致细胞成分和钾离子的泄漏[22-23];影响细菌的生物膜形成,对变异链球菌Streptococcus mutans、副溶血形弧菌Vibrio parahaemolyticus等具有抑制作用[24],还有报道发现不同的黄皮精油组分中,富含β-水芹烯组分对多种念珠菌具有良好的抗真菌活性[25];主要成分为α-姜油烯(34.48%),β-倍半萜烯(22.90%)和α-姜黄素(16.17%)的精油具有抗微生物活性。革兰氏阳性菌比革兰氏阴性菌对精油更敏感,同时该精油还具有抗氧化能力[26]。此外,在深绿木霉HB20111产挥发性物质中还含有2.98%的橙花叔醇,这是一种天然存在的倍半萜烯醇,存在于各种带有花香的植物中,它是合成(3E)-4,8-dimethy-1,3,7-壬二烯(DMNT)的中间体,能够吸引草食动物捕食者,从而保护植物免受草食动物损害[27]。
本研究表明,深绿木霉HB20111产挥发性物质对多种植物病原真菌具有明显拮抗作用,同时对小麦幼苗有促生作用,其产生的挥发性物质中除广为熟知的6PP成分外,还有功能性烯萜类挥发性物质,这些物质具有潜在的抗菌、抗氧化等功能,是我们今后需要进一步研究的内容。
参考文献
[1] HARMAN G E, HOWELL C R, VITERBO A, et al. Trichoderma species-opportunistic avirulent plant symbionts [J]. Nature Reviews Microbiology, 2004, 2(1):43-56.
[2] WEINDLING R. Trichoderma lignorum as a parasite of other soil fungi [J]. Phytopathology,1932, 22(10): 837-845.
[3] SOOD M, KAPOOR D, KUMAR V, et al. Trichoderma: The “secrets” of a multitalented biocontrol agent [J/OL]. Plants, 2020, 9(6):762. DOI:10.3390/plants9060762.
[4] BREWER D, MASON F G, TAYLOR A. The production of alamethicins by Trichoderma spp. [J]. Canadian Journal of Microbiology, 1987, 33(7): 619-625.
[5] BODO B, REBUFF S, EL HAJJI M. Structure of trichorzianine A ⅢC, an antifungal peptide from Trichoderma harzianum [J]. Journal of the American Chemical Society, 1985, 107(21): 6011-6017.
[6] REBUFFAT S, CONRAUX L, MASSIAS M, et al. Sequence and solution conformation of the 20-residue peptaibols, saturnisporins SA Ⅱ and SA Ⅳ [J]. International Journal of Peptide and Protein Research, 1993, 41(1): 74-84.
[7] REBUFFAT S, GOULARD C, BODO B. Antibiotic peptides from Trichoderma harzianum: harzianins HC, proline-rich 14-residue peptaibols [J]. Journal of the Chemical Society Perkin Transactions, 1995, 14:1849-1855.
[8] SONG Xiaoyan, SHEN Qingtao, XIE Shutao, et al. Broad-spectrum antimicrobial activity and high stability of trichokonins from Trichoderma koningii SMF2 against plant pathogens [J]. FEMS Microbiology Letters, 2006, 260(1): 119-125.
[9] WORASATIT N, SIVASITHAMPARAM K, GHISALBERTI E L, et al. Variation in pyrone production, pectic enzymes and control of rhizoctonia root rot of wheat among single-spore isolates of Trichoderma koningii mycological research [J]. Mycological Research, 1994, 98(12):1357-1363.
[10]MASTOURI F, BJRKMAN T, HARMAN G E. Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedling [J]. Phytopathology, 2010, 100(11):1213-1221.
[11]SPECKBACHER V, RUZSANYI V, WIGGER M, et al. The Trichoderma atroviride strains P1 and IMI 206040 differ in their light-response and VOC production [J/OL]. Molecules, 2020, 25(1): 208. DOI: 10.3390/molecules25010208.
[12]陶玲蕓, 张怡雯, 李雅乾,等. 棘孢木霉挥发性次级代谢产物检测及抑菌活性分析[J]. 生物工程学报, 2020, 36(6):166-174.
[13]CONTRERAS-CORNEJO H A, MACAS-RODRGUEZ L, HERRERA-ESTRELLA A, et al. The 4-phosphopantetheinyl transferase of Trichoderma virens plays a role in plant protection against Botrytis cinerea through volatile organic compound emission [J]. Plant and Soil, 2014, 379(1/2): 261-274.
[14]穆静娟,焦加国,葛新成, 等.植物病原真菌广谱拮抗菌M29的筛选、鉴定及其抑菌机制[J].南京农业大学学报, 2017, 40(1): 84-92.
[15]VINALE F, SIVASITHAMPARAM K, GHISALBERTI E L, et al. A novel role for Trichoderma secondary metabolites in the interactions with plants [J]. Physiological & Molecular Plant Pathology, 2008, 72(1/2/3):80-86.
[16]GUPTA V K, SCHMOLL M, HERRERA-ESTRELLA A, et al. In biotechnology and biology of Trichoderma [M]. The Netherlands Amsterdam: Elsevier, 2014: 139-175.
[17]AMIN F, RAZDAN V K, MOHIDDIN F A, et al. Effect of volatile metabolites of Trichoderma species against seven fungal plant pathogens in-vitro [J]. Journal of Phytology, 2010, 2(10): 34-37.
[18]HUNG R, LEE S, BENNETT J W. Arabidopsis thaliana as a model system for testing the effect of Trichoderma volatile organic compounds [J]. Fungal Ecology, 2013, 6(1):19-26.
[19]SCHNRER J, OLSSON J, BRJESSON T. Fungal volatiles as indicators of food and feeds spoilage [J]. Fungal Genetics and Biology, 1999, 27(2/3):209-217.
[20]TYAGI A K, PRASAD S, YUAN W, et al. Identification of a novel compound (β-sesquiphellandrene) from turmeric (Curcuma longa) with anticancer potential: comparison with curcumin [J]. Investigational New Drugs, 2015, 33(6):1175-86.
[21]KOO H J, GANG D R. Suites of terpene synthases explain differential terpenoid production in ginger and turmeric tissues [J/OL]. PLoS ONE, 2012, 7(12):e51481. DOI:10.1371/journal.pone.0051481.
[22]DIAO Wenrui, HU Qingping, ZHANG Hong, et al. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.)[J]. Food Control, 2014, 35(1): 109-116.
[23]ZHANG Jing, YE Keping, ZHANG Xin, et al. Antibacterial activity and mechanism of action of black pepper essential oil on meat-borne Escherichia coli [J/OL]. Frontiers in Microbiology, 2017, 7: 2094. DOI: 10.3389/fmicb.2016.02094.
[24]MENDES J L, DE ARAU'JO T F, DE CARVALHO M G, et al. Chemical composition and mechanism of vibriocidal action of essential oil from resin of Protium heptaphyllum [J/OL]. Scientific World Journal, 2019, 2019: 9563213. DOI: 10.1155/2019/9563213.
[25]HE Xiaowen, ZHANG Lantong, CHEN Jinping, et al. Correlation between chemical composition and antifungal activity of Clausena lansium essential oil against Candida spp. [J/OL]. Molecules, 2019, 24(7):1394. DOI: 10.3390/molecules24071394.
[26]PANDINI J A, PINTO F G S, SCUR M C, et al. Chemical composition, antimicrobial and antioxidant potential of the essential oil of Guarea kunthiana A.Juss [J]. Brazilian Journal of Biology, 2018, 78(1): 53-60.
[27]CHAN W K, TAN L T, CHAN K G, et al. Nerolidol: A sesquiterpene alcohol with multi-faceted pharmacological and biological activities [J/OL]. Molecules, 2016, 21(5): 529. DOI: 10.3390/molecules21050529.
(責任编辑:杨明丽)
