A metal-free carbon dots for wastewater treatment by visible light active photo-Fenton-like reaction in the broad pH range
2021-10-12DongxueYangDanQuLiAnXupengZongZaichengSun
Dongxue Yang,Dan Qu*,Li An,Xupeng Zong,Zaicheng Sun*
Center of Excellence for Environmental Safety and Biological Effects, Beijing Key Laboratory for Green Catalysis and Separation, Department of Chemistry and Biology, Beijing University of Technology, Beijing 100124, China
ABSTRACT Carbon dots(Cdots)has been proved to possess the catalytic decomposition of H2O2 in the photocatalytic system.It is a potential photo-Fenton catalyst.Since multiple emissive Cdots have different light response range.There is rarely investigation on the performance of Cdots based photo-Fenton on the light wavelength.Herein,blue,green and red emissive carbon dots were synthesized from the different ratio of o-phenylenediamine and catechol by the solvothermal method.They exhibit different light adsorption range from UV to visible light.Furthermore, the photo-Fenton reactivity of Cdots was studied for catalyzing the decomposition of H2O2 to generate free hydroxyl radicals and consequently applying for the removal of methyl blue.The results exhibit that Cdots with the broader light adsorption rang possess the stronger catalytic activity for the photo-Fenton reaction.The H2O2 decomposition rate of red emissive Cdots is 0.074 min-1,which is 2.64 and 1.46 times than the blue and green emissive Cdots,respectively.And the radical detection results confirm that the photo-Fenton happens in the reaction.In addition,the Cdots photo-Fenton can be carried out in the broad pH range from acidic to basic solution,which has a great potential to treat wastewater in the neutral system.
Keywords:Carbon dot Photo-Fenton reaction H2O2 Visible light Wastewater treatment
Advanced oxidation processes (AOPs) are considered as powerful methods for the wastewater treatment due to their ability for removing most of organic contaminant [1–3].In wastewater treatment,AOPs generally refer to a group of processes that cover H2O2as oxidant with the assistance of catalyst, light,ultrasonication and/or thermal input [4].Fenton reaction is defined as Fe2+catalytic reaction of H2O2that predominantly produces·OH radicals as the central oxidizing species.The major drawbacks that limit the practical applications of the traditional Fenton process include:(i)a narrow working pH range(pH<3);(ii)high iron concentrations in the final effluent that requires costly removal treatment and generate iron-containing sludge;and(iii)a high demand for H2O2[5,6].The regeneration of catalyst is not only impracticable but also a large amount of catalytic metal is misplaced in the sludge.To reduce the iron amount, various efforts have been demonstrated to promote the degradation of H2O2by adding extra carboxylic acid,like oxalate[7,8]and citrate[9], carbon materials like graphene oxide [10,11].Photo-Fenton process is a combination of Fenton reagents and UV–vis radiation to reduce the catalytic metal [12–15].Recently, semiconductors(like TiO2[12,16,17], BiVO4[18,19], g-C3N4[20–24], AgX [25–27],Ag3PO4[28], CdS [29]) can directly transfer the photogenerated electron to the heterogeneous catalyst or H2O2under light irradiation.In most cases, Fe2+or other metal species is still required to catalytic degradation of H2O2.It is highly desired to taking the place of Fe2+with a metal-free catalyst.
Carbon dots (Cdots) have become a burgeoning carbon nanomaterial.They are composed of a graphitic core and functional groups, which endow Cdots with unique and tunable chemical,optical and electronic properties, super stability, and excellent biocompatibility [30–33].It can be worked as a light absorber for the photocatalyst and photovoltaic device [34–36].In addition, N doped Cdots exhibit catalytic properties due to the addition of N.Previous investigations on graphene have indicated that doped nitrogen atoms in carbon materials, especially in the form of pyridinium moieties, play a critical role in enhancing their electrocatalytic activities toward ORR [37].One of the pioneering reports on the use of Cdots as electrocatalysts for oxygen reduction reaction (ORR) was by Qu and Dai groups [38–40].Cdots-based hybrid materials such as Cdots-NiFe-layered double-hydroxide composites have shown some promise in oxygen evolution reaction(OER) [41].Recently, Kang et al.reported the Cdots work as a catalyst for H2O2degradation in the photocatalyst Cdots/g-C3N4[42,43].That inspires us Cdots may be a highly efficient catalyst for the Fenton reaction instead of ferrous ion.On the other hand, Cdots can adsorb broadly visible light adsorption, the photogenerated electron may be used for the reduction of H2O2.However,Cdots based photo-Fenton reaction is rarely investigated,especially its light response range.Inspired by these,we proposed that Cdots may work as a catalyst for photo-Fenton-like reaction(ph-F)for wastewater treatment to avoid the production of metalcontaining sludge.
In this report, we synthesized a series of Cdots with tunable adsorption range and emission.The blue, green and red emissive Cdots are named as B-, G- and R-Cdots, respectively.The optical properties of Cdots disclose the light adsorption range can be tuned by synthesis conditions.The ph-F reactions were carried out in the absence of ferrous ion under the light irradiation.The result indicates that Cdots can work as a catalyst for the ph-F reaction very well.The reaction rate strongly depends on the optical properties such as the light adsorption range.In other words,the RCdots exhibit the highest ph-F reaction rate due to it has the broadest light adsorption range.In addition, we also proved the H2O2was decomposed by Cdots and produce the hydroxyl radicals,which is employed to oxidize the waste organic molecules.The ph-F reaction can carry out in abroad pH range which overcomes the shortcoming of traditional Fenton process requiring for low pH.
Typically, catechol and o-phenylenediamine are employed to prepare Cdots by hydrothermal method.The Cdots prepared from pure catechol, pure o-phenylenediamine and both can emit blue,green and red light,respectively.Thus,they are simply denoted as B-, G- and R-Cdots.(Figs.1a-c) showed the transmission electron microscopy (TEM) images of the as-prepared Cdots.B-, G- and R-Cdots showed an average diameter of about 1.92±0.55,2.31±0.47 and 2.59±0.61 nm(Fig.S1 in Supporting information),and the particles were uniformly distributed.Their high-resolution TEM (HR-TEM) images (insets) reveal that all three Cdots show a well resolved 0.24 nm lattice spacing, which corresponds to the(100) crystal plane of graphitic carbon, indicating their high crystallinity and similar graphitic structure.Cdots was further characterized by Raman spectroscopy and X-ray diffraction(XRD)patterns (Fig.1).The Raman spectra of the Cdots exhibit the disordered band (D band) at 1580 cm-1and the graphitic band(G band) at 1360 cm-1.The intensity ratios of the D band and the G band(ID/IG)of the B-,G-and R-Cdots are about 1.1,1.02 and 0.9,respectively,indicating that the R-Cdots have the highest degree of the graphitization among three types of Cdots.The XRD patterns of all Cdots show a broad peak at 24.04°which is related to the(002)plane of the graphite crystal structure.The diffraction angle is slightly smaller than the standard diffraction peak of graphite indicating that the distance is slightly larger than the inter-plane distance (0.35 nm) due to the presence of surface groups.In addition, the surface functional groups and composition of the three types of Cdots were investigated by Fourier transform infrared spectrum (FT-IR, Fig.S2 in Supporting information).The stretching vibration for OH (3436 cm-1), CH2(2934 cm-1), C=C(1617 cm-1),C=N(1662 cm-1)indicate the presence of conjugated aromatic structure together with O- and N-containing groups.To disclose the elemental composition Cdots, X-ray photoelectron spectroscopy (XPS) was performed.Fig.S3 (Supporting information) exhibits the full survey XPS of all three Cdots.Three characteristic peaks are observed at 284.0 eV, 400.0 eV and 532.4 eV for G-and R-Cdots,respectively,which are assigned to C 1s,N 1s and O 1s.It indicates that G-and R-Cdots are composed of C, N and O elements.No N signal is observed in the B-Cdots implying that the B-Cdots are only composed of C and O elements.The high-resolution XPS are presented in Fig.2.The sp2C (C=C/C-C)and sp3C(C-C,C-O or C-N)coexist in the high-resolution C 1s XPS spectra of the B- and G-Cdots.The carbonyl C (-C=O) at 287.8 eV also is observed in the R-Cdots besides the sp2C and sp3C.The high-resolution N 1s XPS spectra present that the valance state of N in G-and R-Cdots.The pyridinic N(-N=)at 399.4 eV takes the dominant place in the G-Cdots, the minor pyrrolic N (-NH-) at 400.5 eV is detected.Pyridinic,pyrrolic and graphitic N(401.8 eV)coexist in the R-Cdots.The O 1s XPS spectra exhibit the O exists in the format of C=O (531.7 eV) and C-O (533.6 eV) in the Cdots.

Fig.1.TEM image of blue(a),green(b),and red(c)emissive Cdots,respectively.The insets are corresponding(HR)-TEM images.Raman spectra(d)and XRD pattern(e)of three Cdots, respectively.
As shown in Fig.3a, UV–vis absorption spectra of B-Cdots exhibits a strong absorption band at ~365 nm, which is derived from the n-π transition.For G-and R-Cdots,the similar absorption band appears at about 460 nm and 570 nm, respectively.That indicates that the adsorption range gradually expands from the UV to the visible light region.The adsorption range of R-Cdots covers UV and visible light.The excitation-emission matrixes of the Cdots are presented in Figs.3b-d.Only one emission center is observed in the B-Cdots, which locates in the blue light (400-470 nm) under the UV light excitation (300-380 nm).For the G-Cdots, the main emission center is at the 500 nm under the excitation wavelength of 400-500 nm.In the case of R-Cdots, its emission peak is concentrated at 625-680 nm.The relatively simple emission implies the Cdots have relative purity of Cdots.The emission centers of Cdots also red-shift as the exciton band shifts to red,which means that the multi-color emission originates from the band edge transition in the Cdots.
The ph-F reaction was carried out in the 10 ppm MB solution and Cdots with the presence of H2O2under light irradiation.Fig.4 and Fig.S4(Supporting information)show the ph-F degradation of MB with B-, G- and R-Cdots.The relative concentration of MB almost keeps constant within 2 h in the pure MB solution and Cdots,indicating that Cdots have no degradation effect on the MB.H2O2was added in the solution by itself under the light irradiation,the concentration of MB slightly decreases.That implies that H2O2can be decomposed by light, the MB can be degraded by the hydroxyl radical produced from H2O2.The apparent reaction rate(k)is 0.0014 min-1.When the Cdots and H2O2were added into the MB solution in absence of light, the MB can be decomposed to some extent.Its apparent reaction rate is higher than that in the pure H2O2, indicating that H2O2can be catalytically decomposed by Cdots even in the dark.The MB is significantly decolored by Cdots-H2O2under the light irradiation.The apparent degradation reaction rate increase about 1 order to 0.028, 0.041 and 0.074 min-1implying that H2O2decomposition rate is significantly enhanced when the light is shining on the solution.The R-Cdots has the highest catalytic efficiency for the ph-F process among all three types of Cdots.
In order to understand the different catalytic properties of B-,Gand R- Cdots for the ph-F reaction, we conducted photo-Fenton experiments with three carbon dots with different wavelength range.Due to the Cdots have different adsorption range based on the UV–vis spectra of Cdots.The adsorption bands are ~365 nm,~460 nm and ~570 nm for B-, G- and R-Cdots,respectively.Fig.5 showed the degradation curve of MB catalyzed by Cdots-H2O2with different cutoff filters.For B-Cdots, the decolor rate almost keeps constant when the 420,485 and 600 nm optical cutoff filters were applied.The decolor rate did not increase implying that the B-Cdots has no response to the visible light (λ>420 nm).In the case of G-Cdots, the relative concentration of MB decrease in a linear way for applying 600 and 485 nm cutoff filters.That means the G-Cdots has a light response to the light with λ<485 nm.For R-Cdots,the decolor rate keeps constant when 600 nm cutoff filter was applied.Thus the R-Cdots exhibit more broad light response range.Figs.5d–f showed that the MB apparent degradation rate(k)by ph-F reaction with different cutoff filters.When 600 nm cutoff optical filter was applied, the k values are similar to the k in the dark cases,indicating all three Cdots have no response to the light which wavelength is larger than 600 nm.In the case of 485 nm cutoff filter,the B-and G-Cdots exhibit similar degradation rate as the Cdots-H2O2in the dark condition.The R-Cdots-H2O2demonstrated as high as 0.075 min-1indicating that the light between 480 nm and 600 nm can accelerate the H2O2decomposition.For the 420 nm cutoff filter, the degradation rate is 0.0034 min-1for B-Cdots.It clearly shows B-Cdots-H2O2still have no response to the light with λ>420 nm,but the catalytic degradation rates of MB by G-and R-Cdots-H2O2are 0.0051 and 0.022 min-1,which are higher than that in dark state.These results indicate that B-Cdots-H2O2has a light response on the light which wavelength is less than 420 nm.The light response range of G-and R-Cdots-H2O2are lower than 480 nm and 600 nm, respectively, which match the light adsorption rang of B-, G- and R-CDs.
Besides,the pH of reaction is also investigated to disclose the pH influence on the photo-Fenton.Fig.S5 (Supporting information)shows the apparent decomposition rate of CDs based photo Fenton reaction.It clearly shows the dependence of the decomposition rate on the pH of the solution.The decomposition rate decreases with the increase of the pH of the solution.It mainly because the redox potential is strongly depends on the pH of the solution.Comparing the traditional Fenton process, the CDs based photo-Fenton process has much abroad working window, which covers pH 3~9.That means the Cdots are potential.
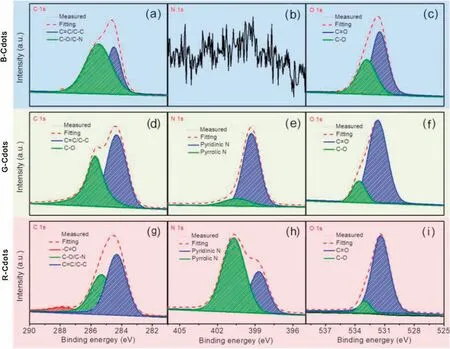
Fig.2.High resolution C 1s (a, d, g), N 1s (b, e, h) and O 1s (c, f, i) X-ray photoelectron spectra (XPS) of B-, G- and R-Cdots, respectively.
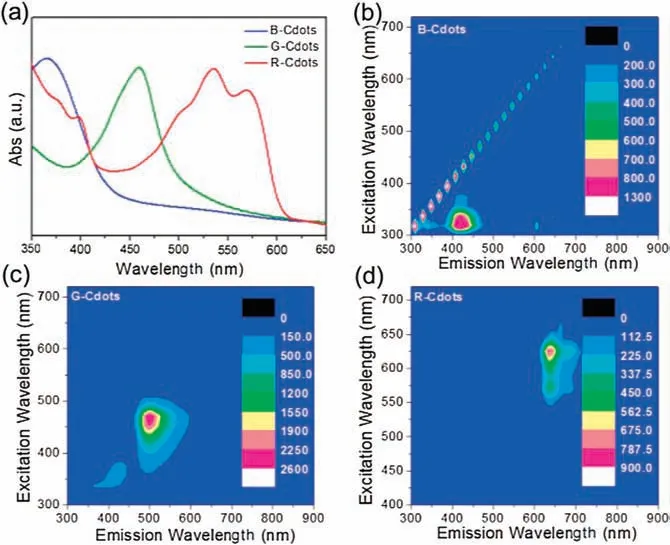
Fig.3.UV–vis spectra(a)and excitation-emission matrix(EEM)of B-(b),G-(c)and R-Cdots (d), respectively.
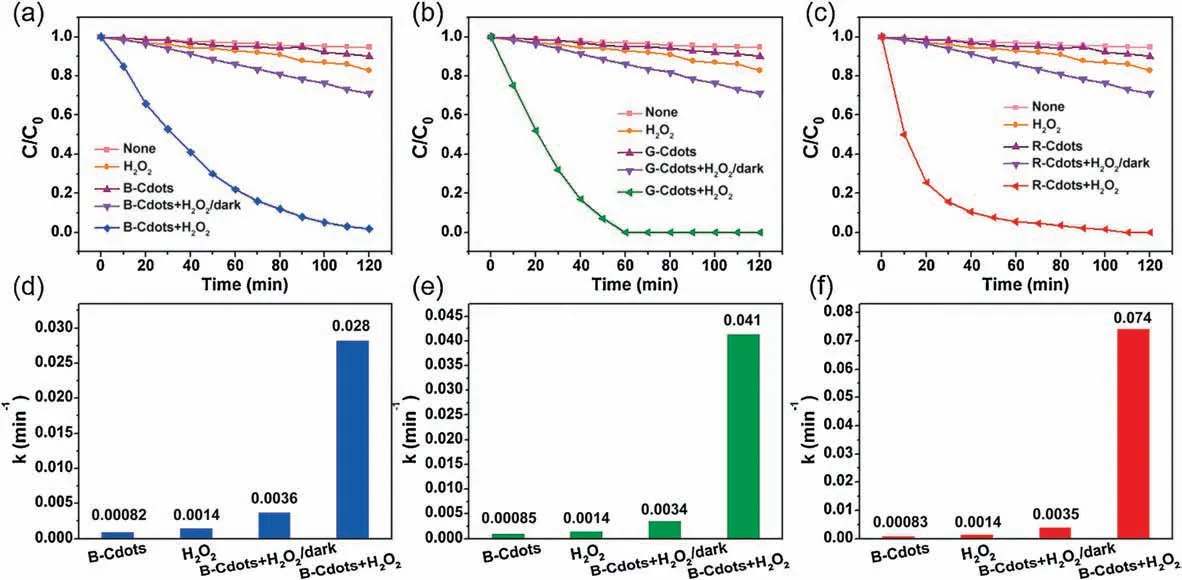
Fig.4.Photodegradation of methylene blue (MB) (a, c, e) and the apparent reaction rate constants k (b, d, f) with the presence of H2O2 and Cdots.
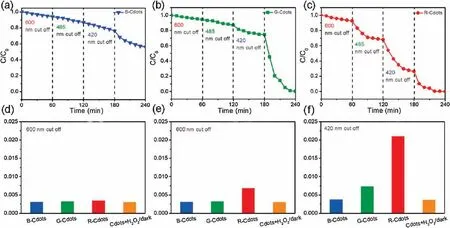
Fig.5.The decomposition of MB by photo Fenton of B- (a), G- (b) and R-Cdots (c) with different optical cutoff filters (420 nm, 485 nm and 600 nm cutoff).The apparent decomposition rate(k)of B-,G-and R-Cdots-H2O2 photo Fenton reaction and R-Cdots-H2O2 without light irradiation,and the apparent reaction rate constants k(d,e,f)with the presence of H2O2 and Cdots.
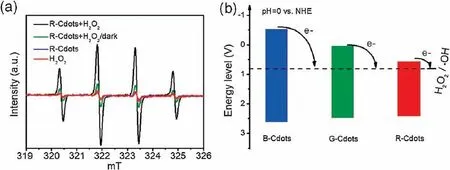
Fig.6.(a)Electron paramagnetic resonance(EPR)spectra of DMPO trapped radical in the R-Cdots-H2O2 system.(b)The schematic diagram of the energy diagram of three types of Cdots and redox potential of H2O2.
To confirm the radical type produced by Cdots-H2O2,the electron paramagnetic resonance(EPR),which detects the unpaired electron using DMPOasa spin-trappingreagent[38],wascarriedouttoverify the type of radical produced in the reaction.Fig.6 shows the typical EPR spectrum obtained R-Cdots-H2O2with DMPO solution.The spectrawere composed of quartet lines having a peak height ratio of 1:2:2:1.The EPR parameters (hyperfine constants aN=1.49 mT,aH=1.49 mT) coincided with those of the DMPO-OH adduct as demonstrated previously[38],confirming that the quartet signal is the DMPO-OH adduct.R-Cdots-H2O2under light irradiation exhibit the highest intensity among all different condition,indicating that the light illustration greatly promotes the catalytic performance of Cdots.Tounderstandtheworkingmechanism,thevalance band(VB)XPS spectra were conducted on three types of Cdots.Fig.S6(Supporting information)shows the VB XPS spectra of B-,G-and RCdots indicating the VB of B-,G-and R-Cdots is 2.5,2.4 and 2.3 eV,respectively.The band gaps of Cdots obtained by Tauc plot of Cdots are 2.59, 2.51 and 2.06 eV for B-, G- and R-Cdots, respectively.Figs.S6d-fshowthe correspondingenergylevelofB-,G-and R-Cdots and the redox potential of H2O2.It clearly shows that the conduction band of three types of Cdots is higher than the redox potential of H2O2.The Cdots adsorb the light and produce the excited electron.The photoexcited electron can transfer to the H2O2and promote its decomposition.That is the reason why Cdots can catalyze the decomposition of H2O2by light irradiation.
In summary, three types of Cdots with different adsorption band are synthesized from o-phenylenediamine and/or catechol as precursors by solvothermal method.The ph-F reactions were carried out to demonstrate Cdots had a capability to promote the H2O2decomposition.And the R-Cdots exhibit the highest ph-F reactivity attributing to the broader light absorption.What is more,the radical trapping results indicate the hydroxyl radical are produced from Cdots-H2O2system, which confirms the Cdots-H2O2is a type of photo-Fenton reaction.As the conduction band of Cdots is higher than the redox potential of H2O2,the mechanism of Cdots-H2O2is proposed base the energy diagram of Cdots.The photogenerated electron is transferred to the H2O2and promoted the decomposition of H2O2.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.21805004, 21671011, 21872001 and 51801006),theBeijing MunicipalHigh Level InnovativeTeamBuilding Program (No.IDHT20180504), the Beijing Outstanding Young Scientists Program (No.BJJWZYJH01201910005017), the Beijing Natural Science Foundation (No.2192005), the Beijing Municipal Science and Natural Science Fund Project(No.KM201910005016).
Appendix A.Supplementary data
Supplementary material related to this article canbefound, in the online version,at doi:https://doi.org/10.1016/j.cclet.2021.02.005.
杂志排行
Chinese Chemical Letters的其它文章
- A millimeter-sized negatively charged polymer embedded with molybdenum disulfide nanosheets for efficient removal of Pb(II) from aqueous solution
- Hyperterpenoids A and B: Two pairs of unprecedented 6/6/4/6/6 polycyclic cyclobutane meroterpenoids with potent neuroprotective and anti-inflammatory activities from Hypericum beanii
- Ammonia leaching mechanism and kinetics of LiCoO2 material from spent lithium-ion batteries
- Design, synthesis and biological evaluation of pyridyl substituted benzoxazepinones as potent and selective inhibitors of aldosterone synthase
- A DNA G-quadruplex converts SOD1 into fibrillar aggregates
- Built-in piezoelectric field improved photocatalytic performance of nanoflower-like Bi2WO6 using low-power white LEDs
