Highly-active, metal-free, carbon-based ORR cathode for efficient organics removal and electricity generation in a PFC system
2021-10-12QingyiZengShengChngMingqiWngMiLiQinwenDengZhuXiongBoxueZhouYnioLiu
Qingyi Zeng*,Sheng ChngMingqi Wng,Mi Li,Qinwen Deng,Zhu Xiong,Boxue Zhou,Ynio Liu**
a School of Resources & Environment and Safety Engineering, University of South China, Hengyang 421001, China
b Institute of Environmental Research at Greater Bay, Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education,Guangzhou University, Guangzhou 510006, China
c School of Environmental Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China
d Textile Pollution Controlling Engineering Center of Ministry of Environmental Protection, College of Environmental Science and Engineering, Donghua University, Shanghai 201620, China
ABSTRACT A highly-active, metal-free, carbon-based oxygen reduction reaction(ORR) cathode, i.e., graphitized Ndoped carbon felt (GNCF), was prepared, for the first time, by in-situ modifying the doping species of polyacrylonitrile (PAN)-based carbon felt (CF) via a facile annealing process in Ar atmosphere.It was applied for dramatically enhanced organics degradation and electricity generation in a photocatalytic fuel cell (PFC) system.The GNCF showed enhanced specific surface area, improved graphitization and raised ratio of graphitic N,therefore resulting in excellently improved ORR performance compared to the CF.When applying the GNCF as a cathode in a PFC system, the proposed PFC showed significant improvement in degrading various model organic contaminants and outputing electricity simultaneously when compared with the PFC with CF.For instance, the apparent rate constant and electricity output efficiency showed ~10.6 times and ~7.2 times,respectively,improvement when using rhodamine B as model waste.Further improved performance was also achieved by aeration of air or O2 due to the further enhanced ORR.The proposed PFC was also efficient in a wide pH,and kept outstanding stability in long-term utilization.
Keywords:Photocatalytic fuel cell Oxygen reduction reaction Electricity Graphitization N-doping
Resource utilization of wastewater(RUW)is a very meaningful and promising way for the sustainable development of human beings, because the situations of water pollution and resource shortage become increasingly urgent along with the development of industrialization and population growth [1–3].Photocatalytic fuel cell (PFC) is one of the most attractive RUW technology that has attracted worldwide interest due to its ability to remove refractory organic pollutants and generate electricity simultaneously[4–11].The PFC is characterized by the oxidation of organic waste as fuel by photoanodes and oxygen reduction reaction(ORR,Eq. 1) at the cathode accompanied by electricity generation in an external circuit under light illumination [4–7].

As a composite system, the performance of PFC is affected by several factors, such as the photocatalytic activity of photoanode,radical reactions in the solution and ORR at the cathode[9–11].The ORR at the cathode reflects the consumption of electrons in the PFC, which in turn affects the amount of photogenerated holes at the photoanode and their induced oxidation reactions.Therefore,improving the ORR efficiency at the cathode is an important way to enhance the performance of PFC.However,oxygen reduction rate at cathode is much slower than oxidation rate or electron generation rate at the photoanode.Pt based catalyst is still the most widely used cathode material in PFC due to its high ORR activity [4–7].Considering the limited availability and high cost,Pt-based cathode is not suitable for practical large-scale applications.
Carbon based ORR catalysts are raised a lot of attentions in recent decades, because they are more economical than Pt-based catalysts[12–17].Among them,non-noble metal(Fe,Co,Ni or Mn)doped carbon materials, such as carbon nanotubes (CNTs),graphene, graphitic arrays, and amorphous carbon were found to exhibit excellent electrocatalytic performance for ORR and widely studied in proton-exchange membrane fuel cells [13–17].However, metal doped catalysts have potential risks of releasing metal ions into the water.Nonmetal heteroatom (N, B, S, or P)doping is another widely studied method to prepare high efficient and low-cost ORR catalysts [18,19].It has been revealed that the electrocatalytic ORR activity originates from heteroatoms present in the graphitic framework, making the catalysts non-electronneutral and thus improving the oxygen molecular adsorption and its reduction [17,20,21].For example, N-doping essentially introduces nitrogen into the carbon structure, either at the edges or replacing one of the sp2-hybridized carbon atoms(graphitic N)in the graphitic structure[18].Lots of works have reported that N doped carbon materials are promising succedaneums of noble,commercial Pt/C catalysts [20–22].These non-toxic, low-cost and metal-free carbon ORR catalysts possess great potential in replace noble Pt-based catalyst.
Carbon felt(CF)has been widely used as electrode substrate in many domains,especially in the energy field,because of their high mechanical stiffness, well regulated porous structure, good chemical stability, environmental friendliness and excellent electrical conductivity [23–25].The commercial CF is usually prepared through carbonization of a polyacrylonitrile (PAN,–[C3H3N]n–) fabric, which is low-cost, chemically stable, easily produced and shaped.However, it is not pure carbon but containing lots of N and O elements [23].The doped N species include pyridinic N, pyrrolic N, graphitic N, and oxidized N [24].Although the commercial CF has lots of doped N species, the occupied species is normally pyrrolic N,which is not favor to ORR[19,20],and lots of O species are adverse to the charge transfer of the carbon material[26,27].Therefore,it is inaptitude to apply the commercial CF as electrode directly.
In this work,we developed a novel and simple treating process to prepare low-cost,highly-active,surface graphitized N-doped CF(GNCF)by an in-situ modification of the doped species of the PANbased CF via inert atmosphere annealing (Preparation of GNCF cathode in Supporting information).The GNCF possessed increased specific surface area, improved graphitization level and raised ratio of graphitic N,and showed significantly improved ORR activity.When applying the GNCF as a cathode in the PFC and using an anodized TiO2nanotube array (TNT, Fig.S1 in Supporting information) as a photoanode, the proposed PFC (TNT-GNCF)showed significant improvement in degrading various model organic contaminants and producing electricity when compared with the PFC with CF (TNT-CF).
As shown in Fig.1a, the carbon fibers of CF were interlaced to construct a porous network structure, which provided a conductive,polyporous and flexible substrate.The carbon fiber showed a diameter of ~22 μm(Fig.1b)and a relatively smooth surface with some longitudinal “ravines” (Fig.1c), which should be originated from the extrusion process of the PAN fibers.After annealing in Ar atmosphere at 1000°C, the network was not destroyed (Fig.1d),and the diameter of the fibers was also ~22 μm(Fig.1e).However,compared to the pristine CF, the surface of GNCF became rougher with a lot of tiny pits(Figs.1e and f),which significantly improves its specific surface area(Fig.S2 in Supporting information).X-ray photoelectron spectroscopy(XPS)survey further indicated that the facile inert atmosphere annealing modified the doping species of the PAN-based CF,resulting in an improved surface graphitization and ratio of graphitic N (Fig.2, detailed analysis is given), which was further demonstrated by the Fourier transform infrared spectroscopy (Fig.S3 in Supporting information) and Raman(Fig.S4 in Supporting information) measurements.All these results indicated that the GNCF possesses increased specific surface area,improved graphitization and raised ratio of graphitic N at its surface(Fig.S5 in Supporting information),which would be conducive to ORR.
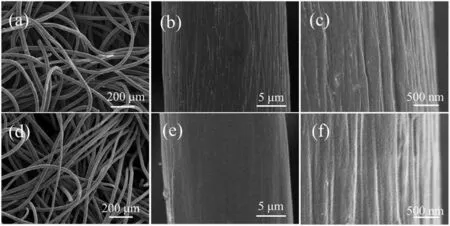
Fig.1.SEM images of (a–c) pristine CF and (d–f) GNCF under different magnifications.
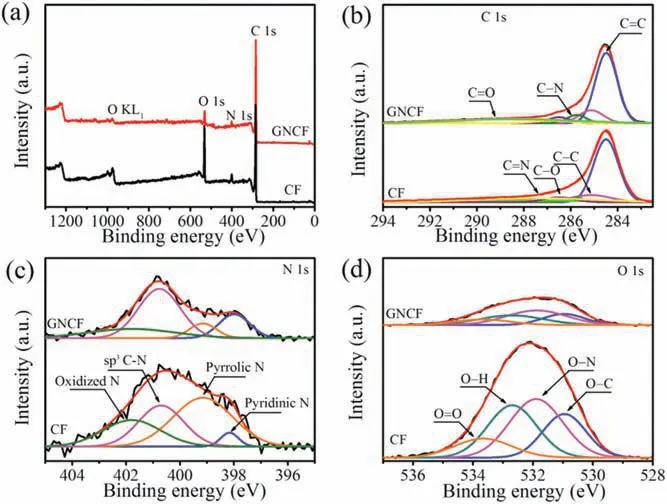
Fig.2.XPS spectra of CF and GNCF: (a) wide region scanning XPS spectra, and deconvoluted XPS spectra at (b) C 1s, (c) N 1s and (d) O 1s region.
The ORR performance of GNCF was investigated by cyclic voltammetry (CV) tests in various conditions.As shown in Fig.S6(Supporting information),compared with the CF,the GNCF showed an improved electrochemical characterization under these four conditions.From the negative scan curves,the ORR current density was excellent enhanced for the GNCF[19].When without blowing(Fig.3a),the reducing current density was relatively small and the ORR peak (around -0.4 V vs.Ag/AgCl) was relatively distinct,which should be due to the sluggish O2diffusion at the cathode/electrolyte interface [17].The ORR current density was only~0.55 mA/cm2for the CF,while it was improved to ~8.75 mA/cm2for the GNCF, which was as much as ~15.9 times higher.The current density was obviously increased when blowing gas(Figs.S6b-d in Supporting information), following the order of O2-blowing>air-blowing>N2-blowing,which demonstrated that increasing the concentration of dissolved O2could enhance the reducing current densities of both the CF and GNCF [22].Furthermore, during these tests, hydrogen was not detected,which indicated that both the CF and GNCF were sluggishness for HER [12].Therefore, these results clarified the efficient ORR performance of the GNCF compared with the pristine CF, and demonstrated that the proposed facile inert atmosphere annealing of PAN-based CF is a novel efficient method to prepared highlyactive, metal-free, carbon-based ORR materials.
To evaluate the feasibility of the TNT-GNCF for removing organic wastes,we firstly performed the degradation experiments by using rhodamine B(RhB)as the probe molecule,and the TNT-CF as a comparison.The preparation parameters, such as calcination temperature and duration,of the GNCF on the removal ratio of RhB were first evaluated (Fig.S7 in Supporting information), and the results indicated that the optimized values were 1000°C and 5 h,respectively.Therefore, all the mentioned GNCF was prepared at this optimized condition in this work.The removal ratio of RhB in the TNT-CF and the TNT-GNCF were shown in Fig.3a.For comparison, the removal ratios of RhB using single electrode,TNT, CF and GNCF were also tested (Fig.S8 in Supporting information), and they are 20.2%, 5.7% and 12.8%, respectively.However, the removal ratio of RhB reached ~98% after about 30 min operation and almost ~100%after 60 min in the TNT-GNCF,whereas the TNT-CF only showed a removal ratio of 41%.And the total organic carbon (TOC) removal rates were 35% and 11% for TNT–GNCF and TNT–CF, respectively.The apparent rate constant for the TNT-GNCF was ~0.1057 min-1, which was ~10.6 times higher than the TNT-CF (Fig.S9 in Supporting information).Obviously, the GNCF cathode significantly improved the performance of organic degradation in PFC systems.
During degrading RhB, the photocurrent density (J) of the PFC systems were tested under chopped light illumination model(Fig.3b).The steady J value of TNT–CF was only ~38 μA/cm2,while it was ~129 μA/cm2for the TNT-GNCF,which was ~3.4 folds that of TNT–CF.The relatively higher transient initial current for these PFC systems would be originated partially from the concentrated substrates’ concentration at the electrode surface and partially from the capacitance current of the PFC system at the initial stage after lighting on[4,6].The current–potential(J–V)curves as shown in Fig.3c further indicated that the TNT–GNCF showed excellent electricity output performance.The short–circuit current ( Jsc)densities were ~38.8 μA/cm2for the TNT–CF and ~127.1 μA/cm2for the TNT–GNCF.The open–circuit potentials (Voc) were 0.57 V and 0.73 V for the TNT–CF and for the TNT–GNCF,respectively.The maximum power density(Pmax) was ~3.98 μW/cm2for the TNT–CF (Fig.3d).However, the TNT–GNCF showed a Pmaxof ~32.77 μW/cm2, which was ~8.2 folds that of the TNT –CF.The increased Jscindicated that the GNCF significantly enhanced the transformation of electrons from the TNT to the cathode for ORR,and the higher Vocvalue of TNT-GNCF revealed that the GNCF could facilitate the activation of oxygen for ORR [12,13].Furthermore,the fill factor(FF)of the TNT–GNCF was ~35.3%while it was only 17.9% for the TNT–CF, which further demonstrates that the GNCF can accelerate the ORR process at the cathode while the CF has a sluggish ORR activity.During the degradation of RhB,steady and long-term current output was also observed for these PFCs(Fig.S10 in Supporting information).When using other kinds of model organic contaminants (Figs.S11 and S12 in Supporting information),the TNT-GNCF also showed significant improvement in degrading organics and outputting electricity.The TNT-CF showed the highest removal rate at a neutral condition(Fig.S13a in Supporting information), and the removal of RhB should be more efficient in acidic conditions compared with alkaline conditions.Furthermore, it showed excellent long-term stability in repeated use (Fig.S13b in Supporting information).
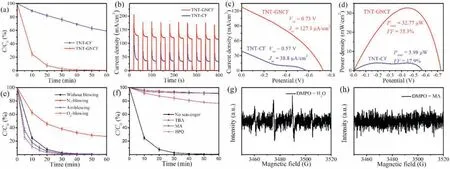
Fig.3.(a)Degradation of RhB,(b)chopped I–t curves,(c)I–V curves(10 mV/s)and(d)P–V curves of PFCs with various cathode tested at 20 mg/L RhB solution with 0.1 mol/L Na2SO4 as electrolyte and pH 7.0 under AM 1.5 illumination.(e)Degradation of RhB in the TNT–GNCF under different experimental conditions.(f)Effect of radical scavengers on the degradation of RhB.BMPO spin–trapping EPR spectra for the TNT–GNCF (g) in water for· OH and (h) in methanol for O2·-.
To clarify the possible mechanism of the rapid degradation of organics in the TNT-GNCF,we first evaluated the effect of gas purge on the removal of RhB.Generally, in the PFC system, the photogenerated holes of photoanode can directly oxidize organics and/or H2O to·OH, while the cathode collects electrons from the photoanode to reduce dissolved O2into H2O or O2·-[6–8].When purged the model wastewater with N2, air and O2, respectively,during the degradation tests, varied removal rates of RhB were observed (Fig.3e).
The removal rate of RhB was abated under the N2blowing,while it was raised when purged with air or O2, and the highest removal rate was obtained by purged with O2.Therefore,the result indicated that the dissolved O2played an important role in the degradation of RhB in PFC system, which should be attributed to the improved the consumption of electrons at the cathode when increasing the concentration of dissolved O2,accordingly improved the oxidization of organics at the photoanode.However, the ORR process would be hindered at the cathode when blowing N2,therefore restricting the separation rate of electron/hole pairs in the photoanode, which in turn weaken the organic degradation.
Furthermore, the effects of various scavengers (tert-butyl alcohol(TBA)for·OH,methyl alcohol(MA)for h+,p-benzoquinone(PBQ) for O2·-) on the removal rate of RhB were investigated to detect the main reactive oxidation species(ROS)in the TNT–GNCF[26,27].The results as shown in Fig.3f indicated that the addition of scavengers obviously inhibited the degradation of RhB, which should be due to the competitive consumption of ROS between RhB and scavengers.However,the inhibiting effects of TBA and MA were stronger than that of BPQ, which suggested that·OH and h+played a more important role in removing RhB than O2·-[28,29].
To further pinpoint the ROS in the TNT–GNCF, the BMPOtrapped electron spin resonance(EPR)technique was employed to detect ROS generated on the photoanode and cathode [30].As shown in Fig.3g,the signals ascribed to·OH were observed,which indicated that the PFC system could generate·OH during the degradation of RhB.To evidence this assumption, the·OH formation was further assessed by adding fluorescent probe,terephthalic acid (TA), into the TNT–GNCF, and the result also certified the steady generation of·OH (Fig.S14 in Supporting information) [31].However, no obvious signal was detected for O2·-(a one-electron process for reducing dissolved O2,Eq. 2)in the TNT–GNCF (Fig.3h) [32].Additionally, H2O2(a two-electron process for reducing dissolved O2, Eq. 3) was also not detected in TNT–GNCF using a N,N-diethyl-p-phenylenediamine (DPD) method (the result is not given), which implied that four-electron reduction of molecular oxygen (oxygen reduction reaction, ORR,Eq. 1) should be occurred at the GNCF [30].These result further demonstrated that the main ROS in the TNT–GNCF should be·OH and h+, and the GNCF was an efficient cathode for ORR.
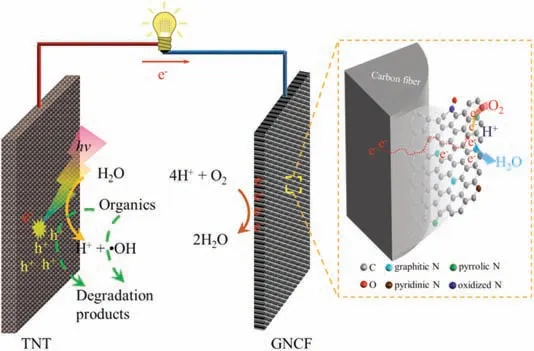
Fig.4.Schematic illustration of efficiently degrading organics and electricity generation in TNT–GNCF system.

The possible mechanism of TNT-GNCF for simultaneously degrading organic pollutants and generating electricity are showed in Fig.4.Under light illumination, the TNT can generate electron/hole(e-/h+)pairs.Under the effect of inter-bias between photoanode and cathode, the h+would transfer to the surface of TNT, and the e-would transfer to the GNCF cathode via the external circuit.Consequently, the holes should oxidize the chemisorbed H2O or OH-on TNT and generate·OH (Eqs.4 and 5 [6]) to mineralize organics, and the electrons should reduce dissolved O2to H2O(Eq. 1)at the GNCF and generate electricity at the external circuit.The improved graphitization and ratio of graphitic N on the surface of GNCF could facilitate the ORR at the cathode, implying the enhanced consumption of electrons, which in turn improve the separation of electron/hole pairs in the photoanode.Therefore, compared to the TNT-CF, the TNT-GNCF achieved an improved performance on organic degradation and electricity production.

In summary, we developed a novel, facile, cost-effective,industrially applicable strategy to prepare highly-active, metalfree, carbon-based ORR cathode (GNCF), and applied it in a PFC system for dramatically enhanced organics degradation and electricity generation.The strategy was designed by in-situ modifying PAN-based CF via a facile annealing process in Ar atmosphere, which successfully modified its surface, inducing enhanced specific surface area,improved graphitization and raised ratio of graphitic N.Therefore, the obtained GNCF showed excellently improved ORR performance compared the CF.The assembled TNT–GNCF also showed excellent improved performance for simultaneously degrading various refractory organic contaminants and outputting electricity when compared with the TNT–CF,because of the accelerated ORR at the cathode.In the TNT–GNCF,the main ROS to oxidize organic was detected as·OH,and the GNCF functioned as an efficient electron consumption electrode for ORR.The TNT–GNCF was also efficient in a wide pH, and neutral condition was the optimized.Its performance was further improved by aeration of air or O2owing to the further enhanced ORR, and kept outstanding stability in long-term utilization.This provides a new insight in developing highly-efficient, low-cost,scalable and stable cathode material for PFC system in simultaneous wastewater treatment and energy recovery.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.51808143), and the Natural Science Foundation of Guangdong Province (No.2018A030313367).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.12.062.
杂志排行
Chinese Chemical Letters的其它文章
- A millimeter-sized negatively charged polymer embedded with molybdenum disulfide nanosheets for efficient removal of Pb(II) from aqueous solution
- Hyperterpenoids A and B: Two pairs of unprecedented 6/6/4/6/6 polycyclic cyclobutane meroterpenoids with potent neuroprotective and anti-inflammatory activities from Hypericum beanii
- Ammonia leaching mechanism and kinetics of LiCoO2 material from spent lithium-ion batteries
- Design, synthesis and biological evaluation of pyridyl substituted benzoxazepinones as potent and selective inhibitors of aldosterone synthase
- A DNA G-quadruplex converts SOD1 into fibrillar aggregates
- Built-in piezoelectric field improved photocatalytic performance of nanoflower-like Bi2WO6 using low-power white LEDs
