Tritonia iocasica sp.nov.,a new tritoniid species from a seamount in the tropical Western Pacific (Heterobranchia:Nudibranchia)*
2021-10-12ShuqianZHANGSupingZHANG
Shuqian ZHANG ,Suping ZHANG
1 Laboratory of Marine Organism Taxonomy and Phylogeny, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Abstract During an expedition to a seamount at Caroline Plate in the tropical Western Pacific,a new species of Tritonia was sampled from the upper bathyal zone at depth of 970–1 262 m.This new species,Tritonia iocasica sp.nov.,represents the first tritoniid nudibranch known to feed on octocoral of the family Melithaeidae.The species is up to 120 mm in length,pinkish in color;with the rhinophoral sheath divided into several petaliform extensions;veil with about 18 elongate digitiform processes;notal margin with 17–19 pairs of secondary gills;anus located below the 5th and 6th secondary gills,and the genitalia below the 3rd secondary gill on the right side of the body.Based on these external features,T.iocasica sp.nov.can be clearly distinguished from all previously described members of the genus.Phylogenetic analyses of two mitochondrial (COI,16S rRNA) and a nuclear (H3) genes using Bayesian inference,maximum likelihood,and species delimitation analysis also support the separation of T.iocasica sp.nov.from its congeners.
Keyword:Tritoniidae;Mariana Trench;upper bathyal zone;Melithaeidae
1 INTRODUCTION
The genusTritoniaCuvier,1798 is a relatively diverse group of tritoniid nudibranchs that currently includes about 27 accepted species (MolluscaBase,2021).The genus has a worldwide distribution,ranging from the Atlantic and Mediterranean (Marcus,1983;Gosliner and Ghiselin,1987;Silva et al.,2014;Trainito and Doneddu,2014;Chimienti et al.,2020;Ortea and Moro,2020),eastern Pacific (Bertsch,2014;Valdés et al.,2018),Western Pacific (Baba,1969;Smith and Gosliner,2003),to Antarctica(Wägele,1995;Schrödl,2003;Ballesteros and Avila,2006),from the intertidal zone to abyssal depths(2 550 m,T.nigromaculataRoginskaya,1984).Most species are known or suspected specialist predators on octocorals,often limited to a single species or group of species of a single family (Smith and Gosliner,2003;García-Matucheski and Muniain,2011;Trainito and Doneddu,2014;Chimienti et al.,2020).The genus has been relatively well studied in the Atlantic Ocean,with nearly 20 species described.In contrast,however,the fauna in the Pacific region,especially in the Western Pacific,remains poorly studied,with only four described species.
Over the recent years,increasing explorations to deep-sea benthic ecosystems have yielded several novel species to the genusTritonia(e.g.Valdés et al.,2017,2018;Chimienti et al.,2020;Ortea and Moro,2020),indicating that the knowledge of deep-water fauna of the genus is still incomplete.In 2019,the Institute of Oceanology,Chinese Academy of Sciences (IOCAS) conducted a scientific cruise to a seamount at the Caroline Plate in the tropical Western Pacific.During this investigation,three individuals of a sea slug were collected.Examinations of their external morphology,radular features,and gross internal anatomy indicated that they represent an undescribed species belonging to the genusTritonia.Examination of gut contents revealed several coral fragments and sclerites that belong to the coral genusMelithaeaMilne Edwards,1857.This is the first record of a tritoniid species feeding on corals of the family Melithaeidae Gray,1870.Phylogenetic analyses based on two mitochondrial (COI,16S rRNA) and a nuclear (H3) genes using Bayesian inference,maximum likelihood,and species delimitation analysis also support the placement of the new species in the genusTritonia,and its separation from related congeners.
2 MATERIAL AND METHOD
2.1 Specimens sampling and preservation
Specimens were photographed and then collected from the upper bathyal zone (970–1 262 m) during two dives of the ROVFaxian(mother ship R/VKexue)(IOCAS) at a seamount near the Mariana Trench(Fig.1a).The specimens were photographed alive on board.One specimen was preserved in 99.5% ethanol(here designated as holotype MBM285105) and two others were frozen at -80 ℃ for genomic study.
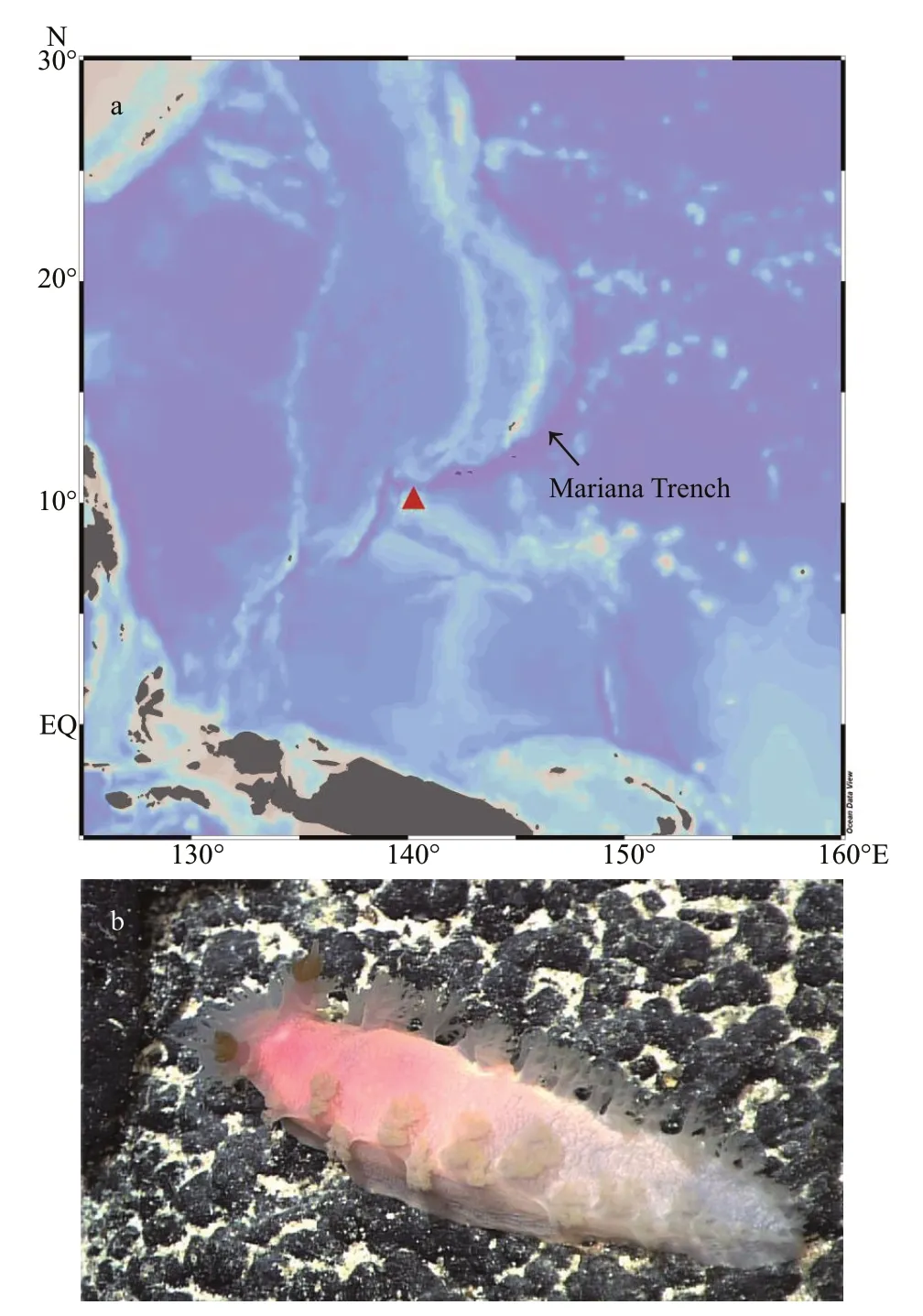
Fig.1 Geographical location of the type locality (a,solid red triangle) and the living specimen (paratype MBM285106) of Tritonia iocasa sp.nov.on a basalt rock at the type locality (b)
In the course of genomic study,one of the two specimens frozen at -80 ℃ was triturated thoroughly after dissecting its jaws,radula,and digestive gland.The jaws,radula,and digestive gland,together with a photograph of the living animal,are herein designated as paratype MBM285106 as a whole.The other specimen was entirely subjected to the genomic study,with no taxonomical observation and no gene fragments (i.e.COI,16S,and H3) sequenced.
The holotype and paratype have been deposited at the Marine Biological Museum of Chinese Academy of Sciences (MBMCAS).
2.2 Morphological examination
External morphology and internal anatomy were observed under a Zeiss Stemi SV 11 Apo stereomicroscope.Drawings of the digestive and reproductive systems were made with the aid of a Zeiss camera Lucida.For scanning electron microscopy (SEM) studies,jaws and radula were extracted from buccal bulb by gross dissection,cleaned using 10% NaOH,rinsed in distilled water,air-dried,coated with gold,and examined with SEM at an accelerating voltage of 5kV.Gut content and coral fragments were treated with undiluted commercial bleach in a centrifugal tube to obtain sclerites.These were washed several times with distilled water,transferred to aluminum stubs,coated with gold,and examined with Hitachi S-3400N SEM at an accelerating voltage of 5kV.
2.3 Molecular analyses
2.3.1 Extraction,amplification,and DNA sequencing
Holotype and paratype (see below) were selected for molecular studies.Genomic DNA from each individual was extracted with the Column Genomic DNA Isolation Kit (Beijing TIANGEN,China)according to the manufacturer’s instructions.DNA was eluted in elution buffer and stored at -20 ℃ until use.PCR reactions were carried out in a total volume of 50 μL,including 2-μL DNA template,1.5-mmol/L MgCl2,0.2 mmol/L of each dNTPs,1 μL of bothforward and reverse PCR primers,10× buffer and 2.5-U Taq DNA polymerase.Thermal cycling was performed under the following conditions:94 ℃ for 3 min (initial denaturation),followed by 35 cycles of 94 ℃ for 30 s (denaturation),40 s at primer-specific annealing temperatures,72 ℃ for 60 s (extension)and a final extension at 72 ℃ for 10 min.PCR and sequencing primers for COI,16S rRNA,and H3 genes are listed in Table 1.PCR products were verified on a GelRed-stained 1.5% agarose gel and purified with the Column PCR Product Purification Kit(Shanghai Sangon,China).
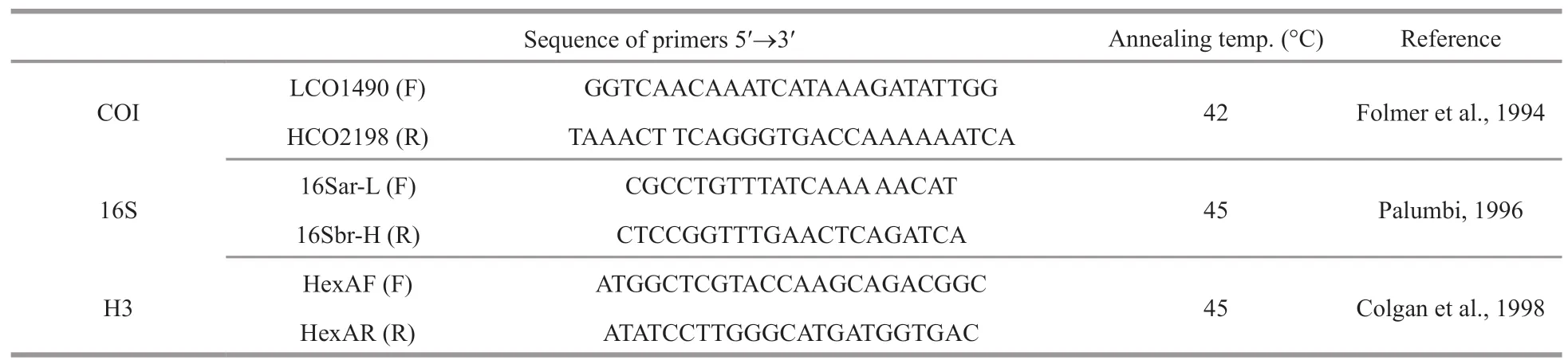
Table 1 PCR primers used to amplify regions of the mitochondrial COI,16S genes,and nuclear H3 gene
2.3.2 Phylogenetic analyses
Two specimens (MBM285105 and MBM285106)were subjected to molecular analysis.In addition,other sequences ofTritoniaspp.were retrieved from GenBank and used for phylogenetic analyses(Table 2).We followed Korshunova and Martynov(2020) to include other tritoniid species in the analyses for comparison,and have usedPleurobranchaeameckeli(De Blainville,1825) to root the tree.
The faithful servant, however, told the queen that it was the king s command that during the years he was absent in the war she should keep herself concealed26 in the castle, so that no one but himself should see her and the little princes
Sequence alignments were generated with the MAFFT algorithm (Katoh and Standley,2013).COI and H3 sequences were translated into amino acids for confirmation of the alignment.Separate analyses were conducted for COI (567 bp),16S (413 bp),H3(288 bp),and concatenated data (1 268 bp).Ambiguously aligned fragments of 16S alignment was removed using Gblocks (Talavera and Castresana,2007) with the following parameter settings:minimum number of sequences for a conserved/flank position(53/53),maximum number of contiguous nonconserved positions (8),minimum length of a block(10),allowed gap positions (with half).Maximum likelihood phylogenies were inferred using IQ-TREE(Nguyen et al.,2015) under Edge-unlinked partition model for 20000 ultrafast (Minh et al.,2013)bootstraps,as well as the Shimodaira-Hasegawa-like approximate likelihood-ratio test (Guindon et al.,2010).Bayesian Inference phylogenies were inferred using MrBayes 3.2.6 (Ronquist et al.,2012) under partition model (2 parallel runs,5 000 000 generations),in which the initial 25% of sampled data were discarded as burn-in.The best-fit models of evolution (GTR+I+G for COI and 16S,GTR for H3)were determined using PartitionFinder2 (Lanfear et al.,2017),with greedy algorithm and the Akaike information criterion AIC (Akaike,1998).Thepdistances within and among each species grouping were estimated with MEGA X (Kumar et al.,2018).
To discriminate species,we included 27 COI sequences for analyses based on the Kimura 2-parameter model.Bayesian inference (BI) was conducted using the software MrBayes v.3.2.6(Ronquist et al.,2012) based on the GTR+I+G model.The Metropolis-coupled Monte Carlo Markov chains were run for 5 000 000 generations,with sampling every 100 generations;split frequencies were less than 0.01 before the analyses were terminated.A 25%burn-in was applied before constructing the majorityrule consensus tree.Results were visualized using FigTree V.1.4.3.
2.4 Species delimitation analysis
The Automatic Barcode Gap Discovery (ABGD)method (Puillandre et al.,2012) was used to assess the number ofTritoniaspecies studied herein.The alignment from the fast-evolving COI gene was uploaded to the online server of ABGD.The analysis was performed with the model of Jukes-Cantor (JC69)with the following settings (Pmin=0.001,Pmax=0.1,steps=10,X=1.0,Nb bins=20).
3 RESULT
3.1 Phylogenetic analyses
Partial sequences (COI and H3) were obtained from the holotype (MBM285105) and paratype(MBM285106),while that of 16S was from the holotype only (see Table 2 for accession numbers).The two consensus trees inferred using Bayesianinference (BI) and maximum likelihood (ML) criteria were generally congruent (Figs.2 &3).The genusTritonia,including the new species,was recovered as monophyletic with strong support (posterior probability,PP=1;bootstrap value,BS=89).The analyses based on concatenated dataset (COI,16S,and H3) showed thatT.iocasicasp.nov.form a separate clade within the genus.
Table 2 List of species and GenBank accession numbers of sequences used in the present study
The BI analysis based on the concatenated COI,16S and H3 markers does not solve (PP=0.62) the relationships amongT.iocasicasp.nov.,T.challengeriana,and the remainingTritoniaspp.included in this study,although the monophyly of the genus is recovered.The phylogenetic tree inferred using BI criteria based on single gene COI(Supplementary Fig.S1) only support a joint relationship ofT.iocasicasp.nov andT.challengeriana,but does not recover the monophyly ofTritonia.
On the other hand,the ML analysis based on the concatenated COI,16S,and H3 markers support a joint relationship amongT.iocasicasp.nov.withT.challengeriana.These two species clustered together form a clade sister to otherTritioniaspp.(BS=89).
These results support the systematic placement of the new species in the genusTritoniaand its separation from other congeners.
3.2 Species delimitation analyses
The ABGD analysis of the COI sequences resulted in the delimitation of seven species (Fig.2),with values of prior intraspecific divergence (P) being≥0.001 0.These groups correspond to clades recovered by BI and ML analyses.Within the genus,the two specimens of the new species are closest related to each other,with 0.3% pairwise distance;with the sequences studied herein,the pairwise distances amongT.iocasicasp.nov.and other species ofTritoniaare 17.8%–20.5% (Table 3).
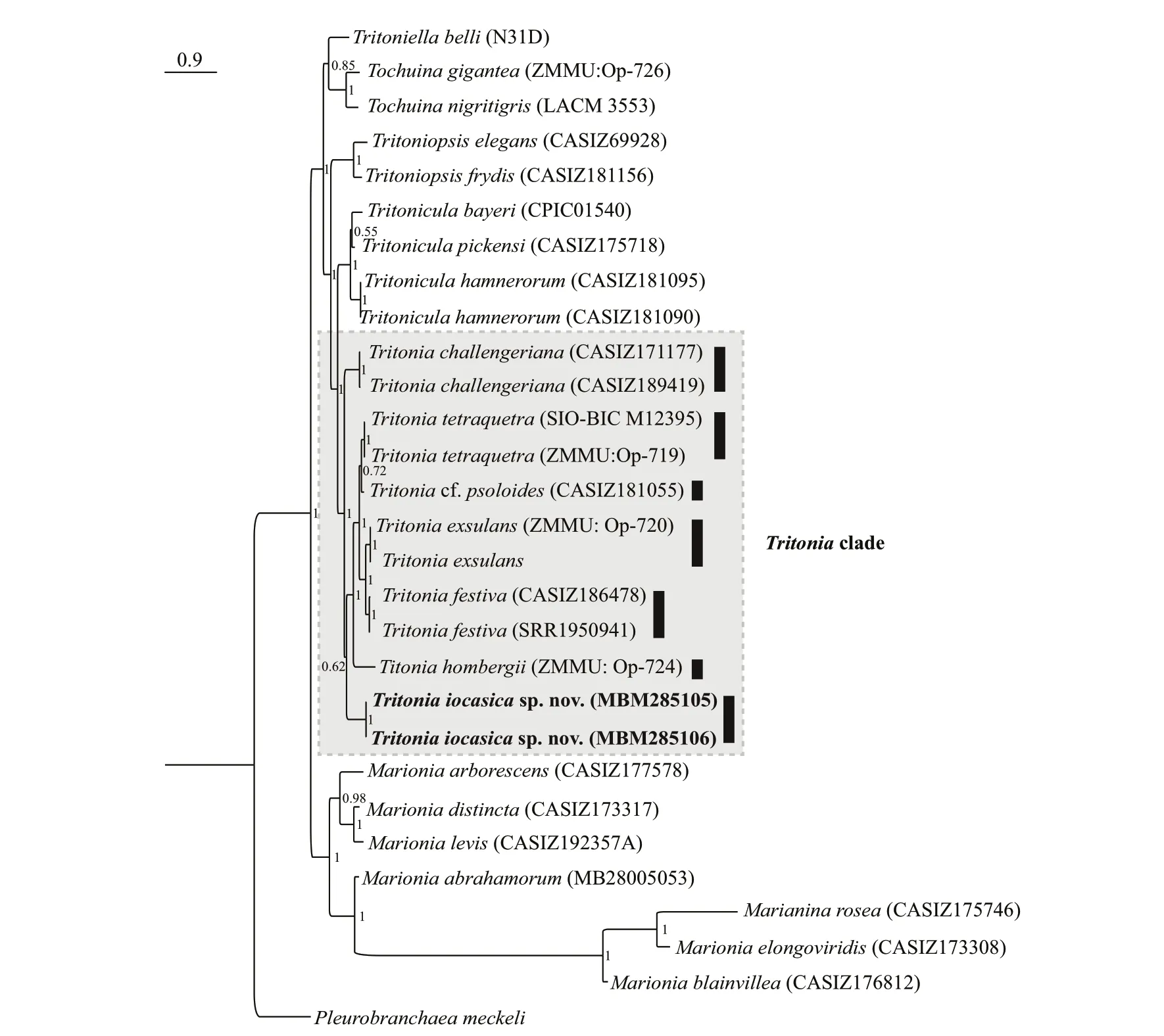
Fig.2 Phylogenetic tree inferred by Bayesian analysis (BI) based on concatenated dataset of COI,16S,and H3 genes
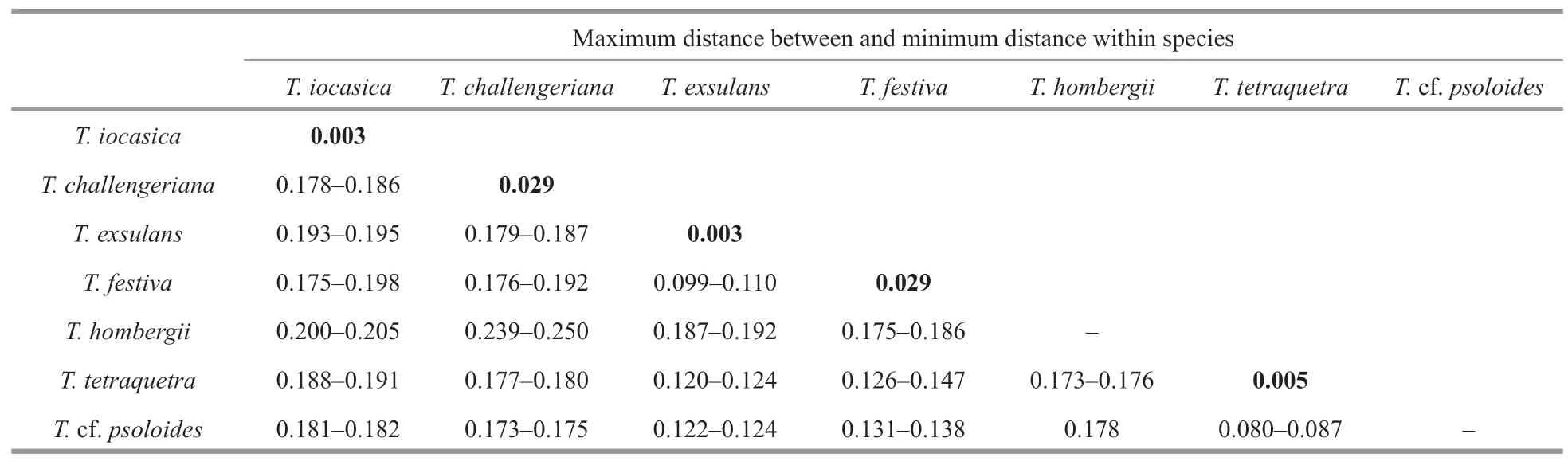
Table 3 Estimates of p-distances of mitochondrial COI gene among Tritonia species and studied sequences
3.3 Systematics
Superfamily Tritonioidea Lamarck,1809
Family Tritoniidae Lamarck,1809
GenusTritoniaCuvier,1797
Type species:TritoniahombergiiCuvier,1803,subsequent designation by Lemehe (1961).
Tritonia iocasica sp.nov.(Figs.1b,4–6)
http://zoobank.org/urn:lsid:zoobank.org:act:C29C82DB-08A5-490C-8512-3976CCD28132
Material examined:Holotype MBM285105,live length 120 mm,dissected and sequenced,Station FXDive 212,(10°03ʹN 140°11ʹE) at depth of 1 262-m,30 May 2019.
Paratype MBM285106,live length 120 mm,partly used for genomic analysis,with only jaws,radula,and digestive gland remaining for taxonomical study,Station FX-Dive 209,(11°36ʹN,140°12ʹE) at depth of 970-m,27 May 2019.
An additional specimen,live length 110 mm,entirely used for the genomic study,with no taxonomical observation and no gene fragmentssequenced.Collected together with the paratype.
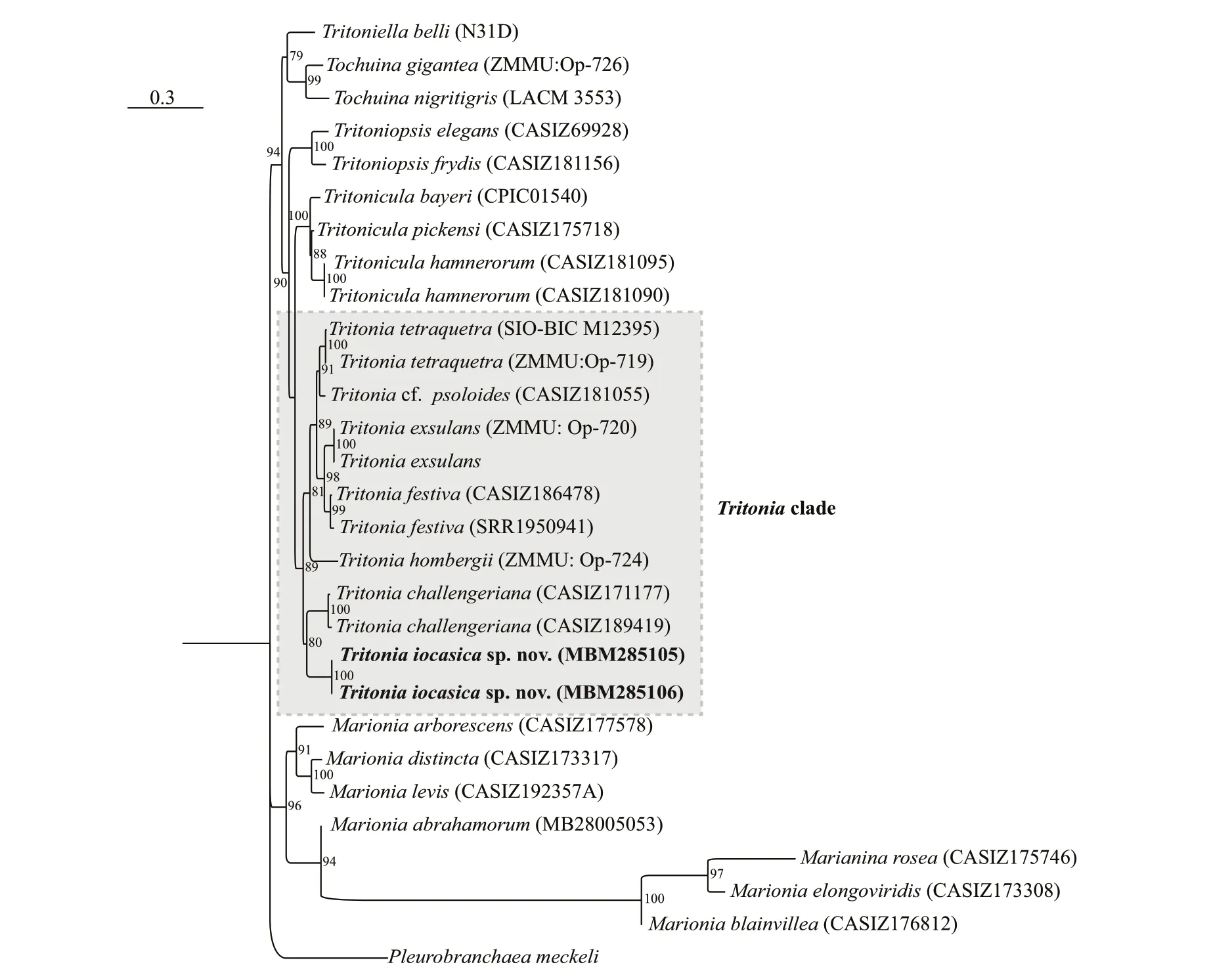
Fig.3 Phylogenetic tree inferred by maximum likelihood (ML) based on concatenated dataset of COI,16S,and H3 genes
Type locality:A seamount at the Caroline Plate near the Mariana Trench,10°03ʹN,140°11ʹE.
Etymology:The new species is named after IOCAS (Institute of Oceanology,Chinese Academy of Sciences),in celebration of its 70thanniversary.
Description:
EXTERNAL ANATOMY (Figs.1b,4a–c,paratype MBM285106):Body elongate,slender.Dorsum covered with weak reticulate pattern of low ridges,giving dorsal surface a fish-scale appearance.Velum rounded,with about 18 elongate digitiform velar tentacles (Fig.4b).Notal margin with 17–19 pairs of secondary gills.Rhinophores long,located on anterior end of notum,bearing a cluster of pinnate processes that surrounds cylindrical apical papilla.Rhinophoral sheath wide,divided into several (~10) petaliform lobes.Foot smooth,anterior end rounded,gradually tapering posteriorly.Genital,anal opening on the right side of body (Fig.4c).Anus situated below the 5thand 6thsecondary gills,genital opening below 3rdsecondary gills.Background color of living animal uniformly pinkish (same color as prey) except for pinnate processes on rhinophores which is brownish.Lighter pink on posterior 2/3 of the notum,secondary gills,and velum.
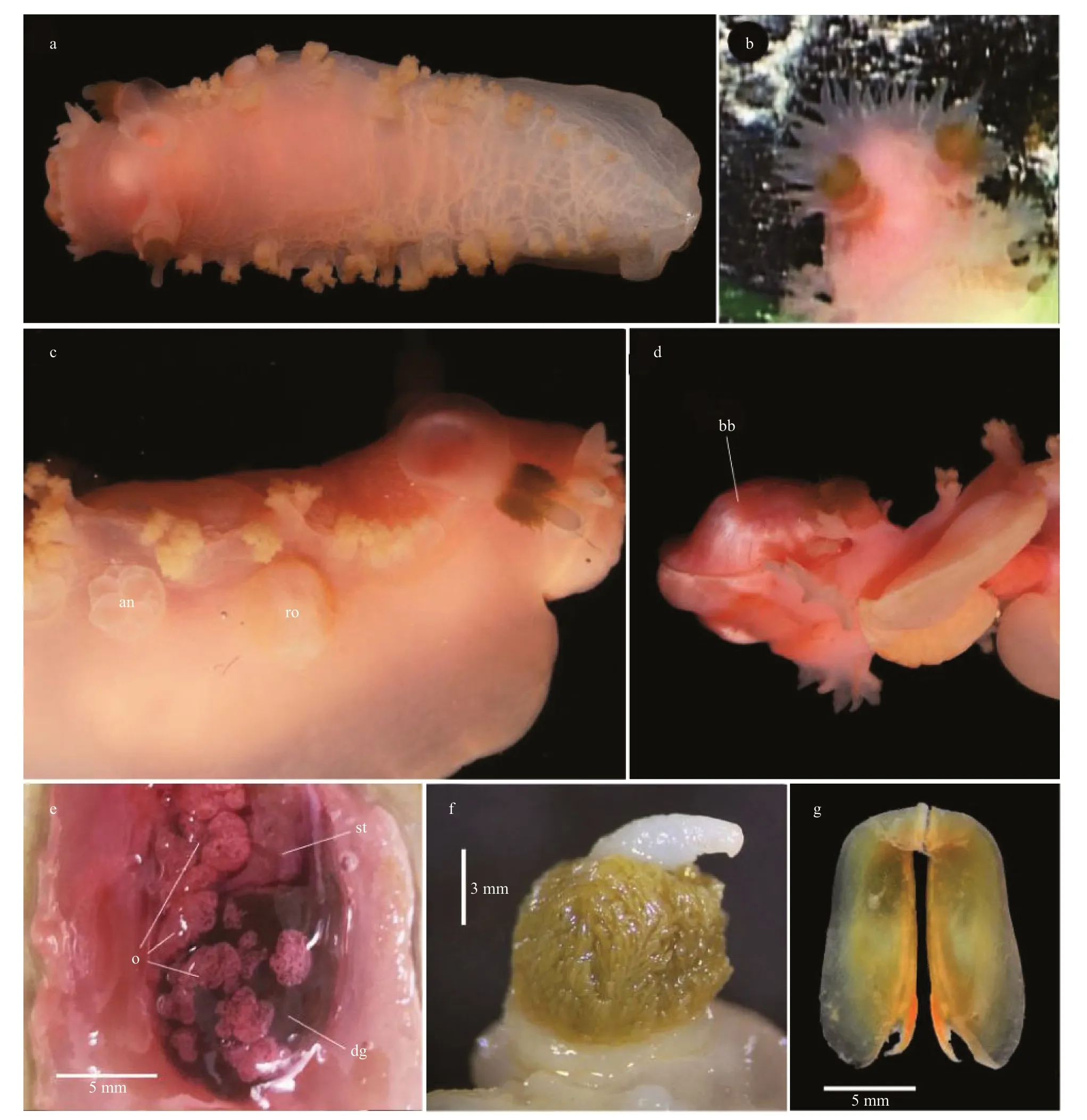
Fig.4 Tritonia iocasica sp.nov.
INTERNAL ANATOMY:Digestive system(Fig.5a,holotype MBM285105) with thick,muscular buccal bulb (Figs.4d &5a).Esophagus wide,anterior part connecting into buccal bulb.Posteriorly,it expands into a wider tube connecting to the stomach.The stomach oval in shape and its posterior part embedded ventrally into the digestive gland.Two long salivary glands connecting with the buccal bulb at each side of the esophageal junction.Intestine emerging dorsally from left side of digestive gland,forming simple loop towards right side of body,where it opens into the anus.Stomach simple,no stomach plates present.Digestive gland reddish brown,large(Fig.4e).Jaws (Figs.4g,6a–c,paratype MBM285106)thin,elongate,amber-colored,inner margin(masticatory margin) terminating distally in small pointed process.The masticatory margin apparently worn at upper end;1/3 lower part with about 18 or more rows of rodlets.Maximum length of rodlets about 200 μm in length,and maximum width about 40 μm.Most rodlets with irregularly pentagonal or hexagonal cross section.Radula (Fig.6d–h,paratype MBM285106) with a formula of 62×100.1.100.Rachidian teeth tricuspid,with nearly rectangular bases.Central cusp sharply pointed and longer;outer cusps blunt and shorter,fused to margin of base.Distinct,deep incision between central cusp and right outer cusp (Fig.6f).First lateral teeth with thicker base,sturdy,hoodlike cusps (Fig.6g).Remaining lateral teeth sickle-shaped,with sharp cusps,becoming thin towards outer margins of radula (Fig.6h).
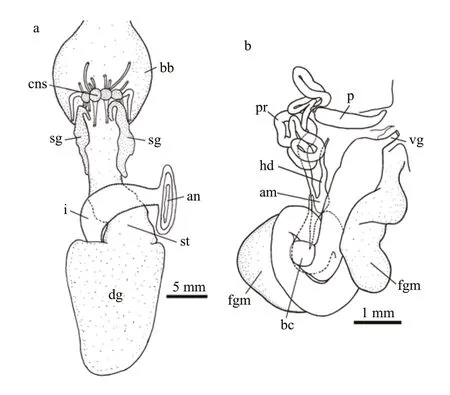
Fig.5 Tritonia iocasica sp.nov.,holotype,MBM285105
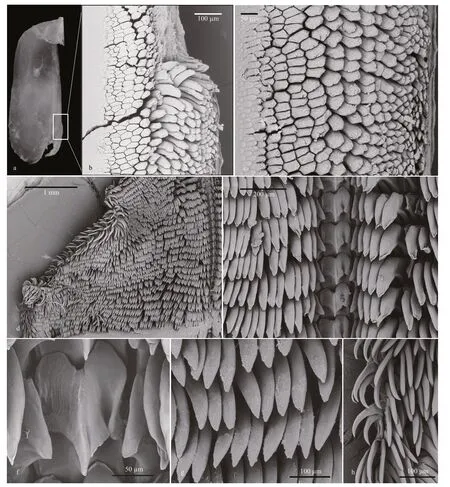
Fig.6 Micrographs of jaw and radula of Tritonia iocasica sp.nov.,specimen MBM285106
Reproductive system (Fig.5b,holotype MBM285105) androdiaulic.Ovotestis covering most of surface of digestive gland,uniformly pinkish(Fig.4e).Ampulla short,simply convoluted,opening on female gland mass where prostate connects.Prostate narrow,elongate,forming several loops and ending into narrow deferent duct,which connects with conical penial sac.Penis simple,elongate.Vagina elongate,broad,opening into the spherical bursa copulatrix.
Diet:Intestinal tract contained coral fragments and calcareous sclerites (Fig.7) of an unidentified coral species,Melithaeasp.(Melithaeidae).
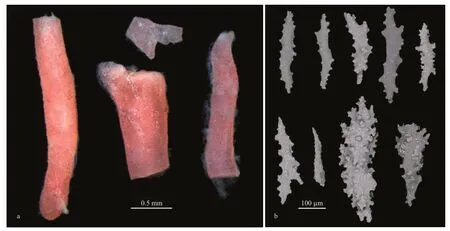
Fig.7 Coral fragments belonging to Melithaea sp.dissected from intestinal tract of specimen MBM285106 (a) and different forms of sclerites of coral (b)
4 DISCUSSION
The systematics of Tritoniidae has long been considered problematic despite the revisions by Odhner (1936,1963) and Marcus (1983).Relationships among the genera are still unclear and the placement of some species remain tentative(Willan,1988;Schrödl,2003).The fact that previous authors utilized different characters to distinguish genera may account for the problem.Nevertheless,recent phylogenetic studies also showed that most tritoniid genera currently recognized are not monophyletic (Bertsch et al.,2009;Almón et al.,2018).A revision of the whole family based on more detailed anatomical information and molecular evidence is therefore needed to define generic boundaries (Korshunova and Martynov,2020).Gosliner and Ghiselin (1987) treated all species of tritoniids with well developed secondary gills,with a tricuspid rachidian tooth,and without stomach plates as members ofTritonia.In the present study,we follow this classification,to assign the new species to the genusTritonia.This assignation was supported by molecular evidence.In the phylogenetic tree reconstructed herein (Figs.2 &3),T.hombergii,the type species of the genus,together with otherTritoniaspp.,was recovered as a monophyletic clade (PP=1,BS=89).The trees based on concatenated data and single COI gene all showed thatT.iocasicasp.nov.form a separate clade sister to otherTritoniaspp.The species delimitation andp-distance analyses strongly support the separation ofT.iocasicasp.nov.from its congeners.Within the genus,T.iocasicasp.nov.is unique by its external morphology and distinct color pattern.At present,no other known species,to our knowledge,can be confused with the new species.In the Western Pacific region,there are only four otherTritoniaspecies that have been described (Table 4),includingT.bollandiSmith and Gosliner,2003 from Okinawa,Japan and Indonesia (58–88-m depth),T.festiva(Stearns,1873),an intertidal species native to Pacific coast of North America but also occurs in Japan Sea (Baba,1969),T.olivaceaBergh,1905 from Indonesia (32-m depth) andT.tetraquetra(Pallas,1788) from Northwestern Pacific (1–700-m depth).T.iocasicasp.nov.thus represents the first ofTritoniaknown from deep sea of the tropical Western Pacific,and,to our knowledge,the first for the family Tritoniidae.Unlike in the Atlantic and the eastern Pacific,the taxonomic knowledge of the genus in the Western Pacific is very poor,although several additional undescribed species were reported and illustrated by Gosliner et al.(2008,2018).This probably results from the fact that deep-sea fauna of the Western Pacific was relatively poorly investigated.Another explanation is that many recordedTritoniaspecies were unidentified or misidentified,as previous taxonomic studies were based primarily on few external characters that always result in taxonomic confusions and in turn,an inaccurate estimate of biodiversity.In central Pacific,an individual likely belonging to the genus was photographed by ROVDeep Discoverer(NOAA)from Musicians Seamounts (see https://www.ncei.noaa.gov/waf/okeanos-animal-guide/Gastropoda030.html).This individual is highly similar toT.iocasicasp.nov.in coloration pattern,body shape,numbers of secondary gills and velar tentacles,and thus may be conspecific with the new species described herein.If this is the case,it would extends the distribution ofT.iocasicasp.nov.to the central Pacific.

Table 4 Comparison of the main characteristics of some related species of Tritonia
Internally,T.iocasicasp.nov.has a jaw armed with multiple rows of rodlets (Fig.5b &c).This type of denticulation has been only recorded for a single species in the genus (Chimienti et al.,2020).In the whole family,only two other species,MarioniabathycarolinensisSmith and Gosliner,2005 andMarioniatediEv.Marcus,1983,were known to have a jaw armed with rows of rodlets (Smith and Gosliner,2005;Valdés,2006).Compared to these three species,the rodlets in the new species is much longer.The type of this denticulation may facilitate the distribution of stress that might damage the thin,delicate inner edge of the jaw during cutting the coral stem.
Examination of the digestive tract ofT.iocasicasp.nov.revealed that the gut contains several coral fragments and calcareous sclerites that belong toMelithaeasp.The three specimens studied herein were collected from a single seamount near the Mariana Trench during two ROV dives.Two of the specimens (Fig.1b) were discovered crawling on a platform of basalt rock.The entire area is partially colonized by primnoid octocorals,flabelliid hexacorals,the hydroidStegolariasp.,and pheronematid sponges.Two or three groups ofMelithaeasp.(Melithaeidae) were observed (not collected) from distance of about 1.5–2 m.The diet of theTritoniaspecies was reviewed by García-Matucheski and Muniain (2011);the members exclusively feed on octocorals,especially on the gorgonians,often limited to a single species or group of species within a single family.The present study represents the first record of predation by a tritoniid species on octocoral of family Melithaeidae,which expands our knowledge of the predation range ofTritonia.
The majority ofTritoniaspecies live in intertidal or shallow subtidal waters,and only five have been reported down to 500 m (Bergh,1899;Bouchet,1977;Roginskaya,1984;Valdés et al.,2017,2018).Until now,the evolutionary history of species inTritoniais still little known.Actually,it is diffi cult to make speculation as to the time of theTritoniaspecies originated from shallow water or deep sea,due to lack of a fossil record for these shell-less mollusks.
5 DATA AVAILABILITY STATEMENT
The authors declare that all the data supporting the findings of this study are available within the article.
6 ACKNOWLEDGMENT
The data and samples were collected onboard R/VKexue.We would like to express our sincere thanks to the crews for their assistance during the survey.We are very grateful to Prof.Kuidong XU and Dr.Xuwen WU for their eff orts in collecting the specimens.Dr.Yang LI identified the coral and helped in conducting the SEM study of the coral sclerites.Four anonymous reviewers provided valuable comments and suggestions that greatly improved the manuscript.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Screening of stable internal reference genes by quantitative real-time PCR in humpback grouper Cromileptes altivelis*
- Morphology and multifractal features of a guyot in specific topographic vicinity in the Caroline Ridge,West Pacific*
- Geochemical characteristics and geological implication of ferromanganese crust from CM6 Seamount of the Caroline Ridge in the Western Pacific*
- Deep-sea coral evidence for dissolved mercury evolution in the deep North Pacific Ocean over the last 700 years*
- Physical oceanography of the Caroline M4 seamount in the tropical Western Pacific Ocean in summer 2017*
- Characteristics and biogeochemical effects of oxygen minimum zones in typical seamount areas,Tropical Western Pacific*
