Hydrogen peroxide-generating nanomedicine for enhanced chemodynamic therapy
2021-10-12PengYuXiodongLiGuohuiChengXuZhngDnWuJinChngShengWng
Peng Yu,Xiodong Li,Guohui Cheng,Xu Zhng,Dn Wu*,Jin Chng,*,Sheng Wng,*
a School of Life Sciences, Tianjin University, Tianjin 300072, China
b College of Chemical and Biological Engineering, Zhejiang University of Technology, Hangzhou 310014, China
c The Second Hospital, Tianjin Medical University, Tianjin 300211, China
ABSTRACT Chemodynamic therapy (CDT) is an emerging endogenous stimulation activated tumor treatment approach that exploiting iron-containing nanomedicine as catalyst to convert hydrogen peroxide(H2O2)into toxic hydroxyl radical(·OH)through Fenton reaction.Due to the unique characteristics(weak acidity and the high H2O2 level)of the tumor microenvironment,CDT has advantages of high selectivity and low side effect.However,as an important substrate of Fenton reaction,the endogenous H2O2 in tumor is still insufficient, which may be an important factor limiting the efficacy of CDT.In order to optimize CDT,various H2O2-generating nanomedicines that can promote the production of H2O2 in tumor have been designed and developed for enhanced CDT.In this review, we summarize recently developed nanomedicines based on catalytic enzymes, nanozymes, drugs, metal peroxides and bacteria.Finally,the challenges and possible development directions for further enhancing CDT are prospected.
Keywords:Nanomedicine ROS Chemodynamic therapy Fenton reaction Hydrogen peroxide
1.Introduction
Reactive oxygen species(ROS),such as superoxide anion(O2-),hydrogen peroxide (H2O2) and hydroxyl radical (·OH), generally refer to a class of highly active reduction products of oxygen with one or more unpaired electrons [1].Normal body metabolism produces part of ROS, and ROS at normal concentration are beneficial to cell growth and proliferation[2].However,high level of ROS will damage nucleic acids and proteins, which is detrimental to cell survival.Furthermore, cancer cells often lack some key antioxidant enzymes, so they are more susceptible to damage by increased intracellular ROS concentration [3].Take advantage of this characteristic of cancer cells,various ROS-based treatment approaches that kill cancer cells by further promoting the generation of ROS at tumor sites have received increasing attention in recent years [4–8].This treatment strategy based on the different responses of cancer cells and normal cells to ROS has brought relatively specific tumor targeting and protection to normal cells.
Among the ROS generation approaches, iron-mediated Fenton reaction is a promising method that converts less-reactive H2O2into highly toxic·OH in acidic condition [9].Based on Fenton reaction,chemodynamic therapy (CDT), which uses iron-containing nanomedicine as catalyst to generate toxic·OH at the tumor site, has attracted much attention in recent years [10,11].The amount of ROS production is critical in ROS-based treatment strategies because insufficient amount of ROS can even promote the development of the tumor [12].In general, H2O2is overexpressed in weak acidic tumor microenvironment (TME); while insufficient in weak alkaline normal tissues[13].The environmental difference between tumor tissue and normal tissue ensures high selectivity and low side effect of CDT [14].The therapeutic effect of CDT often depends on the strength of Fenton response[15].Therefore,it is increasingly important to find suitable ways to promote the Fenton response to improve efficacy.
Various methods such as optimizing nanomaterials [16–19],adjusting the reaction environment[20],and introducing external energy[21,22]have been developed for improving Fenton reaction efficacy.However,as an important substrate of the Fenton reaction,the endogenous H2O2in tumor is still insufficient to generate sufficient·OH, which may be an important factor limiting the efficacy of CDT.In order to increase the concentration of H2O2in tumor, targeted delivery of exogenous H2O2through nanocarriers has been studied[23].But the leakage of H2O2during transportation may cause low delivery efficiency and damage to normal cells.Another method to increase intratumoral H2O2level is promoting the production of H2O2in tumor.In order to ensure the selective production of a large amount of H2O2at the tumor site, various H2O2-generating nanomedicines containing catalytic enzymes or nanozymes, drugs, metal peroxides and even bacteria have been designed and developed for enhanced CDT(Fig.1).Herein,we will briefly introduce the working mechanisms and applications of these nanomedicines (Table 1) [2,24–42].The prospects and challenges are also discussed.
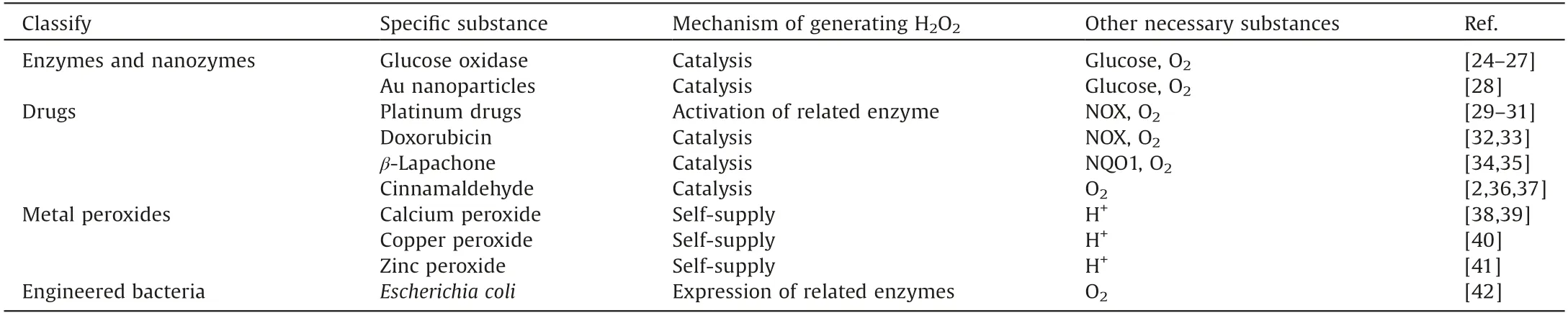
Table 1 A summary of various methods to increase H2O2 level.
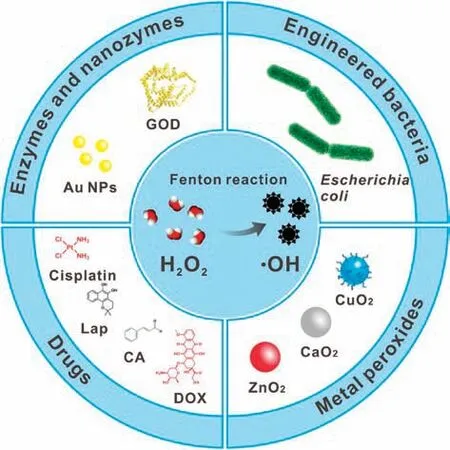
Fig.1.Schematic illustration of H2O2-generating nanomedicines for enhanced CDT.
2.Enzymes and nanozymes
One of the main methods for generating H2O2in situ is utilizing catalysis of enzymes, of which the most widely used is glucose oxidase (GOD) [43].GOD can convert glucose into H2O2and glucose acid in the presence of oxygen, which provides the possibility to solve the hydrogen peroxide deficiency [44].The large amount of glucose in TME provides favorable conditions for GOD to produce H2O2[45].And the production of glucose acid further enhances the acidity of TME, which can promote the efficiency of Fenton reaction.
Recently, a large number of studies have reported GODmediated production of H2O2for CDT [44].Lin et al.prepared a manganese dioxide (MnO2)-coated iron carbide (Fe5C2) magnetic nanoparticle for GOD loading, the resulted Fe5C2-GOD@MnO2could be used for CDT through Fenton reaction (Fig.2a) [27].In response to the weak acidity of TME,MnO2would decompose into Mn2+and O2, leading to increased O2concentration and GOD release.The O2can promote the catalytic efficiency of GOD to produce sufficient H2O2in tumor region.Finally,·OH could be generated through Fe5C2-mediated Fenton reaction for triggering tumor cells apoptosis and death.As shown in Fig.2b, in the presence of glucose,the characteristic peaks of·OH were observed in electron spin resonance (ESR) spectrum of the Fe5C2-GOD@-MnO2,indicated that·OH was generated in this situation.However,no obvious signals were detected without glucose, indicating the key role of glucose in·OH generation.The generation of·OH in different conditions were further quantitatively analyzed by chromogenic 1,3-diphenylisobenzofuran (DPBF), which can be oxidized by·OH into colorless radicals.As shown in Fig.2c,under acidic condition with glucose, the absorbance of DPBF was decreased obviously, indicating a large amount of·OH was generated;in contrast,under neutral conditions or acidic condition without glucose, the absorbance of DPBF did not decrease significantly.These results demonstrated that GOD-mediated H2O2producing can effective enhance the·OH generation.The intracellular ROS was detected by using a ROS probe, 2′,7′-dichlorofluorescin diacetate (DCFH-DA).As shown in Figs.2d and e,cells incubated with Fe5C2@MnO2did not show intracellular ROS level change when compared with control group, indicated that the GOD was the critical part in ROS generation process.In vitro cell viability and live/dead cell staining results proved that Fe5C2@MnO2has good biocompatibility and negligible cytotoxicity;while Fe5C2-GOD@MnO2exhibited strong inhibitory activity on HeLa cells(Figs.2f and g).Furthermore,the therapy effect could be improved by magnetic targeting.The anticancer effect of Fe5C2-GOD@MnO2was further demonstrated by in vivo experiment.The tumor growth was significantly inhibited by Fe5C2-GOD@MnO2,indicating the effectiveness of CDT.Importantly,magnetic targeted Fe5C2-GOD@MnO2group displayed the best antitumor efficacy with the inhibition rate of 92% (Fig.2h).Therefore, the nanocatalytic particles have the characteristics of magnetic targeting,TME response, high safety, and strong anti-cancer effect.
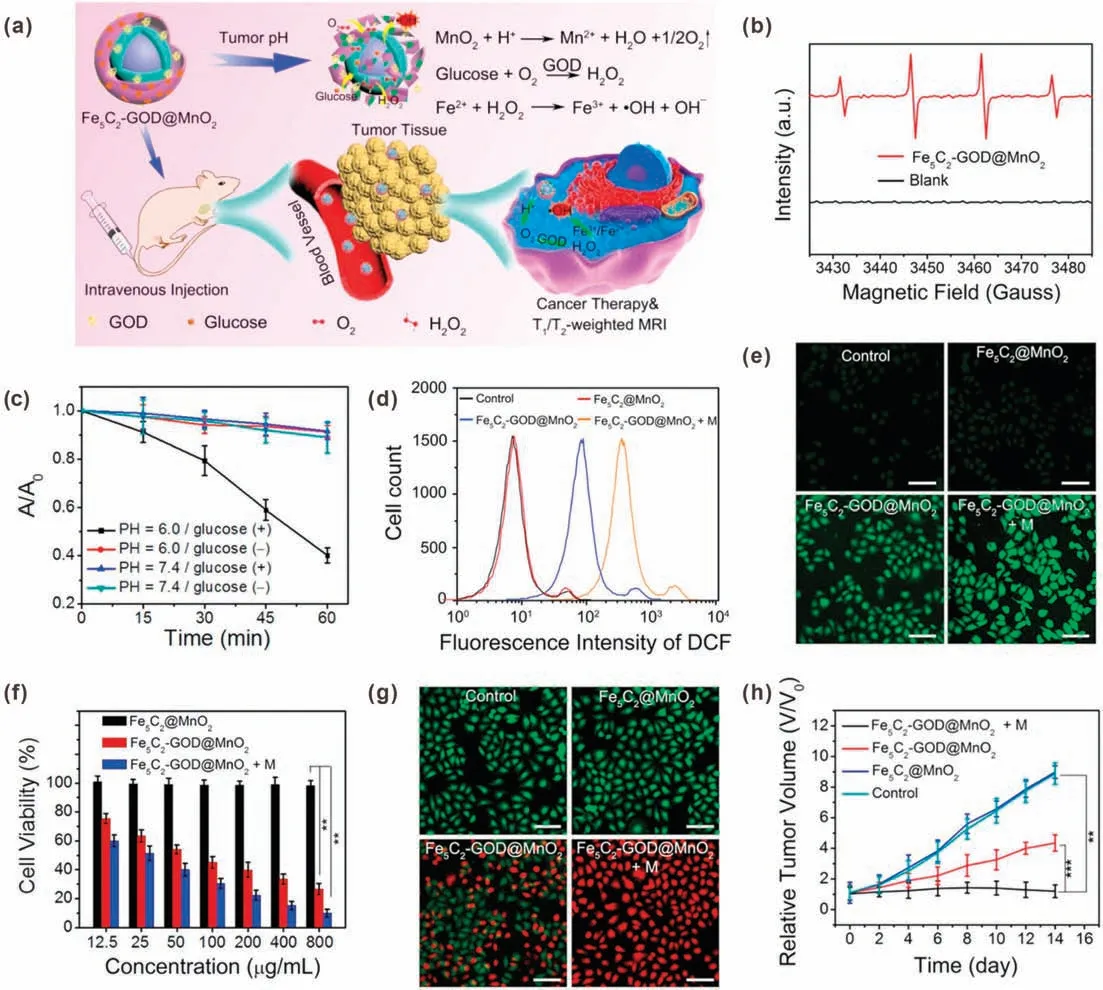
Fig.2.(a)Schematic illustration of Fe5C2-GOD@MnO2 nanocatalysts for H2O2 generation-enhanced CDT.(b)ESR spectra of Fe5C2-GOD@MnO2 in the presence and absence of glucose(10 mmol/L)at pH 6.0.(c)Normalized absorption changes of DPBF after incubation with Fe5C2-GOD@MnO2 at different pH.(d)Flow cytometry analysis of DCFH-DA stained HeLa cells with different treatments.(e) Fluorescence images of DCFH-DA stained HeLa cells with different treatments.(f) Viability of HeLa cells upon different treatments.(g) Live/dead cell staining results of HeLa cells with different treatments.(h) Relative tumor volume of mice after different treatments.Reproduced with permission [27].Copyright 2018, American Chemical Society.
In addition, GOD has been combined with a variety of Fecontaining nanomaterials including ultra-small Fe3O4nanoparticles and metal–organic frameworks for H2O2productionenhanced CDT[24,26].However,as a natural protein,GOD suffers from the disadvantages of immunogenicity, high cost and short half-life in vivo [44].These shortcomings severely hinder its bioapplications.Recently,alternatives to natural enzymes,catalytically active nanomaterials, which are often called "artificial enzymes"or"nanozymes",have become more and more attractive due to their advantages of easy manufacturing,low cost,and strong adaptability [46].Nowadays, a large number of mimetic enzymes have been developed.For example, ultra-small gold (Au) nanoparticles (NPs) have been demonstrated to have catalytic properties similar to GOD.In the presence of dissolved oxygen, ultrasmall Au NPs can oxidize glucose into glucose acid and H2O2,which is fully in line with the characteristics of GOD substitutes[47].Shi et al.reported a dual inorganic nanozyme system for efficient tumor CDT [28].As sown in Fig.3a, ultra-small Au NPs and iron oxide (Fe3O4) NPs were dispersed in the pores of dendriticmesoporous silica NPs(DMSN).After surface poly(ethylene glycol)(PEG) modification, the resulting nanozyme system (DMSN-Au-Fe3O4-PEG)without any toxic substances was obtained.The Au NPs can be used as a GOD mimic to catalyze H2O2generation(process I);while Fe3O4NPs can be used as a peroxidase mimic to catalyze·OH generation(process II).To demonstrate the function of Au NPs,a colorimetric assay was performed to detect gluconic acid,which is a typical product of glucose oxidation reaction.As shown in Fig.3b, the absorbance of DMSN-Au NPs solution increased with increasing glucose concentration,indicated that gluconic acid was produced through Au NPs-catalyzed reaction.In addition,with the process of reaction, the oxygen level of the solution containing DMSN-Au decreased rapidly, confirming oxygen consumption during catalytic process(Fig.3c).These results confirmed that Au NPs have the GOD mimic function of catalyzing the production of H2O2from glucose.Then 3,3′,5,5′-tetramethylbenzidine (TMB)colorimetric assay was used to confirm·OH generation in process II.In the presence of DMSN-Au-Fe3O4and glucose, absorption peaks were observed, indicating the cascade catalytic effects of DMSN-Au-Fe3O4(Fig.3d).The·OH generation was also evaluated in 4T1 cells by using DCFH-DA.Strong green fluorescence was observed inside cells incubated with glucose and DMSN-Au-Fe3O4,demonstrated that the DCFH-DA was oxidized into fluorescent 2′,7′-dichlorofluorescein(DCF)by generated·OH(Fig.3e).Based on the efficient cascade catalysis of Au NPs and Fe3O4NPs,the DMSNAu-Fe3O4showed great tumor-growth inhibition effect(Fig.3f).It should be noted that the GOD-like activity of Au NPs was affected by many factors, such as size, shape, surface chemistry, concentration and environmental pH value.For example, the catalytic activity of Au NPs could be blocked by surface modification.Furthermore, it has been reported that Au nanorods (and other metals including Ag,Pd and Pt)also have intrinsic peroxidase-like and catalase-like activities.Under acidic and neutral conditions,Au nanorods mainly exerted peroxidase-like activity; while under alkaline condition they exerted catalase-like activity [48].Therefore, in the development and application of nanozymes, their physical and chemical properties and application environment of nanozyme should be considered.In addition, the GOD- and nanozyme-mediated H2O2generation is an oxygen-consuming process;therefore,improving the hypoxia of tumor microenvironment may be beneficial to the performance of these enzymes and nanozymes [49].
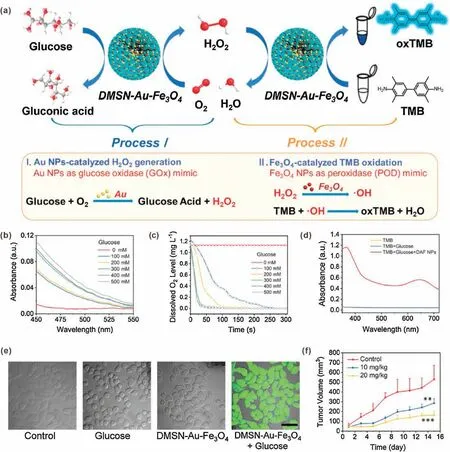
Fig.3.(a)Schematic illustration of DMSN-Au-Fe3O4 nanoplatform for·OH generation by two reaction processes.(b)Absorption spectra of DMSN-Au solutions incubated with different concentrations of glucose in the presence of hydroxylamine and FeCl3.(c)Oxygen level changes of DMSN-Au solutions incubated with different concentrations of glucose.(d)Absorption spectra of TMB after different treatments at pH 6.5.(e)Fluorescence imaging of DCFH-DA stained 4T1 cells after different treatments.(f)Tumor growth curves of mice after different treatments.Reproduced with permission [28].Copyright 2019, Wiley-VCH.
In another study, Qu et al.designed a nanozyme that can promote the effective accumulation of H2O2at the tumor site by perturbing H2O2balance [50].The nanozyme (PZIF67-AT) was composed of metal–organic framework (ZIF-67) and inhibitor 3-amino-1,2,4-triazole (AT).The PZIF67-AT had superoxide dismutase-like activity, which can promote the production of H2O2.Meanwhile,the loaded AT can inhibit activity of catalase,resulting in decreased H2O2decomposition.Therefore,the nanozyme could effectively promote the accumulation of H2O2.In addition, the PZIF67-AT can further convert H2O2into·OH through Fenton-like reaction to realize efficient CDT.
3.Drugs
Besides enzymes and nanozymes, various chemotherapeutic drugs such as platinum drugs,doxorubicin(DOX),cinnamaldehyde(CA) and β-lapachone (Lap) have been used to promote the production of H2O2.
Cisplatin is not only the first metal-based chemotherapy drug,but also one of the most effective chemotherapy drugs[51].A large number of experiments have proved that cisplatin has a good inhibitory effect on a variety of tumors,making it a representative of chemotherapy drugs.The nature of cisplatin that can induce the generation of ROS has attracted much attention [52].Specifically,cisplatin activates a family of enzymes called nicotinamide adenine dinucleotide phosphate(NADPH)oxidase(NOX)in cancer cells[53].The activated NOX can catalyze NADPH to NADP+,and at the same time give an electron to O2to convert it into O2-[54],and then disproportionate by superoxide dismutase (SOD) to produce H2O2.Therefore, due to the characteristic of cisplatin to promote the generation of H2O2,the combination of cisplatin chemotherapy and CDT has become a promising approach,which can reduce the toxic and side effects of cisplatin and improve the efficacy.For example,Mao et al.constructed an organic nano-drug(PTCG NPs)by using epigallocatechin-3-gallate(EGCG),phenolic platinum(IV)prodrug (Pt-OH), ferric chloride and polyphenol-modified block copolymer (PEG-b-PPOH) for synergistic chemotherapy and CDT(Fig.4a) [29].In response to low pH and glutathione (GSH)environments,the PTCG NPs will be disassembled and Pt-OH will be activated, leading to rapid release of cisplatin (Figs.4b and c).The released active cisplatin will promote the generation of H2O2through the abovementioned processes.Catalyzed by Fe ions, the·OH will be further generated through Fenton reaction for CDT.The intracellular ROS levels inside cells with different treatments were determined by DCFH-DA.As shown in the confocal laser scanning microscopy(CLSM)images(Fig.4d),very weak green fluorescence was observed in FeCl3group, demonstrated that the endogenous H2O2was insufficient for·OH generation.It can be clearly seen that the PTCG NPs-treated HepG2 cells showed the strongest intracellular green fluorescence, indicating the enhancement of ROS generation.Both vitamin C(VC,ROS scavenger)and deferoxamine mesylate (iron chelator) could block the effect of PTCG NPs,demonstrated that the ROS was generated through an ironinvolved reaction.Due to the synergistic effect of chemotherapy and CDT,the HepG2 cells incubated with PTCG NPs showed higher levels of apoptosis and necrosis than that incubated with EGCG or Pt-OH(Fig.4e).Animal experiments also proved that PTCG NPs had the best inhibitory effect on tumors,and the inhibition rate of PTCG NPs was as high as 81.6%.Owing to the great therapeutic performance and biological safety of PTCG NPs, the survival time of PTCG NPs-treated mice was obviously prolonged.
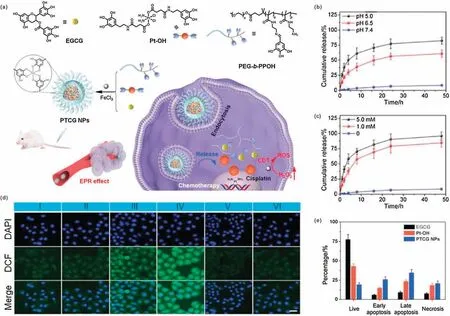
Fig.4.(a)Schematic illustration of PTCG NPs for synergistic chemotherapy/CDT.(b)In vitro platinum release from PTCG NPs at different pH.(c)In vitro platinum release from PTCG NPs at different concentrations of GSH (mM: mmol/L).(d) CLSM images of DCFH-DA stained HepG2 cells after different treatments (Goups I-VI: Control; FeCl3;cisplatin+FeCl3;PTCG NPs;PTCG NPs+VC;PTCG NPs+DFO).(e)Cell apoptosis results of HepG2 cells after different treatments.Reproduced with permission[29].Copyright 2020, Wiley-VCH.
DOX is a clinical anthracycline drug for treatment of malignant tumor by inhibiting the synthesis of DNA and RNA.Due to the broad-spectrum anti-cancer and good curative effect of DOX,it has been widely used in the treatment of malignant lymphoma,leukemia,prostate cancer and various solid tumors[55].However,excellent antitumor effect is often accompanied by serious side effects.The strong cardiotoxicity of DOX can cause heart failure[56].Therefore, the development of combination treatment strategies is highly desirable for optimizing DOX-based therapy[57].The effect of DOX on the promotion of ROS generation has attracted the attention of researchers [58–60].The working mechanism of DOX is similar to that of cisplatin.Based on this property of DOX, Sun et al.developed a DOX and AT (a catalase inhibitor) co-loaded iron oxide mesoporous silica core–shell NPs(Fe3O4@MSN) for chemo/chemodynamic combination therapy[33].After folate (FA) and triphenylphosphonium (TPP) modification,the DOX/AT@Fe3O4@MSN-TPP/PEG-FA can realize cancer cell and mitochondrial targeted therapy.In cancer cells, the released DOX could stimulate the activation of NOXs, converting O2into O2.-.Then the production of H2O2was promoted by SOD.Moreover, AT could reduce the decomposition of H2O2through inhibiting catalase activity.By combining the promotion of H2O2production and inhibition of H2O2decomposition,the intracellular H2O2level could be significantly improved for efficient Fenton reaction(Fig.5a).Quantitative analysis of intracellular ROS by flow cytometry showed that the ROS levels in both breast adenocarcinoma cells(MCF-7)and human gastric carcinoma cells(MGC-803)were obviously improved by the incubation of DOX-containing nanomedicines(Fig.5d).The addition of Fe3O4can further amplify ROS level, which may attribute to the·OH generation through Fenton reaction.The cytotoxicity results showed that the DOX/AT@Fe3O4@MSN-TPP/PEG-FA had a significant inhibitory effect on both MCF-7 and MGC-803 cancer cells, which was stronger than that of other groups (Figs.5b and c).This result proved that the chemo/CDT combination treatment outcome could be further enhanced by AT.In addition,the induced apoptosis rate of the DOX/AT@Fe3O4@MSN-TPP/PEG-FA reached 88.1%,confirming the higher efficacy of the synergistic therapy.The characteristic of this study is not only the combination of chemo/chemodynamic therapy,but also the introduction of multi-level targeting and catalase inhibitors, which improves the targeting while further improving safety and therapeutic effects.In another study, Chen et al.developed a kind of metal-phenolic network NP by using DOX and cisplatin together to realize better O2-production capacity [31].The SOD-like activity of polyphenols could further promote the conversion of O2-to H2O2,resulting in effective tumor inhibition.
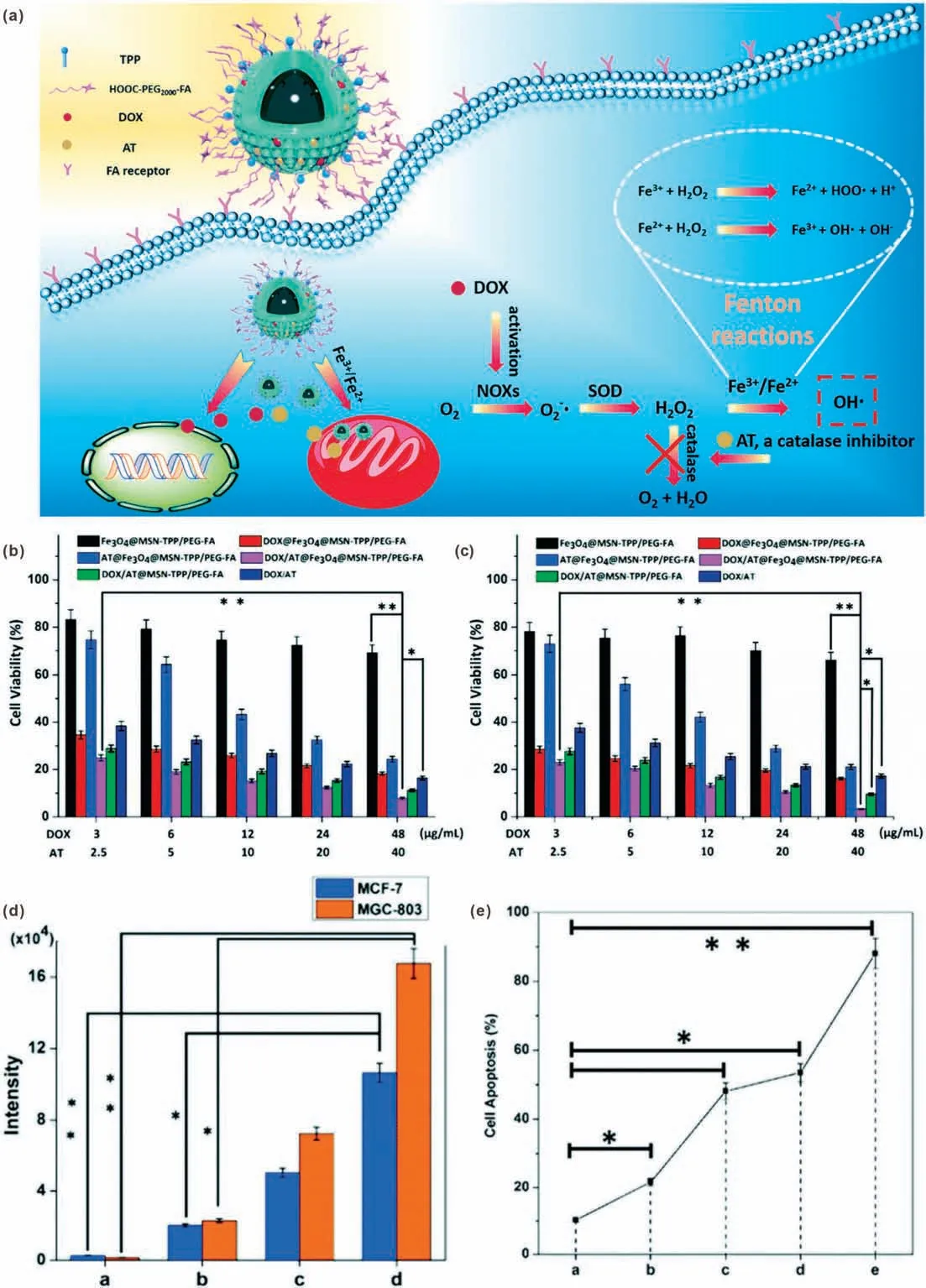
Fig.5.(a)Schematic illustration of DOX/AT loaded Fe3O4@MSN-TPP/PEG-FA for mitochondria-targeted CDT.Viabilities of MCF-7 cells(b)and MGC-803 cells(c)treated with different samples.(d)Intracellular ROS levels detected by DCFH-DA staining and flow cytometry analysis(Groups a-d:control;DOX/AT-loaded MSN-TPP/PEG-FA;DOX/ATloaded Fe3O4@MSN;DOX/AT-loaded Fe3O4@MSN-TPP/PEG-FA).(e)Cell apoptosis results of HepG2 cells after different treatments(Groups a-e:Fe3O4@MSN-TPP/PEG-FA;ATloaded Fe3O4@MSN-TPP/PEG-FA; DOX-loaded Fe3O4@MSN-TPP/PEG-FA; DOX/AT-loaded MSN-TPP/PEG-FA; DOX/AT-loaded Fe3O4@MSN-TPP/PEG-FA).Reproduced with permission [33].Copyright 2018, Royal Society of Chemistry.
Lap, as an o-naphthoquinone, has been found to have a lethal effect on cancer cells in the late 20thcentury [61].Since then, indepth research has been carried out and the Lap has been demonstrated to have therapeutic effect on a variety of cancers such as pancreatic cancer, breast cancer and liver cancer [62].In the process of studying Lap,its ability to promote the production of H2O2in the presence of NAD(P)H: quinone oxidoreductase-1(NQO1)was found.This property of Lap can be exploited to develop nanomedicines for CDT [63,64].Furthermore, this process occurs specifically in cancer cells that overexpress NQO1, which has a protective effect on normal cells.Chen et al.developed a pH/ROS dual-responsive nanomedicine (denoted as PtkDOX-NM), which brought new ideas for the combined application of chemotherapy and CDT[34].In this study,the PtkDOX-NMs were prepared by selfassembly of two types of amphiphilic polymers, pH-responsive PEG-b-polydiisopropylaminoethyl methacrylate-b-polydopamine(PEG-PDPA-PDA) and ROS-responsive PEG-b-polythioketal DOX prodrug (PEG-PtkDOX).Lap and ferric ions were co-loaded in the PtkDOX-NMs.After accumulating in the tumor area through the enhanced permeability and retention (EPR) effect and entering cancer cells, under the influence of the TME acidity, the PtkDOXNMs were disassembled due to hydrophobic-to-hydrophilic conversion of PDPA segments, so that the loaded contents were quickly released.Then a large amount of H2O2would be produced through Lap catalysis for Fenton reaction and·OH generation.The generated·OH could further cause DOX release by breaking thioketal bonds and thus realize combination therapy(Fig.6a).The Lap-induced intracellular H2O2production was confirmed by DCFH-DA staining.With the increase of Lap concentration,the ROS level inside NQO1-overespressed A549 cells(Fig.6b).Cytotoxicity study results showed that the inhibitory effect of nanomedicines without Lap on A549 cells was limited;while the anticancer effect of the nanomedicines was significantly enhanced by addition of Lap (Figs.6c and d).These results indicated that the Lap-induced H2O2production was the critical process in the cascade of·OH generation and DOX release.Compared with control groups, the Lap-loaded PtkDOX-NMs showed the most potent anticancer effect,indicating combination effect of chemotherapy and CDT.In vivo experiments also demonstrated the great effect of PtkDOXNMs on tumor inhibition (Fig.6e).
CA is an unsaturated aromatic aldehyde widely present in plants such as cinnamon.It is not only widely used as a flavoring agent in various foods,but also used in the field of medicine due to its antifungal and peripheral blood vessel relaxation effects[65,66].In addition, at the beginning of the 21stcentury, the ability of CA to induce cell apoptosis by promoting the production of ROS was discovered [67].In recent studies, CA-based drug/nanomedicine was developed to amplify oxidative stress by promoting H2O2production and downregulating antioxidant systems, resulting in enhanced tumor cells killing effect [2,36].By combining with Fenton catalysts, CA has also been used to enhance the efficacy of CDT by increasing the H2O2level.For example, Zhao et al.reported a nanoreactor (HA-CD/Fc-CA NP)prepared by host-guest interaction of the bifunctional ferrocenemodified cinnamaldehyde prodrug (Fc-CA) with cyclodextrinmodified hyaluronic acid conjugate (HA-CD) [37].HA not only improved the biocompatibility and biodegradability of the nanoreactor,but also endowed it with a strong targeting ability for CD44 receptor-overexpressed tumor cells.After cellular internalization,in response to an acidic environment,CA and ferrocene(Fc)will be released due to cleavage of pH-sensitive hydrazone bonds.Attributing to the CA-promoted H2O2generation and Fc-mediated Fenton reaction,highly cytotoxic·OH was generated for enhanced CDT (Fig.7a).The flow cytometry analysis proved that the intracellular ROS level could be amplified by CA.Compared with free CA,Fc-CA showed better effect,indicated that Fc could convert H2O2into·OH with stronger oxidation capability.The ROS amplification ability of HA-CD/Fc-CA NPs was comparable to that of free Fc-CA (Fig.7b).The in vitro cytotoxicity results proved the anticancer effect of CA-containing groups.Importantly,the HA-CD/Fc-CA NPs showed higher cytotoxicity against 4T1 cells than free CA, indicating Fenton reaction-enhanced therapeutic effect(Fig.7c).Furthermore, in vivo experiments also demonstrated that the HA-CD/Fc-CA NPs had a significant tumor suppressing effect, which was attributed to effective tumor targeting and synergistic chemo/chemodynamic therapy (Fig.7d).
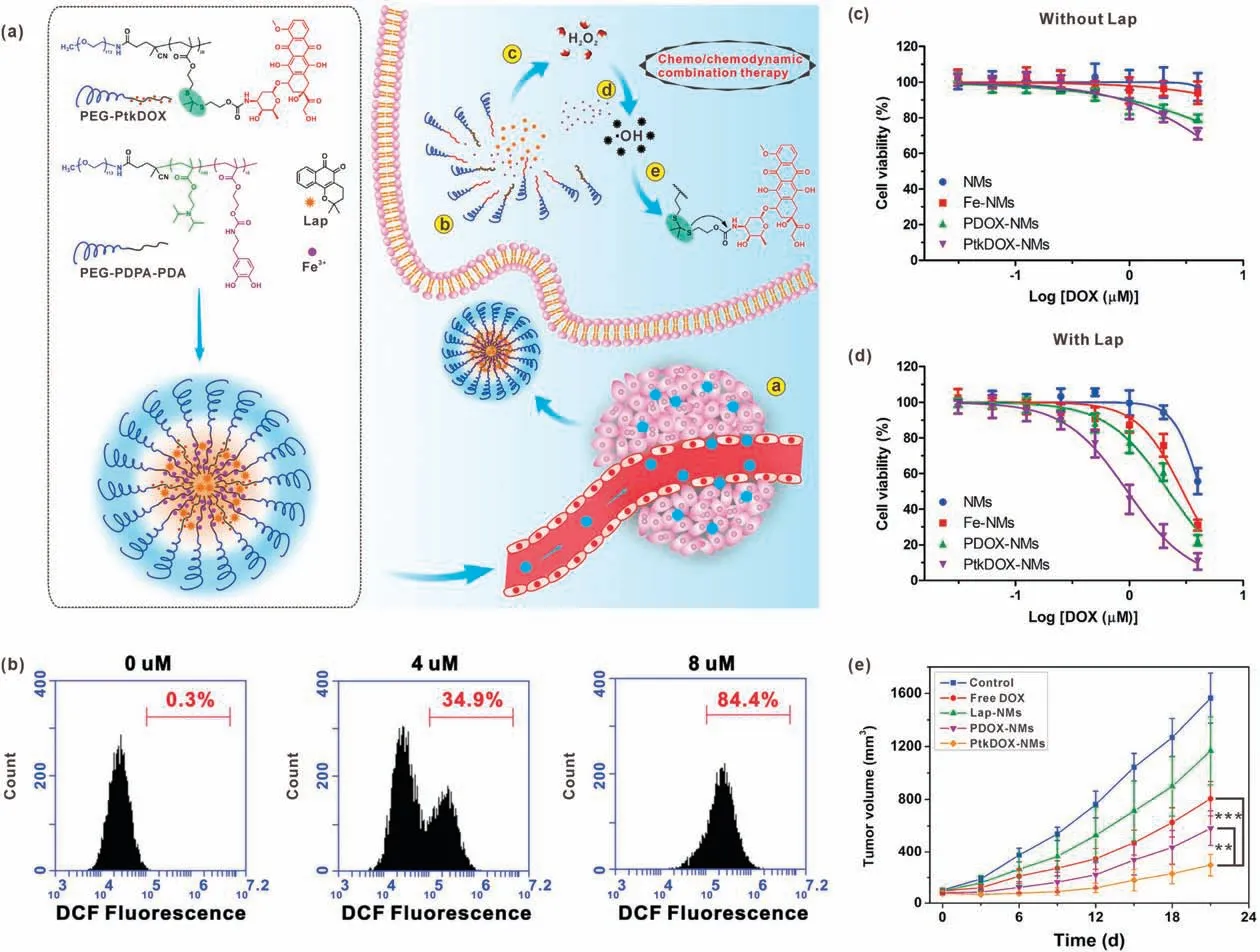
Fig.6.(a) Schematic illustration of Lap-loaded PtkDOX-NMs for H2O2 generation-enhanced chemo/chemodynamic combination therapy.(b) Flow cytometry analysis of DCFH-DA stained A549 cells treated with different concentrations of Lap.Viability of A549 cells incubated with different samples in the absence(c)and presence(d)of Lap.μM:μmol/L.(e) Tumor growth curves of the mice treated with different samples.Reproduced with permission [34].Copyright 2019, Wiley-VCH.
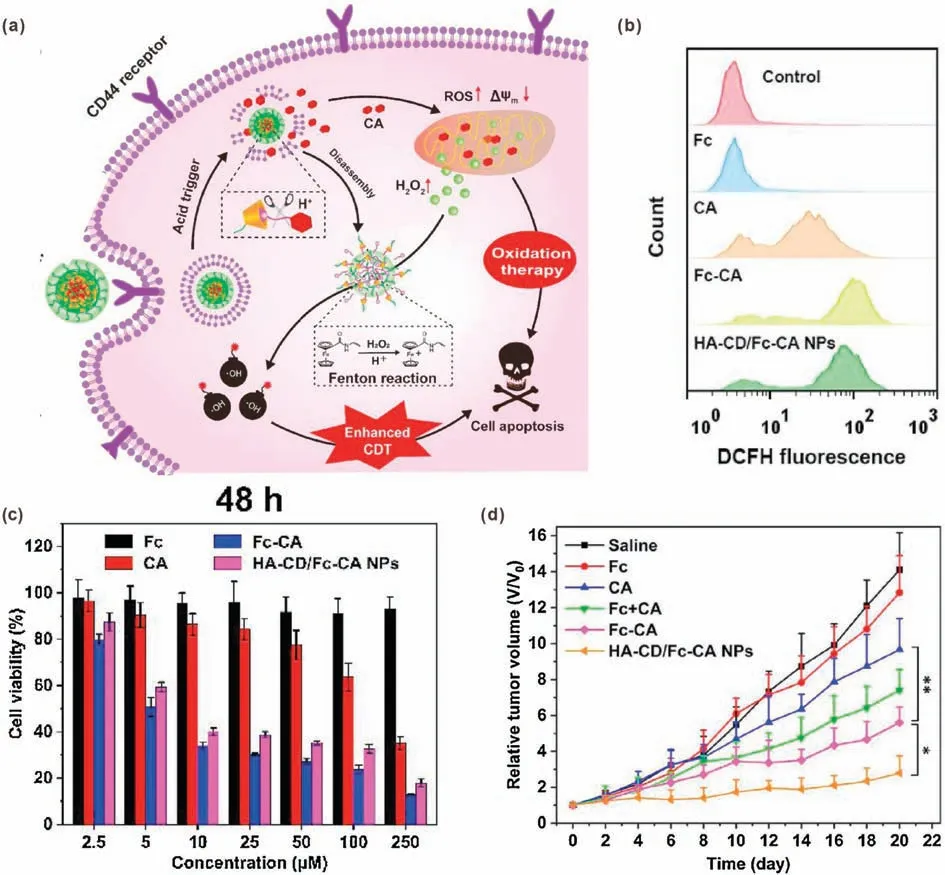
Fig.7.(a)Schematic illustration of HA-CD/Fc-CA NPs for synergistic chemo/chemodynamic therapy.(b)Flow cytometry analysis of intracellular ROS level in 4T1 cells treated with different formulations.(c)In vitro cell cytotoxicity against 4T1 cells of different formulations.μM:μmol/L.(d)Tumor growth curves of the mice treated with different formulations.Reproduced with permission [37].Copyright 2020, Elsevier.
4.Metal peroxide
Although catalysis strategy is effective,the yield of H2O2is often limited by tumor oxygen level.However,most solid tumors suffer from insufficient oxygen concentration.Therefore, the oxygenindependent H2O2generation strategy will be promising in the treatment of hypoxic tumors.Metal peroxides, which can decompose to produce metal ions and H2O2, has attracted more and more attention for developing H2O2self-supplying nanosystems [68].
For example, Dong et al.developed a H2O2self-supplying nanosystem for thermal responsive enhanced CDT[39].To prepare the nanosystem (denoted as Fe-GA/CaO2@PCM NPs), ultra-small calcium peroxide(CaO2)NPs and iron-gallic acid NPs(Fe-GA)were encapsulated by organic phase-change materials (PCMs), which can achieve solid-liquid transition through temperature changes.The outer layer of PCM could improve the stability of CaO2by isolating it from the outside environment.By changing the ratio of fatty acids or fatty alcohols, the melting point of PCMs can be adjusted to 46°C[69,70].Upon 808 nm laser irradiation,the Fe-GA can be used as a photothermal conversion agent to convert light energy into heat,which further induced melting of PCM,leading to rapid release of CaO2NPs and Fe-GA.In an acidic intracellular environment, CaO2would be decomposed into Ca2+and H2O2.Then H2O2would be further converted into·OH through Fe-GAmediated Fenton reaction, achieving H2O2self-sufficient CDT.In addition, the released Ca2+could induce mitochondrial damage,resulting in synergistic therapy (Fig.8a).The CaO2@PCM did not show H2O2generation ability without heating, indicating the protective effect of PCM.However,when CaO2@PCM was heated to about 50°C at pH 5.5, a large amount of H2O2was produced,demonstrated that the released CaO2NPs were decomposed under acidic condition(Fig.8b).The H2O2self-sufficient CDT was proved by a methylene blue (MB) assay in vitro.As shown in Fig.8c, in acidic condition with heating, the Fe-GA/CaO2@PCM NPs could cause significant MB degradation, demonstrating effective·OH generation.Because there was no exogenous H2O2, the·OH generation was caused by H2O2self-supplying Fenton reaction.The intracellular ROS detection results demonstrated that the Fe-GA/CaO2@PCM NPs could significantly improve ROS level under laser irradiation;however,no obvious ROS level improvement was observed in Fe-GA@PCM NPs-treated cells(Fig.8d).In addition,Ca2+was only detected in cells treated with Fe-GA@PCM NPs and laser irradiation (Fig.8e), which was consist with the results of ROS detection, indicated that both Ca2+and H2O2came from the decomposition of CaO2.The mitochondrial membrane potential staining results also proved that only the Fe-GA/CaO2@PCM NPs with laser irradiation could cause significant mitochondrial damage(Fig.8f).In vitro cell experiments showed that the viability of cells treated with Fe-GA/CaO2@PCM under laser irradiation was significantly lower than that of Fe-GA@PCM-treated cells,indicating the H2O2self-supplying enhanced CDT (Fig.8g).In vivo antitumor results also proved the efficient tumor inhibition effect of Fe-GA/CaO2@PCM (Fig.8h).
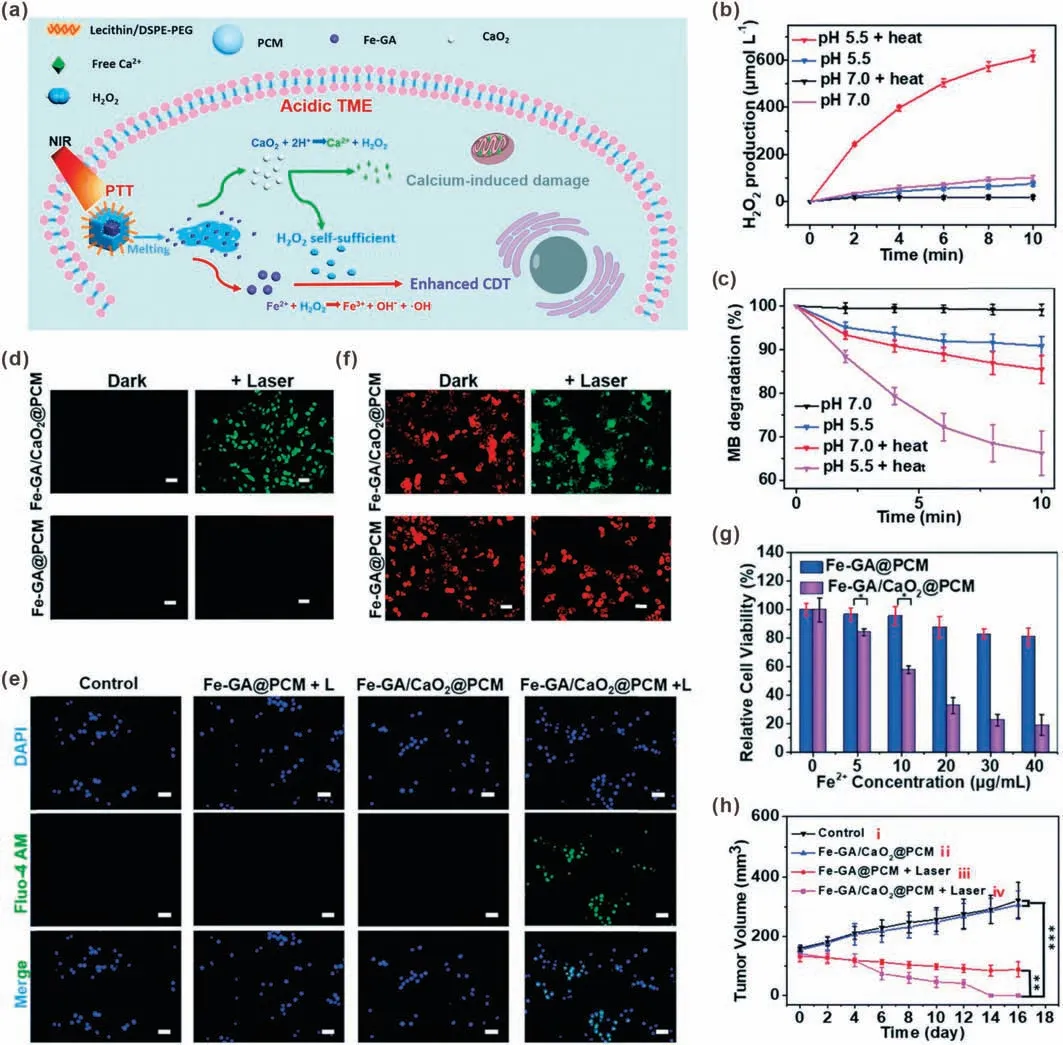
Fig.8.(a)Schematic illustration of Fe–GA/CaO2@PCM for H2O2 self-sufficient CDT.(b)H2O2 production from CaO2@PCM with different treatments.(c)Fe–GA/CaO2@PCM NP caused MB degradation under different conditions.Fluorescence images of DCFH-DA probe (d), JC-1 probe (e) and Fluo-4 AM probe (f) stained HeLa cells after different treatments.(g) Viability of HeLa cells incubated with different samples under laser irradiation.(h) Tumor growth curves of the mice treated with different formulations.Reproduced with permission [39].Copyright 2020, Royal Society of Chemistry.
In another study,Chen et al.developed another metal peroxide,copper peroxide(CP)nanodot,for H2O2self-supplying CDT[40].In acidic endo/lysosome conditions, CP nanodots would be decomposed into Cu2+and H2O2.Compared with other metal peroxides,one major advantage of CP nanodots is that the generated Cu ions can be used as a Fenton catalyst directly.Therefore,both the H2O2and Fenton catalyst were self-supplied simultaneously by CP nanodots without adding other materials,resulting in efficient·OH generation.In addition,the generated·OH would lead to lysosomal lipid peroxidation (LPO) and subsequent release of lysosomal hydrolases to induce cell death(Fig.9a).It was demonstrated that the CP nanodots were stable in physiological environment(pH 7.4);while rapid decomposed to release Cu2+at pH 5.5 (Fig.9b).The TMB assay also showed that CP nanodots would decompose under acidic condition to produce a large amount of·OH(Fig.9c).Based on the H2O2self-supplying property and efficient·OH generation,CP nanodot itself could inhibit tumor cell proliferation.As shown in Figs.9d and e,the treatment of 20 μg/mL of CP nanodots could lead to more than 80%of U87MG cell death.The CDT-induced LPO was verified by acridine orange (AO) indicator, which emits different fluorescence in different organelles(red:acidic lysosomes;green:cytoplasm and nuclei).As shown in Fig.9f, in control group,both green and red fluorescence could be detected;however,almost no red fluorescence was detected in U87MG cells treated with CP nanodots,indicated that the lysosomes could be damaged by·OHinitiated LPO.Attributing to lysosomal damage, the released lysosomal hydrolases would elicit cancer cell apoptosis.
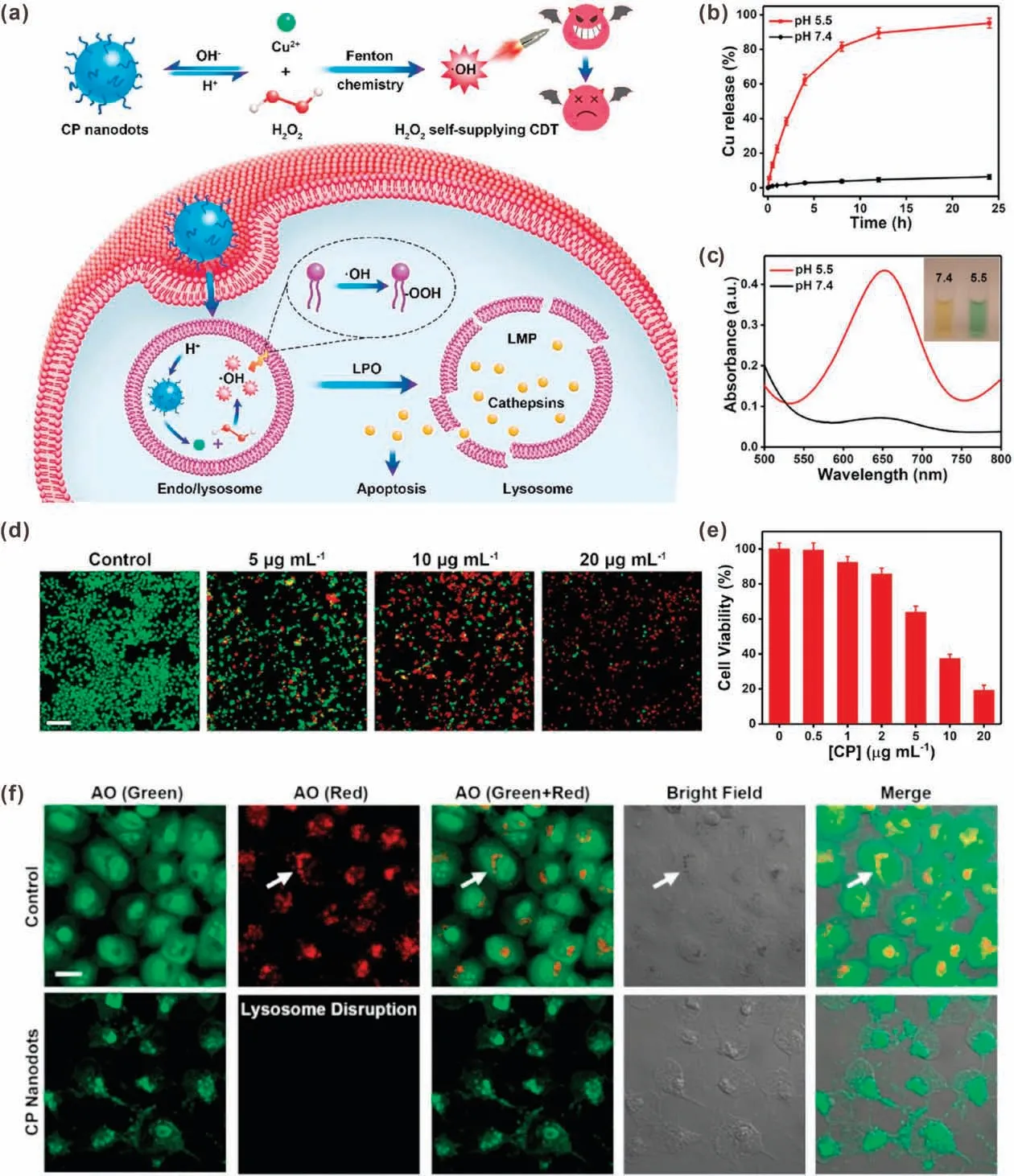
Fig.9.(a) Schematic illustration of CP nanodots for H2O2 self-supplying CDT.(b) In vitro Cu release from CP nanodots at pH 7.4 and 5.5.(c) Absorption spectra of TMB incubated with CP nanodots at pH 7.4 and 5.5.(d)Live/dead cell staining results of U87MG cells treated with different concentrations of CP nanodots.(e)Viability of U87MG cells treated with different concentrations of CP nanodots.(f) CLSM images of U87MG cells treated with AO-staining with or without CP nanodots.Reproduced with permission [40].Copyright 2019, American Chemical Society.
5.Engineered bacterial bioreactor
By the middle of the nineteenth century,some bacteria such as Streptococcus pyogenes have been found to have antitumor effects[71,72].However,the problems of large individual differences,low repetition rate and high toxicity limited further application of bacterial therapy.In recent years,the rise of synthetic biology has brought new opportunities for bacterial therapy.Synthetic biology refers to the design and construction of artificial biological systems from an engineering perspective [73].Specifically, for cancer treatment, genetic modification is used to give bacteria certain abilities, or to weaken some of their abilities, thus achieving safe and stable cancer treatment[74].Recently,Zhang et al.reported an integrative bioreactor (Ec-pE@MNP) composed of engineered bacterium and magnetic Fe3O4nanoparticles (MNP) for cancer CDT [42].In this study, MNP were chemically conjugated on the surface of nonpathogenic bacterium Escherichia coli MG1655 (EcpE)that overexpresses respiratory chain enzyme II(NDH-2).Due to the tropism of bacteria and the magnetic targeting ability of MNP,the Ec-pE@MNP could effectively accumulate in tumor site.The overexpressed NDH-2 could catalyze the production of H2O2through electrons transfer during bacterial respiration.Afterwards,a Fenton-like reaction occurred under the action of the MNP,generating highly toxic·OH for cancer cell killing (Fig.10a).Polyacrylamide gel electrophoresis (PAGE) results showed that NDH-2 was expressed by Ec-pE, especially in the presence of isopropyl-β-D-thiogalactoside(IPTG),an inductive agent(Fig.10b).The intracellular H2O2level was detected by using DCFH-DA.As shown in Fig.10c, intense fluorescence was found in Ec-pE with IPTG induction group, indicating efficient H2O2generation.In addition, a hydrogen peroxide assay kit was used to measure the H2O2concentrations of media.The result indicated that the engineered bacterium Ec-pE successfully promoted the production of H2O2(Fig.10d).In contrast,the amount of H2O2produced by the wild-type strain and the negative control group (Ec-pB) were below the detection limit.According to flow cytometric analysis,the Ec-pE@MNP showed the best effect in inducing cell death(Fig.10e).In short, this study developed a bacterial-based H2O2-generating bioreactor for cancer CDT, and provided new ideas for the future applications of synthetic biology in cancer treatment.
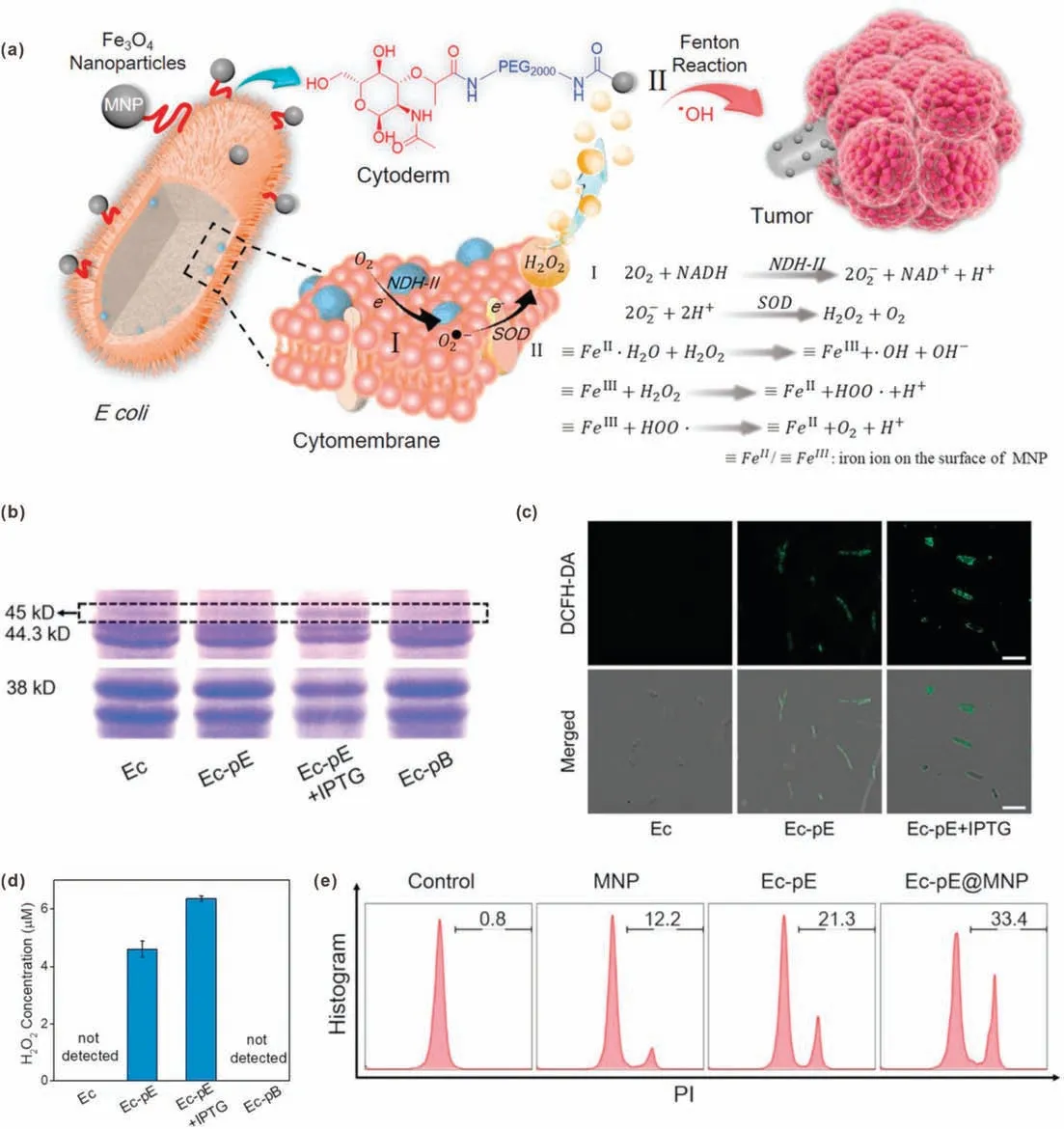
Fig.10.(a) Schematic illustration of engineered bacterial bioreactor for H2O2 production-enhanced CDT.(b) The NDH-2 expression results of different bacteria.(c) CLSM images of different bacteria after DCFH-DA staining.(d)The detection of produced H2O2 in different bacteria suspensions(μM:μmol/L).(e)Flow cytometric analysis of PIstained cells with different treatments.Reproduced with permission [42].Copyright 2019, Wiley-VCH.
6.Conclusion and perspective
CDT is an emerging cancer treatment approach by exploiting Fenton reaction to convert H2O2into highly toxic·OH.In CDT,H2O2,as the source of·OH, plays very important role in·OH generation efficiency.Although H2O2is overexpressed in tumor microenvironment, the concentration of endogenous H2O2is still at a relatively low level.In order to improve the treatment outcome of CDT, various H2O2-generating nanomedicines have been developed in recent years.Through catalysis or self-supply effects,these nanomedicines can promote the H2O2concentration in tumor tissue or cells and thus enhance CDT efficacy.
In this review, we summarized recent advances in H2O2-generating nanomedicines that based on enzymes, nanozymes,drugs, metal peroxides and engineered bacteria.Although enhanced CDT was achieved, the H2O2-generating strategy still faces some challenges.Firstly,the unsatisfactory biosafety of these nanomedicines is a big problem.For example, GOD and GOD-like nanozymes suffer from high systemic toxicity because the catalytic reaction will also take place in the blood circulation and normal tissues.The inherent toxicity of chemotherapeutic drugs will also exist in drug-mediated CDT process.Secondly, most H2O2generation reactions are depend on tumor oxygen content;however, hypoxia is a typical feature of most solid tumors.Therefore,the efficacy of these nanomedicines in the treatment of hypoxic tumors is limited.Thirdly, although metal peroxidesbased self-supply approach is oxygen-independent, the low stability in aqueous environment of metal peroxides may be an obstacle to applications.
For the future work, the following directions need to be taken into consideration: (1) Development of nanomedicines with properties of high tumor targeting efficiency and controlled H2O2production.(2) GSH, as a typical antioxidant overexpressed in cancer cells,can neutralize ROS and protect cells from oxidative damage; therefore, the combination of H2O2production and GSH depletion would be an effective way to further improve CDT treatment efficiency.(3) Combination of oxygen delivery strategy and H2O2-generating agents to attain the sustainable and stable H2O2production.(4) Besides H2O2supply, the Fenton reaction efficiency also affected by other factors such as acidity,slow Fe(III)/Fe(II)conversion,catalyst poisoning.The design of nanomedicines with H2O2production ability and high Fenton reaction efficiency would be a promising direction.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.32000991, 51873150), the Young Elite Scientists Sponsorship Program by Tianjin(No.TJSQNTJ-2020-02),the Key project of Tianjin Foundational Research (JingJinJi)Program, China (No.19JCZDJC64100), the Key Project of Tianjin Nature Science Foundation (No.16JCZDJC35100), the Tianjin Research Innovation Project for Postgraduate Students (No.2020YJSB130).
杂志排行
Chinese Chemical Letters的其它文章
- A millimeter-sized negatively charged polymer embedded with molybdenum disulfide nanosheets for efficient removal of Pb(II) from aqueous solution
- Hyperterpenoids A and B: Two pairs of unprecedented 6/6/4/6/6 polycyclic cyclobutane meroterpenoids with potent neuroprotective and anti-inflammatory activities from Hypericum beanii
- Ammonia leaching mechanism and kinetics of LiCoO2 material from spent lithium-ion batteries
- Design, synthesis and biological evaluation of pyridyl substituted benzoxazepinones as potent and selective inhibitors of aldosterone synthase
- A DNA G-quadruplex converts SOD1 into fibrillar aggregates
- Built-in piezoelectric field improved photocatalytic performance of nanoflower-like Bi2WO6 using low-power white LEDs
