Effects of storage media, supplements and cryopreservation methods on quality of stem cells
2021-10-11OzgurDogusErolBurcuPervinMehmetEminSekerFatimaAertsKaya
Ozgur Dogus Erol, Burcu Pervin, Mehmet Emin Seker, Fatima Aerts-Kaya
Ozgur Dogus Erol, Burcu Pervin, Mehmet Emin Seker, Fatima Aerts-Kaya, Department of Stem Cell Sciences, Hacettepe University Graduate School of Health Sciences, Ankara 06100, Turkey
Ozgur Dogus Erol, Burcu Pervin, Mehmet Emin Seker, Fatima Aerts-Kaya, Center for Stem Cell Research and Development, Hacettepe University, Ankara 06100, Turkey
Abstract Despite a vast amount of different methods, protocols and cryoprotective agents (CPA), stem cells are often frozen using standard protocols that have been optimized for use with cell lines, rather than with stem cells. Relatively few comparative studies have been performed to assess the effects of cryopreservation methods on these stem cells. Dimethyl sulfoxide (DMSO) has been a key agent for the development of cryobiology and has been used universally for cryopreservation. However, the use of DMSO has been associated with in vitro and in vivo toxicity and has been shown to affect many cellular processes due to changes in DNA methylation and dysregulation of gene expression. Despite studies showing that DMSO may affect cell characteristics, DMSO remains the CPA of choice, both in a research setting and in the clinics. However, numerous alternatives to DMSO have been shown to hold promise for use as a CPA and include albumin, trehalose, sucrose, ethylene glycol, polyethylene glycol and many more. Here, we will discuss the use, advantages and disadvantages of these CPAs for cryopreservation of different types of stem cells, including hematopoietic stem cells, mesenchymal stromal/stem cells and induced pluripotent stem cells.
Key Words: Cryoprotective agents; Dimethyl sulfoxide; Hematopoietic stem cells; Mesenchymal stromal/stem cells; Induced pluripotent stem cells
INTRODUCTION
Although optimization of stem cell culture, expansion and differentiation methods has been the main focus of stem cell research, an equally important and largely ignored topic in stem cell research is long term storage and cryopreservation. No matter the quality of the stem cell cultures, without optimization and careful control of cryopreservation, reproducibility and clinical (side) effects may be difficult to interpret. Furthermore, effects may be unexpected and suboptimal if cells are not stored, frozen and thawed under the most favorable conditions. Cryopreservation of cells, tissues and embryos has been common practice since the 1950s and took flight with the development ofin vitrofertilization practices and hematopoietic stem cell (HSC) transplantation.
Storage under low temperature conditions reduces the rates of intracellular enzymatic and chemical reactions that may be harmful and allows the cells to be stored long-term without damage. The basic principle underlying successful cell cryopreservation is prevention of the formation of intra- and extracellular ice crystals during freezing, since this is the primary cause of cell damage[1]. Cryopreservation methods can be classified into slow freezing and fast freezing (vitrification) procedures. Both methods are based on the freezing or solidification of the cells or tissues and may cause cell injury in the process. However, the mechanisms that cause cell damage are quite distinct. Whereas rapid cooling results in the formation of intracellular ice crystals causing physical stress to the cells and mechanical breakdown, slow cooling causes osmotic changes in the cells and mechanical stress due to the formation of extracellular ice[2]. During vitrification a liquid is transformed into a glass-like non-crystalline solid state due to overcooling without freezing. Its most important feature is the prevention of ice formation[3,4]. During vitrification, cells kept in cryoprotectant solutions are briefly exposed to nitrogen vapor and subsequently immersed in liquid nitrogen[5] and usually a permeable cryoprotectant [dimethyl sulfoxide (DMSO) or glycerol] and an impermeable cryoprotectant [hydroxyethyl starch (HES), polyvinyl alcohol, trehalose] are used together[6,7]. During slow freezing, extracellular ice crystals may cause an increase in cellular osmolality and dehydration, and therefore the cooling rate during freezing should be sufficiently slow to allow a suitable amount of water to leave the cell[8,9]. The optimal cooling rate depends on cell size, sample size, water permeability and the presence of nucleating agents, which initiate and catalyze the freezing process. In addition, the cryoprotectant used, the temperature and surface/volume ratio should also be taken into consideration to determine the optimal cooling rate[10]. A cooling rate of 1-3 ℃/min during the initial freezing phase (+4 ℃ to -40 ℃) is optimal for most mammalian cells when frozen in the presence of cryoprotective agents, such as glycerol or DMSO[11]. Automated freezing devices, such as KRYO 10 series III (Planer Products, Sunbury-on-Thames, United Kingdom)[12], CryoMed 1010 (Forma Scientific, Marjetta, OH, United States)[13] and Cryomed (New Baltimore, MD, United States)[14] provide a temperature decrease at a controlled rate. Differences between vitrification and cryopreservation are depicted schematically in Figure 1.
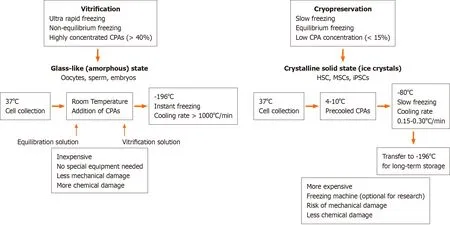
Figure 1 Comparison of vitrification and cryopreservation procedures.
Despite a vast amount of different methods, protocols and cryoprotectants, stem cells are often frozen using protocols optimized for cell lines and relatively few comparative studies have been performed to assess the effects of cryopreservation methods and supplements on stem cell quality and viability. A list of commercially available cryopreservation media is provided as Supplement 1. Here, we summarize the use, advantages and disadvantages of cryopreservation methods used for different types of stem cells, including HSCs, mesenchymal stem cells (MSC) and induced pluripotent stem cells (iPSC).
CRYOPROTECTIVE AGENTS, ADDITIVES AND SOLUTIONS
In order to serve as an effective cellular cryoprotective agent (CPA), the compound should have certain properties, including (1) High water solubility, even at low temperatures; (2) Free penetration of cell membranes; and (3) Low toxicity. Although many compounds may have these properties, including the most commonly used agents DMSO and glycerol, the choice of the compound may differ depending on the type of cell. CPAs are often used in combination with a carrier solution, which may provide different concentrations of (nutritional) salts, a variety of buffers, osmogens and/or apoptosis inhibitors. The contents of this carrier solution further help the cells maintain an isotonic concentration (300 milliosmoles) to prevent swelling or shrinking during the freezing process[15].
DMSO [Me2SO, (CH3)2SO]
DMSO has been a key agent for the development of cryobiology. For cryopreservation of HSCs, use of DMSO, in combination with a temperature-controlled freezing technique followed by a rapid thawing procedure of 1-2 °C/min, is considered the clinical standard[16]. The use of DMSO as a CPA to prevent freezing-related cell damage was first proposed by Lovelock and Bishop[17], who used it during slow cooling of bull sperm. Due to its low hydrophilicity and molecular weight, DMSO freely penetrates cell membranes. It can disrupt ice crystal nucleation by forming hydrogen bonds with intracellular water molecules and prevents dehydration by reducing the amount of water absorbed into ice crystals[18]. However, prolonged exposure to DMSO negatively affects cellular function and growth by interfering with metabolism, enzymatic activity, cell cycle and apoptosis[19]. DMSO is also thought to modulate intracellular calcium concentrations[19,20] and may induce or inhibit cell apoptosis and differentiation, depending on the cell type, the stage of cell growth and differentiation, the concentration of DMSO (typically 5%-10%), duration of exposure and temperature[21,22]. Whereas high concentrations of DMSO may cause instant hemolysis, white cell stacking and fibrinogen precipitation, intravenous administration of DMSO has been associated with local irritation and necrosis[23]. Infusion of cell products that contain DMSO is associated with a wide range of gastrointestinal side effects (nausea, vomiting, abdominal pain, diarrhea)[24-26]; cardiovascular effects (hypertension, bradycardia, tachycardia)[25-27]; respiratory (dyspnea) and dermatological effects (urticaria, itching, and redness)[28,29]. Furthermore, even very low concentrations of DMSO can affect cellular processes by causing differential expression of thousands of genes, changing DNA methylation profiles and tissuespecific deregulation of miRNAs[30,31], and may affect stem cell fate by inducing unwanted differentiation[32].
Glycerol (C3H8O3)
Glycerol is a simple polyol compound. Its cryoprotective effects have been known since the early 1950s, when glycerol was first tested on fowl spermatozoa, rabbit red blood cells and water amoeba[33,34]. Glycerol is a colligative CPA that prevents dehydration damage by increasing the total solute concentration, including sodium ions, thus preventing ice formation and reducing the amount of water absorbed by ice crystals[7,35]. Although glycerol at low concentrations (< 20%) is not sufficient to prevent crystallization completely, it does protect different cells from cell death. High concentrations (70%) of glycerol were used without significant toxicity and were shown to provide substantial protection[36].
Hydroxyethyl starch
Hydroxyethyl starch was synthesized by Ziese W in 1934. The hydroxyethyl starch molecule is a high molecular weight synthetic polymer and can be purified from corn or potatoes[37]. Since high molecular weight CPAs are generally unable to enter cells, HES accumulates in the extracellular space. Here, it regulates water flow during cooling and heating and provides cryoprotection by absorbing the water molecules and keeping them thermally inert. Although HES remains extracellulary, it can minimize intracellular ice crystal formation and provides membrane stabilization[38]. By increasing the extracellular viscosity it further prevents osmotic stress and damage, reducing the rate at which water is withdrawn from the cells during cooling[39,40].
Trehalose
Trehalose is a non-toxic disaccharide and helps maintaining the structural integrity of cells during freezing and thawing[41,42]. Trehalose has high water retaining properties and is found in a large number of organisms, such as nematodes and yeasts that can survive freezing and drying[43] and can be isolated from yeasts, plants and fungi[42,44]. However, trehalose does not display any significant cryoprotective potential by itself and should therefore be used in combination with other CPAs[45].
Albumin
The albumin protein consists of three homologous domains, each with specific structural and functional properties[46]. Human serum albumin (HSA) is present in serum at high quantities and serves as a buffer or depot for hormones, growth factors, fatty acids and metals. Due to its stabilizing function, albumin is an important component of common preservation and cell culture media. During freezing, albumin is used for its ability to coat surfaces, buffer function and binding capacity[47], but, similar to trehalose, albumin is only used as a supplementary cryoprotective agent during freezing of cells and tissues[48].
Dextran
Dextran is a branched polysaccharide with α-1.6 glycosidic links between glucose molecules[49]. Dextran can interact with lipoproteins, enzymes and cells, and has the ability to stabilize proteins[50]. Dextran is non-toxic, only weakly antigenic and usually used at a concentration of 10%[51,52]. Dextran has been used as a cryoprotect during freezing of HSCs and sperm[53,54]. Similar to albumin and trehalose, dextran is only used in combination with other CPAs, such as DMSO or glycerol.
CRYOPRESERVATION OF HEMATOPOIETIC STEM CELLS
Hematopoietic stem cell transplantation (HSCT) is used for the treatment of various malignant and non-malignant diseases affecting the hematopoietic and immune system as well as for the treatment of a variety of inborn errors of metabolism[55]. HSC products derived from bone marrow (BM), peripheral blood or umbilical cord blood (UCB) are usually stored for a brief period that may range from a few days to months but may increase up to several years, depending on the disease state of the patient and treatment schedule[56]. Banking of HSC transplants is becoming increasingly important because of the possibility to use previously stored material even years after collection. In addition, storage of UCB for personal (private banking) or transplantation purposes (biobanks) is becoming increasingly popular and may require banking for up to several decades. For this reason, it is critically important that HSCs retain their potential during the freezing, banking and thawing[57]. HSCs can be stored unprocessed at +4 °C or room temperature for approximately 72 h after collection without massive apoptosis, cell death or loss of stem cell function. Within this time period, they can be transported and engrafted without any problems, but additional protocols may be required for longer storage[22,58,59]. Freezing the cells extends their shelf life greatly and increases the safety of HSC therapy by providing time to perform quality controls (microbiologically) and product testing (HSC content, colony assay, CD34+ enumeration). Despite these benefits, cryopreservation of HSCs poses several challenges, most notably a decrease in cell viability after thawing and side effects in patients due to the CPAs used[60]. An overview of current protocols used for cryopreservation of HSCs has been provided in Table 1.
Throughout the years, DMSO has been the CPA of choice in most studies. It has been tested at different concentrations, ranging from 2.5% to 10% with variable results. Since DMSO is highly hyperosmotic, rapid infusion of the cryopreserved cells into the isosmotic blood system may cause osmotic damage, excessive cell expansion and decreased cell viability. This in turn may cause immediate side effects but can also affect engraftment in the long term[14,22]. Generally, lower doses of DMSO provided less toxicity, but in some cases, this was accompanied by a decrease in cell viability. Nevertheless, observed effects and side-effects of DMSO may differ widely between the protocols used due to the addition of other supplements (HES, HSA, Trehalose), cell dose (ranging from 15 x 106cells/mL-4000 x 106cells/mL), cell source (peripheral blood/BM/UCB), use of controlled rate or uncontrolled rate freezing, duration of storage (< 1 wk to > 1 decade) and the temperature used for long-term storage (-80 °C to -196 °C). To reduce the toxic effects of DMSO-cryopreserved HSCs during transplantation, it has been opted to divide the infusions into multiple portions, given at intervals of several hours or days, or alternatively to concentrate further HSC grafts to reduce cryopreservation volume and DMSO content[61]. In addition, alternatives such as different CPAs to reduce or replace DMSO for cryopreservation[14,62] or complete removal of DMSO prior to infusion[63,64] are being investigated. Even though a concentration of 10% DMSO in HSC cryopreservation is widely accepted as the cryopreservation medium of choice[65,66], similar or even more successful results have been obtained using percentages of DMSO as low as 2.5%-5%, with or without the addition of HES. Using these protocols similar engraftment was observed but with less toxicity[14,67,68]. Use of trehalose in combination with DMSO in UCB-derived HSC freezing has been shown to increase survival and cell differentiation capacity of HSCs in comparison to HSCs frozen without trehalose[53]. Direct comparison of trehalose and DMSO for cryopreservation of BM-HSCs showed no differences on viability between both groups[45]. Similarly, in NOD-SCID mice, the use of low amounts of DMSO (5%) and trehalose (5%) to reduce the toxic effects of DMSO showed a positive effect on HSC survival and engraftment after transplantation[69]. When BM-derived HSCs were frozen using a combination of 7.5% DMSO and 4% HSA, cells displayed high viability and sustained engraftment[70]. Studies using combinations of DMSO with dextran-40 showed increased HSC viability and functionality in comparison to the DMSO only group[71]. In conclusion, a lower concentration of DMSO and addition of a non-toxic second CPA or supplement, such as HSA and trehalose, decreases toxicity related to DMSO, while maintaining high HSC viability and sustaining engraftment.
CRYOPRESERVATION OF MESENCHYMAL STEM/STROMAL CELLS
Multipotent mesenchymal stem/stromal cells (MSCs) can be isolated from many tissues, including the bone marrow (BM-MSC), adipose tissue (adipose tissue derived stem cell), umbilical cord Wharton Jelly (Wharton Jelly-MSC), placenta (placenta-MSC), tooth germ (tooth germ MSC) or dental pulp (dental pulp stem cell) and many other connective tissues[72,73]. MSCs can differentiate into cells from several mesenchymal lineages, including but not limited to osteoblasts, adipocytes and chondrocytes[74,75]. MSCs are highly positive for cell surface molecules like CD29, CD44, CD73, CD90 and CD105[76]. They hold great potential for clinical application due to their capacity for regeneration of damaged or injured tissues, migration to sites of injury and regulation (usually suppression) of local and generalized immune responses. In order to obtain a sufficient amount of MSCs for clinical application, cells are often profoundly expanded in culture. Since MSCs themselves do not express HLA-DR, the cells are considered immunologically inert and expanded MSCs from unrelated, third-party donors can be used for treatment of a variety of diseases, ranging from graftvshost disease to severe acute respiratory distress syndromes[77,78]. These characteristics make MSCs ideal for ready, off-the-shelf treatments but require significant expansion and long-term cryopreservation[79-81]. Similar to the protocols developed for freezing of HSCs, a variety of freezing solutions and protocols has been tested for cryopreservation of MSCs (Table 2). Similar to freezing protocols used for HSCs, MSC freezing media generally consists of a basic medium [alphamodified minimal essential medium, Dulbecco's Modified Eagle's Medium (DMEM) or advanced DMEM], supplemented with 3%-10% DMSO. In most studies expression of MSC surface markers (CD29, CD44, CD73, CD90, CD105 and/or CD166) was assessed before and after cryopreservation, and in almost all cases, MSC phenotype was not affected by cryopreservation, with overall expression levels > 90%. Cell viability ranged from 60% to 95% when fetal bovine serum (FBS) was used in addition to DMSO. In the presence of 10% DMSO, viability was typically very high (80% to 100%) after thawing, regardless of the duration of the freezing period[81-84].
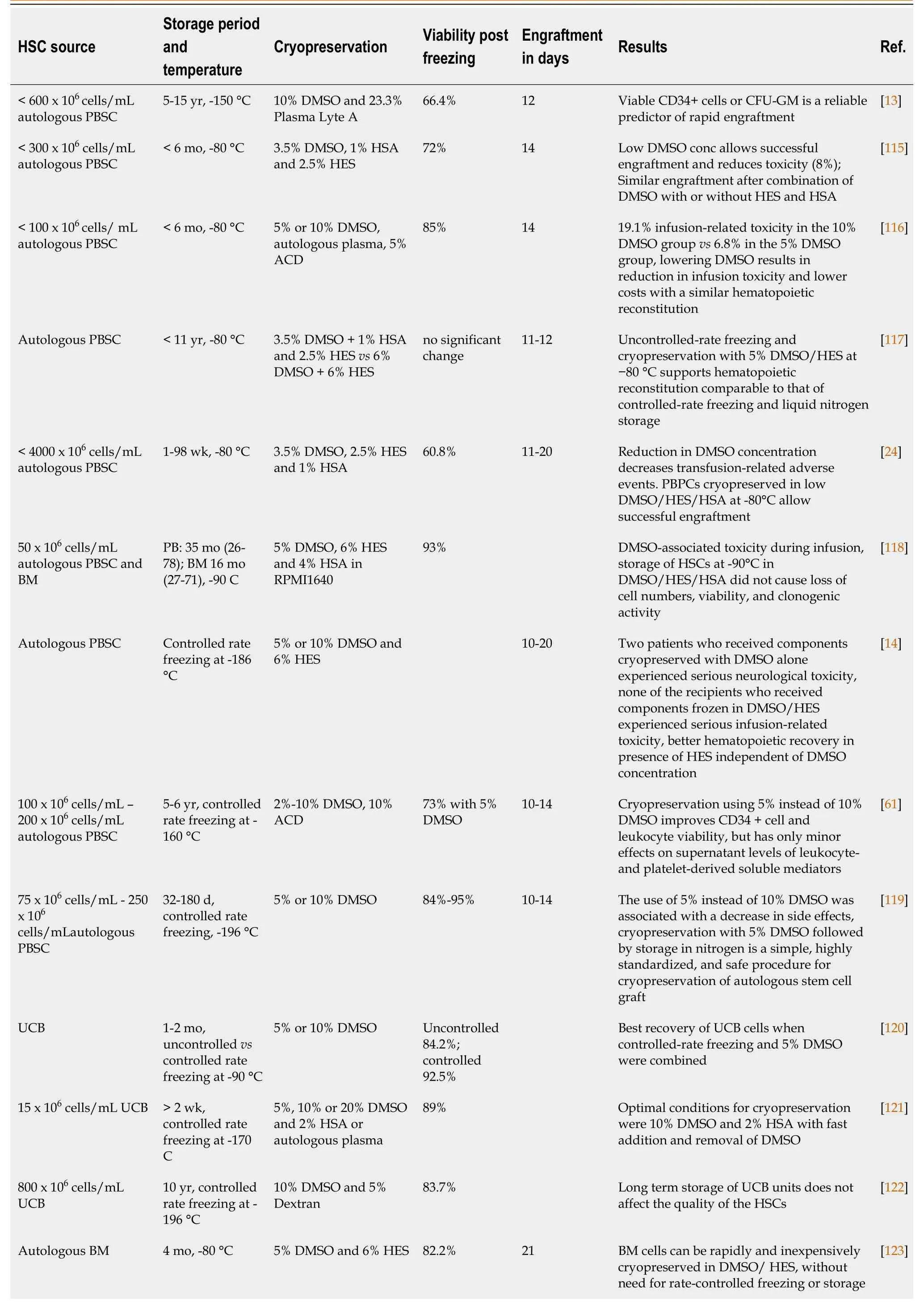
Table 1 Comparison of different protocols used during cryopreservation of hematopoietic stem cells
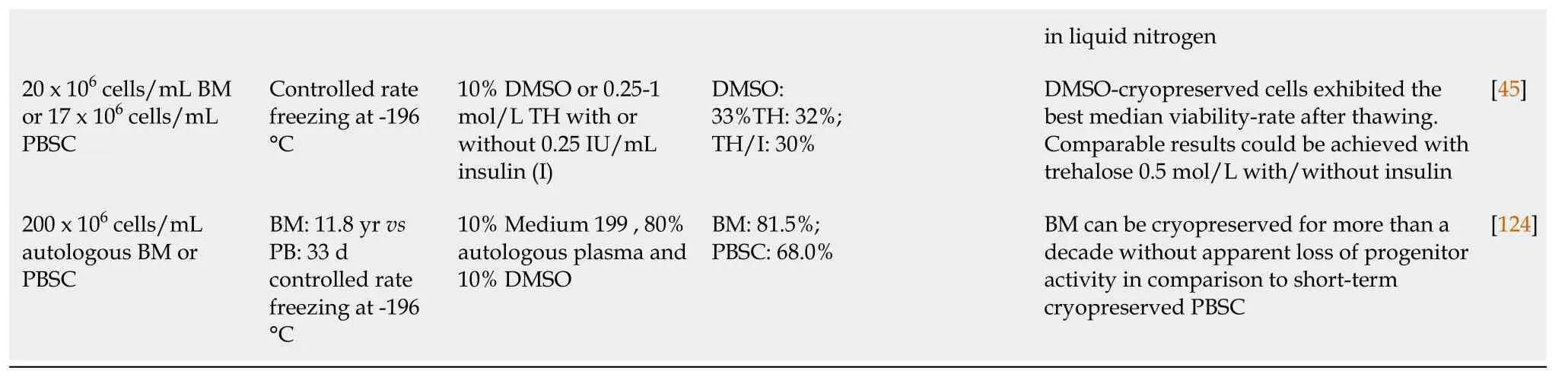
HSC: Hematopoietic stem cell; DMSO: Dimethyl sulfoxide; CFU-GM: Colony Forming Unit-Granulocyte/Macrophage; ACD: Acid citrate dextrose; RPMI: Roswell Park Memorial Institute Medium; HES: Hydroxyethyl starch; HSA: Human serum albumin; BM: Bone marrow; UCB: Umbilical cord blood; TH: Trehalose.
While there was no significant difference between 2% and 10% DMSO in terms of viability after a 1 mo freezing period, a significant portion of the cells frozen in presence of 2% DMSO died after long-term cryopreservation[81]. Therefore, in order to reduce the toxicity related to DMSO, either the percentage of DMSO was reduced or secondary CPAs (trehalose, sucrose, boron) were added to the freezing media[83-85]. Alternatively, high molecular weight macromolecules, such as FBS, polyethylene glycol (PEG) or polyvinylpyrrolidone were added as secondary CPAs to the freezing media[83,84,86]. However, since FBS contains animal components, cell products may contain remnants of FBS despite post-thaw washing that may trigger adverse (immune) reactions when used in a clinical setting[87]. Therefore, animal component free media, such as Cryostor, have been developed as an alternative to standard freezing medium formulations[81]. Studies using adipose tissue-derived MSCs frozen with 10% DMSO, 0.9% NaCl and human serum, HSA or knockout serum replacement (KSR)[88] revealed that all FBS replacements supported a similar multilineage differentiation potential, expression of cell surface markers and gene expression of stem cell markers, indicating that these may be good alternatives for clinical use. Carnevaleet al[89] used 5% DMSO and human serum instead of FBS for cryopreservation of BMMSCs and found no differences in terms of differentiation or phenotype. Cryopreservation of BM-MSCs using 7.5% DMSO, supplemented with 2.5% PEG and 2% BSA or even 5% DMSO, supplemented with 5% PEG and 2% BSA were shown to be almost as good as 10% DMSO in terms of viability and similar in terms of differentiation[84]. Comparison of mixed osmolyte solutions, consisting of sucrose/glycerol/creatine and sucrose/glycerol/isoleucine with standard DMSO containing freezing media further showed the potential of these type of cryopreservation solutions by improving postthawing function of MSCs[31].
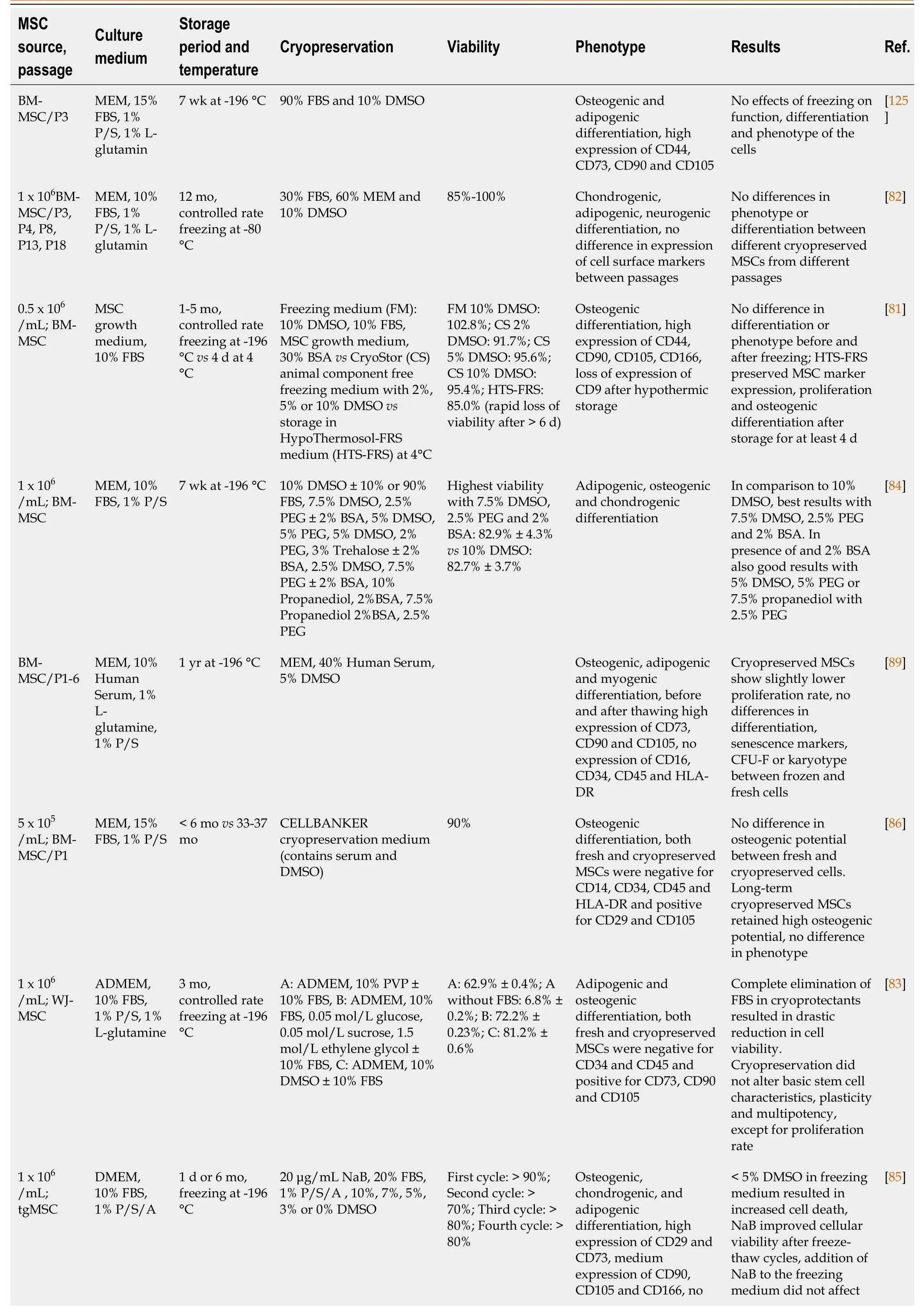
Table 2 Comparison of different protocols used during cryopreservation of mesenchymal stem/stromal cells
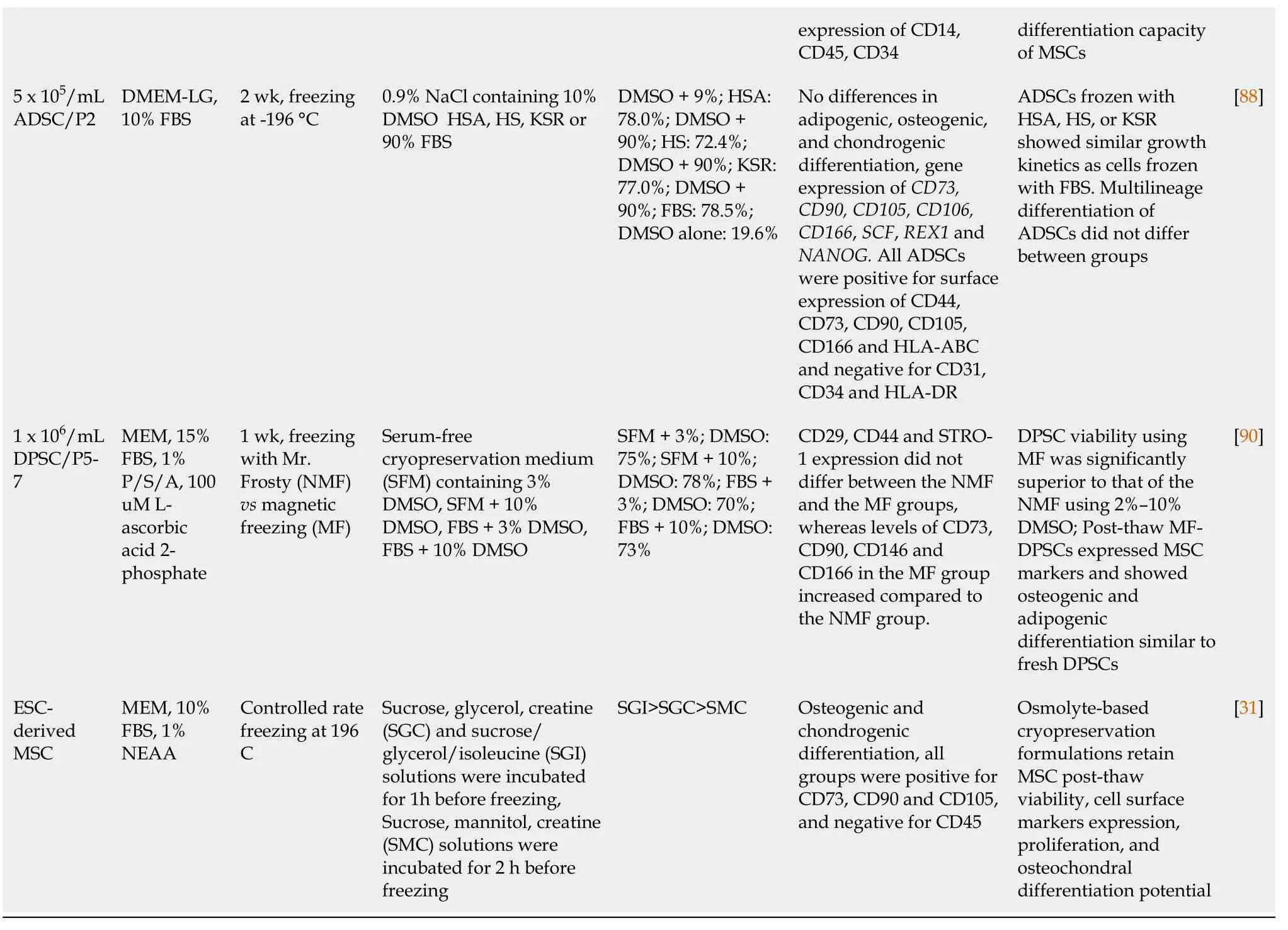
MSC: Mesenchymal stem/stromal cell; FBS: Fetal bovine serum; DMSO: Dimethyl sulfoxide; ESC: Embryonic stem cell; NEAA: Non essential aminoacids; MEM: minimal essential medium; KSR: Knockout serum replacement; BSA: Bovine serum albumin; P/S: Penicillin/Streptomycin; DPSC: Dental pulpa stem cells; ADSCs: Adipose derived stem cells.
For research purposes often non-controlled, simple isopropanol-jacketed freezing containers (such as the Mr. Frosty from NALGENE) are used. Using this system, temperature in cryovials decreases approximately 1 C/min[89,90]. In contrast, for clinical use, temperature controlled freezing devices are often preferred. Leeet al[90] used a programmed freezer with a magnetic field to freeze human dental pulp MSCs. Using the magnetic freezing procedure, the researchers were able to decrease the level of DMSO to 3% without a significant difference in cell viability. Using the magnetic field freezer “Cells Alive System” (CAS) rat BM-MSCs were frozen in serum-free freezing medium (10% DMSO, 5% Albumin, 0.2% D-Glucose, 0.6% NaCl, 0.03% glutamine, 0.2%NaHCO3)[91]. After 3 years, viability andin vivobone formation in the CAS group was significantly higher than that in cells stored in a non-programmed or non-magnetic freezer (87.7% and 48.5%, respectively). These data show the potential for use of alternative freezing systems for cryopreservation of MSCs as well as the use of secondary CPAs that decrease the need for DMSO. Most clinical trials use MSCs from related donors rather than off-the-shelf products. These MSCs are often directly after expansion infused into the patients. However, considering the increasing requirement for readily available MSC products, MSC culture and cryopreservation protocols under good manufacturing practice conditions will need to be revisited and low DMSO protocols that are optimized for clinical use and support MSC function in the absence of animal components remain to be developed.
CRYOPRESERVATION OF INDUCED PLURIPOTENT STEM CELLS
Whereas studies on HSCs have been the focus of stem cell research since the 1960s-70s, studies assessing the role and function of MSCs have intensified since the 1990s. Since 2006, a substantial portion of the focus within the stem cell field has moved steadily towards the use of the new kid on the block,i.e.induced pluripotent stem cells (iPSC). iPSCs are stem cells with embryonic stem cell (ESC)-like properties, but lack the ethical issues involved with the use of ESCs. This is related to the fact that iPSCs are artificially generated from somatic cells by forced overexpression of the pluripotency transcription factors OCT4, SOX2, KLF4 and c-Myc[92,93]. New protocols using different combinations of transcription factors, including NANOG and LIN28[94] and others, devoid of oncogenic potential, as well as different methods for transfer (e.g., integrating lentiviral vectors, non-integrating sendai based vectors, episomal vectors, direct mRNA transfer,etc.)[95] have not affected the characteristics of the derived iPSCs: iPSCs have unlimited self-renewal capacity and the ability to differentiate into cells from all three germ layers (endoderm, mesoderm, ectoderm). iPSCs thus provide the tools to study early developmental biologyin vitroand can be used for disease modeling and drug discovery. In addition, patient-derived iPSCs offer the opportunity to study the pathophysiology of diseases that could not be studied previously and can be used for the development of personalized medicine. All these features further stimulated iPSCs to become an important source of stem cells, and biobanks for storage of healthy and patient-derived iPSCs have now been established in many countries. However, efficient banking requires cell production facilities where cells can be expanded, maintained and cryopreserved under optimal conditions to ensure protection of iPSC characteristics and properties for weeks to years. In contrast to the cryopreservation protocols developed for HSCs and MSCs, current protocols for cryopreservation of iPSCs have focused on different issues, including freezing of cells in small aggregatesvssingle cell freezing in the presence of absence of DMSO[96-99], cell freezing using vitrification or different combinations of CPAs[100-102], cell recovery after cryopreservation using small molecules, such as the Rho kinase (ROCK) inhibitor Y-27632[103-105] and development of animal-component free formulations of culture and cryopreservation media using KSR instead of serum[106-108] (Table 3).
Using Raman spectroscopy to assess intracellular ice formation in iPSCs during cooling, Liet al[96] showed that iPSC aggregates are more sensitive to supercooling than single iPSCs in suspension due to the decreased water permeability of iPSCs in aggregatesvssingle cells. They also showed a greater variation in DMSO concentration across the aggregates than in single cells, suggesting that the size of the aggregates may hinder equal diffusion of the cryoprotectant to the cells. They also found that iPSC aggregates frozen in an optimized solution consisting of non-essential amino acids, sucrose, glycerol, isoleucine and albumin dissolved in a buffer made of poloxamer 188 (P188) in Hank’s Balanced Saline Solution, did not exhibit the same sensitivity to undercooling as those frozen in non-optimized solutions or those containing 7.5% DMSO[97]. In addition, cryopreservation of iPSCs in aggregates requires a significantly modified freezing technique, where iPSC aggregates are first incubated at room temperature for 30 min to 1 h before freezing to allow sufficient internalization of the CPAs[97], in contrast to freezing with DMSO, which usually requires working at low temperatures (4 °C) and rapid mixing of cells.
Miyamotoet al[100] compared the efficacy of a variety of different cryopreservation media on an established murine iPSC line. These media consisted of control 10% DMSO formulations to reduced DMSO solutions, glycerol-containing solutions, combinations of DMSO and glycerol and commercially available cryopreservation media (CELLBANKER 1, 1+, 2 and STEM-CELLBANKER) and were used to freeze mouse iPSCs in suspension. Comparison of viability, proliferation and multipotency after long-term freezing of iPSCs in these media showed optimal results with the serum-free formulations of CELLBANKER (CELLBANKER 2 and STEMCELLBANKER)[100]. However, the precise formulations of these freezing media is proprietary, Hank’s Balanced Saline Solution and the researchers did not mention whether the STEM-CELLBANKER formulation used contained DMSO. Katkovet al[98] compared freezing of iPSCs in aggregates and as single cells using different CPAs including DMSO, ethylene glycol (EG), propylene glycol and glycerol. After extensive comparison, they found that freezing in aggregates resulted in favorable iPSC recovery after thawing. In addition, toxicity tests revealed that EG was not only less toxic than DMSO, it also supported better maintenance of pluripotency than propylene glycol or glycerol[98].
The use of KSR as a serum replacement has shown promising results and is another step in the development of animal component-free cryopreservation solutions. In combination with 10% DMSO, KSR has been used at concentrations of 25%-90% to freeze effectively iPSCs, ESCs and iPSC-derived cells with high post-thaw viability[105,106,108,109]. Inhibition of Rho kinase activity with ROCK inhibitors has shown favorable outcomes after freezing of both ESCs and iPSCs, and although not added during cryopreservation itself, it promotes both plating and cloning efficiency[104,105,108,110,111] by preventing apoptosis of detached cells[112]. Since addition of ROCK inhibitors up to 5 d after thawing still promotes colony formation, and since the effects of ROCK inhibition appear to be reversible, it has been also been suggested that ROCK inhibitors may relieve cellular stress[104].
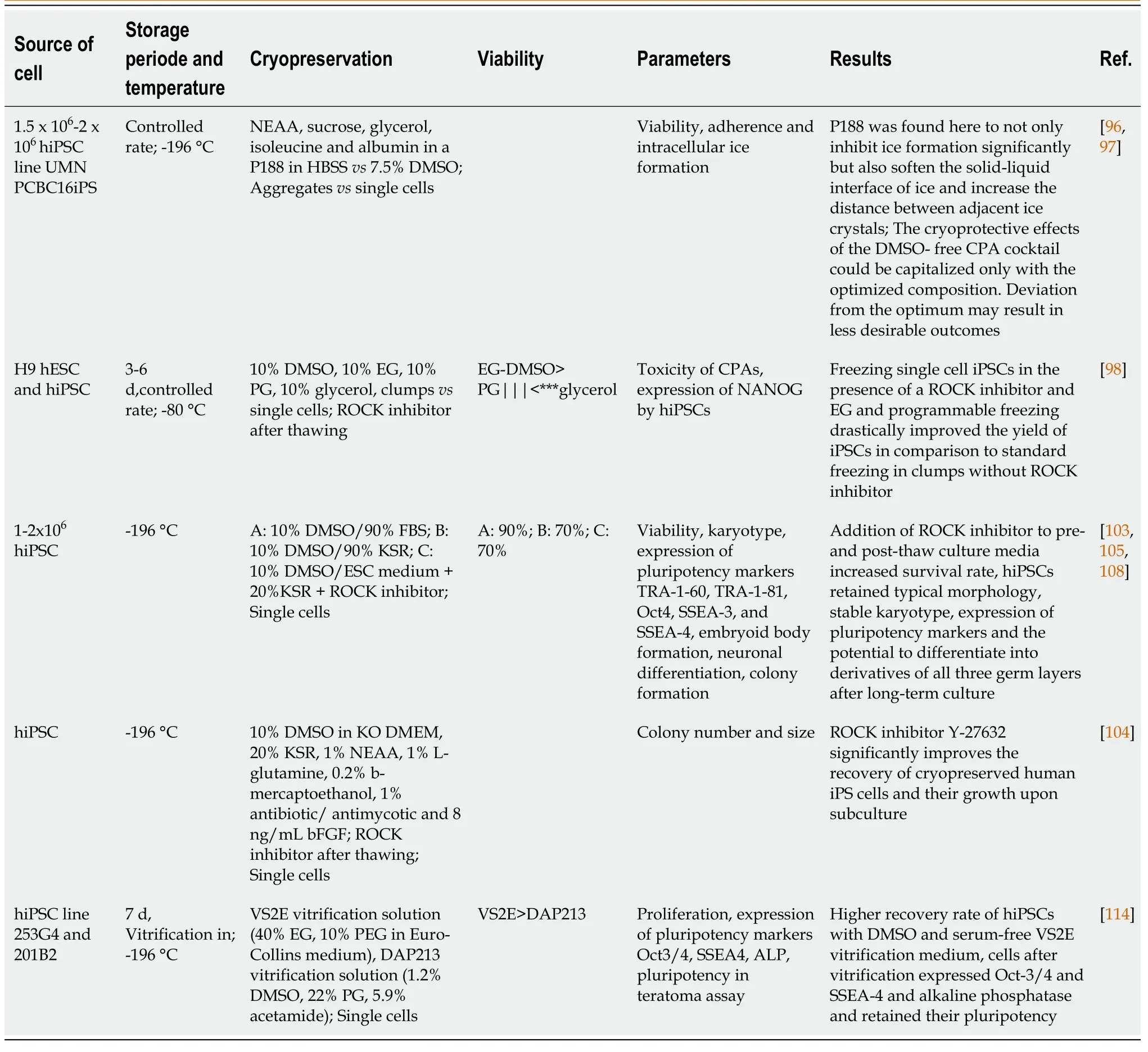
Table 3 Comparison of different protocols used during cryopreservation of induced pluripotent stem cells
Similar to studies in MSCs, the effects of magnetic fields on iPSC recovery after freezing have been assessed. Using the CAS researchers showed improved survival after thawing of iPSCs, but no effect on proliferation, gene expression and multilineage differentiation[113]. Reubinoffet al[101] previously showed that vitrification of both ESCs and iPSCs is feasible, using precooled freezing medium consisting of 90% FBS and 10% DMSO and a cooling rate of 1 C/min. ESC aggregates were preincubated in 80% DMEM, 10% DMSO and 10% EG and then placed into small 1-2 mL droplets containing 60% DMEM, 20% DMSO, 20% EG and 0.5 mol/L sucrose. All vitrified ESC aggregates recovered upon thawing and gave rise to colonies after plating. However, vitrified colonies were significantly smaller and showed increased differentiation compared with control colonies. Nevertheless, colonies generally recovered within 1-2 d of cell culture. Using a similar method for iPSCs, but using a DMSO and serum-free medium based on 40% EG and 10% PEG, Nishigakiet al[114] obtained a higher recovery rate of iPSCs than with a vitrification solution containing DMSO and serum.
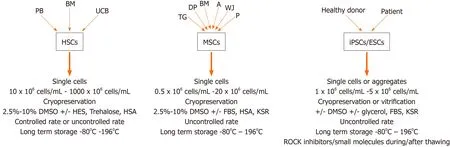
Figure 2 Preferred cryopreservation protocols for different types of stem cells.
CONCLUSION
The universally used cryoprotectant DMSO has been associated within vitroandin vivotoxicity and has been shown to affect many cellular processes through dysregulation of gene expression and changes in DNA methylation. Despite studies showing that DMSO affects cell characteristics including differentiation potential, DMSO remains to be the CPA of choice both in a research setting and in the clinics. Many different protocols have been developed for different types of stem cells and a broad range of alternatives to DMSO have been shown to hold promise for use as a CPA (Figure 2). These alternatives include such molecules as trehalose, sucrose, EG, PEG and many more. It is obvious that a single protocol that can be used for all types of stem cells is not feasible, but the enormous amount of available alternatives should make it possible to adapt and optimize DMSO-free and animal component and serumfree cryopreservation solutions adapted for different types of stem cells in the foreseeable future.
杂志排行
World Journal of Stem Cells的其它文章
- Effects of living and metabolically inactive mesenchymal stromal cells and their derivatives on monocytes and macrophages
- Stem cells' centrosomes: How can organelles identified 130 years ago contribute to the future of regenerative medicine?
- Recent advances in stem cell therapy for neurodegenerative disease: Three dimensional tracing and its emerging use
- Stem cell therapies in cardiac diseases: Current status and future possibilities
- Current evidence on potential of adipose derived stem cells to enhance bone regeneration and future projection
- Neural stem cell therapy for brain disease
