The Search for Putative Hits in Combating Leishmaniasis:The Contributions of Natural Products Over the Last Decade
2021-10-08PatrickSakyiRichardAmewuRobertDevineEmahiIsmailaWheltonMillerSamuelKwofie
Patrick O.Sakyi ·Richard K.Amewu ·Robert N.O.A.Devine ·Emahi Ismaila ·Whelton A.Miller ·Samuel K.Kwof ie
Abstract Despite advancements in the areas of omics and chemoinformatics,potent novel biotherapeutic molecules with new modes of actions are needed for leishmaniasis.The socioeconomic burden of leishmaniasis remains alarming in endemic regions.Currently,reports from existing endemic areas such as Nepal,Iran,Brazil,India,Sudan and Afghanistan,as well as newly aff ected countries such as Peru,Bolivia and Somalia indicate concerns of chemoresistance to the classical antimonial treatment.As a result,eff ective antileishmanial agents which are safe and aff ordable are urgently needed.Natural products from both flora and fauna have contributed immensely to chemotherapeutics and serve as vital sources of new chemical agents.This review focuses on a systematic cross-sectional view of all characterized anti-leishmanial compounds from natural sources over the last decade.Furthermore,IC50 /EC50 ,cytotoxicity and suggested mechanisms of action of some of these natural products are provided.The natural product classification includes alkaloids,terpenes,terpenoids,and phenolics.The plethora of reported mechanisms involve calcium channel inhibition,immunomodulation and apoptosis.Making available enriched data pertaining to bioactivity and mechanisms of natural products complement current eff orts geared towards unraveling potent leishmanicides of therapeutic relevance.
Keywords Chemotherapeutics·Chemoinformatics·Natural products·Cytotoxicity·Leishmaniasis·Phenotypic screening
1 Introduction
The debilitating rate of parasitic infections in the tropical and subtropical regions of developing countries has become alarming [1].Vector-borne neglected tropical diseases and related synergetic co-infections,particularly leishmaniasis are very challenging and sophisticated to treat [2].This is partly due to the existence of diverse parasitic species with diff erent bionomics and sophisticated overlap between virulent factors.Activated immune response during disease exacerbation coupled with emerging resistance by both parasites and vectors against various treatment regimens have also contributed to this challenge [2,3].
Leishmania,the etiological agent of leishmaniasis,is transmitted globally by over 90 diff erent female sand-fly species of the Phlebotomus family,spread across 98 countries and four continents,with annual estimates of 1 million new cases and 30,000 deaths as at 2017 [2,4].The exact disease burden is unknown,but statistics indicate that over 350 million people are at risk,signifying a prominent public health risk [2,5,6].
Leishmaniasis is curable if the disease is diagnosed early and the appropriate medication is administered.Typically,leishmaniasis is initially marked by dermotropic ulcers,which then progress into the visceral tissues,resulting in a late and more debilitating condition that can often lead to death ifleft untreated.In some cases,the destruction of the mucocutaneous membrane especially the nose,throat,and mouth have also been very common [2].The degree of clinical outcome and its corresponding immunopathology depends primarily on the type of causative species,age of host,and the balance between the host immune response and how the parasites subvert these defense mechanisms.In cases where the victim’s immune system is strong,Leishmaniapathogens behave as opportunists by remaining dormant until the host’s immunity is compromised.Additionally,when the host is immunosuppressed,relapses are usually prevalent resulting in treatment failures.
Some challenges associated with the management of leishmaniasis include systemic toxicity of administrated drugs,high cost of existing therapeutic options,lengthy treatment periods and drug resistance.Furthermore,confounding factors such as parasite diversity has hampered various intervention strategies and halted global eff orts,necessitating an immediate search for new drug leads for development as the next generation of antileishmanial agents[7- 9].In lieu of this,the review seeks to bring to the fore the various classes of natural products recently discovered with antileishmanial potentials over the last decade.Even though,the review primarily reported compounds with potent bioactivity,few with low potency were reported since these could be optimized or their scaff olds may serve as skeletons for the development of future leishmanicides.
1.1 Trends in leishmanial chemotherapy and current panorama
Protection against leishmaniasis started with mimicking natural immunity through live inoculations [10] until modernized techniques including killed promastigotes and knocked out parasites came into play.Unfortunately,the presence of persistent lesions and the difficulty in estimating their efficacy rendered these approaches less eff ective [10,11].Eff orts to alleviate leishmaniasis via chemotherapy include the use of pentavalent antimonial,which was essentially a small tartrate complex of antimony first reported in 1925 by Brahmachari [12,13].Although,antimoniate (Sb V ) is still active after reduction by arsenate reductase to Sb III ,Leishmaniaparasites are also susceptible to Sb V via oxidative stress.
Gene amplification studies involving the Adenosine Triphosphate (ATP) binding cassette transporters including the multi-resistance proteins that act as effl ux pumps have been shown to contribute to antimony resistance in clinical isolates [14,15].Likewise,deletions of aquaporin membrane carrier genes and phenotypic changes of the parasite with subsequent induced eff ects on the microbicide activity and the effl ux rate of antimony reaching the macrophages also contribute to the resistance [16].
In the mid-1960’s pentamidine became the second choice to antimony resistant strains [17].However,its utility like the antimonial was hampered due to severe vasomotor side eff ects and complex interactions with the pancreas which leads to the destruction ofβ-cells causing diabetes mellitus[17].
In the quest to expedite the time it takes for drugs to reach the market,strategies such as deciphering the cellular similarities between disease causing pathogens from phenotypic screening were developed.In the early 1960s,the anti-fungal amphotericin B fromStreptomyces nodosuswas used for treating leishmaniasis [18,19].This choice was widely accepted in most endemic areas due to its efficacy but not so in other areas especially East Africa (L.donovani) and South America (L.infantum) [20].
The anticancer agent alkyl phosphocholine (miltefosine)was the first oral formulation with strong protection against visceral leishmaniasis.Miltefosine works by modulating an apoptosis process induced by mitochondria membrane depolarization and phospholipid biosynthesis inhibition [21].The main drawback in administering miltefosine for leishmaniasis treatment includes longer elimination time,lengthy treatment course,and miscarriage in pregnant patients after use[22].
A new and simple formulation of an old antibiotic paromomycin which inhibited translation with diff erent modes of application (enteral,parenteral and topical) was also repurposed for leishmaniasis in 1967 [23,24].Unlike the other treatment options,paromomycin’s toxic eff ects are very minimal,but its efficacy is quite poor.New optimum carriers targeting pathogen macrophage using albumin has recently been reported to increase efficiency [25].
Following the failure of miltefosine,a collaboration between the Walter Reed Army Institute of Research(WRAIR,USA) and GlaxoSmithKline (UK) identified sitamaquine as a promising alternative,but its apparent loss of efficacy in tegumentary leishmaniasis limited its use [26].Subsequently,findings from amphotericin B use and its high curative rate in patients influenced another repurposing strategy using the oral anti-fungal azoles (fluconazole,itraconazole,and ketoconazole) as suitable control and cost-eff ective therapy [27,28].
Due to the therapeutic challenges,new chemotypes with high potency in tandem with immunostimulatory activity targeting new proteins applicable to both visceral and cutaneous leishmaniasis cases are desperately needed.
1.2 Natural products as possible sources of new drugs against leishmaniasis
The lack of eff ective vaccines for control and concerted elimination campaign [2],and recent snail paced progress on leishmanial vaccine development does not guarantee any optimism.With the advancements in synthetic organic chemistry,combinatorial chemistry,and computational de novo drug discovery strategies,as well as high throughput screening techniques,only a few synthetically constructed drugs have been useful in combating leishmaniasis.Even with this,few natural product scaff olds represent major pharmacophores responsible for their curative eff ects.Between 2005 and 2010,about 19 natural products were registered for treatment of infectious diseases [29].Similarly,over 69%of new small molecules used for the treatment of infectious diseases originated from natural products [30,31].
Despite the large molecular weights of natural products which renders some of them less druglike,structural diversity,large chemical space and safety are characteristics that overrides synthetic alternatives.Treatments using extracts from plant families from endemic regions includeFabaceae[32],Annonaceae,Euphorbiaceae[31,33,34],Rutaceae[35- 37],Myrsinaceae[31,38],Liliaceae[39],Araliaceae[38],Simaroubaceae[40],as well as endophytes generaAlternaria[41],Arthrinium,Penicillium,Cochloibus,Fusarium,Colletotrichum,and Gibberella[42].Additionally,the exploration of marine natural products has led to the identification of interesting natural products with diverse biomolecular functions [43,44].
Since the mid-eighties when the search for anti-leishmanial natural products became prominent,numerous metabolites originating from plants to current antileishmanial therapies have been reported.Lately,credible chemical entities from marine sources and endophytic species have also been reviewed [45- 51].This review presents the various classes of natural products from both flora and fauna that have been isolated over the last decade with anti-leishmanial properties.Also,the IC50/EC50values and suggested mechanisms of action of these natural products are discussed.
1.3 Classification of natural products with anti-leishmania properties
1.3.1 Alkaloids
Among the characterized bioactive constituents from nature,alkaloids have provided a broad-spectrum activity against different ailments and demonstrated their suitability as potential drug leads.Phenotypic alterations in ultrastructure form of the infective cells and immunomodulatory investigation studies of isolated alkaloids within the last decade reveal 27 alkaloids (Table 1) with varying efficacies from strong to weak activity.The natural product 3 isolated fromCissampelos sympodialisacts as a calcium channel inhibitor with immunomodulatory eff ects through the enhancement of nitric oxide (NO) production in macrophages [52].Studies of 4 fromCroton pulleireported significant alterations in organelle membranes of the endoplasmic reticulum,kinoplast and golgi body,depicting an apoptosis-like process[53].Treatment with spectaline alkaloids,16 and 17 from dichloromethane fractions of the flowerSenna spectabilisofLeishmaniapromastigotes also portray a similar molecular mechanism like its structurally related piperine amide alkaloid,which either modulates the sterol biosynthetic pathway or acts as an inhibitor of cell proliferation by mitochondrion organelle destruction [54].Although,the exact mode of action has not been fully elucidated,21 fromBerberine vulgarislike the active alkaloid inBerberine aristateperpetuates a similar activity through respiration incapacitation and apoptosis [55].However,21 was identified as a potential cell membrane disruptor via sterol biosynthesis inhibition [56],while 22 induces reactive oxygen species (ROS) generation.Structural activity relation (SAR) studies of high affinity protein kinase inhibitors,staurosporine-based compounds(24-27) revealed the 4th C methyl amine and 7th C hydrogen acceptor as the cause for the reinforced activity observed inL.donovani,which had major morphological changes in the flagella pocket and plasma membrane because of signal blockage via phosphokinase (PK) inhibition.

Table 1 23 alkaloids isolated from various flora and fauna together with their IC50 and toxicity tested on some Leishmania species

Table 1 (continued)

Table 1 (continued)

Table 1 (continued)

Table 1 (continued)
1.3.2 Phenolics
As characterized by hydroxy-phenyl groups,polyphenolics are widely distributed in nature and have been isolated from diff erent plants.In traditional medicine phenolics have received much interest in phyto-therapeutics for the treatment of ailments ranging from non-infectious to infectious diseases.These chemotypes include compounds like coumarins,flavonoids,quinones,lignans,flavone glycosides amongst others (Table 2).Flavonoids fromSelaginella sellowiwhen tested against diff erent forms ofLeishmaniarevealed a pro-drug mechanism for 28 but an activated NO generation for 29 [70].The diff erence in the mode of action of these two flavonoids may be due to their conformational orientations.Similar investigations to understand the possible cause of apoptosis induced by 30 and 31 suggested a mitochondrial dysfunction with no influence on ROS [71].However,evidence from suicidal action of some quercetin analogues have also indicated iron chelation,arginase inhibition,and topoisomerase II intercalation as possible mechanisms [72].From the sameNectandragenus,inhibitory activity of 34 and 43 have been fully elucidated.Results indicated an inactivation of exacerbatory immunogens with reduced calcium levels and depolarized mitochondria potential [73].Studies with similar compounds against melanoma cells indicated an apoptosis process confirming the depolarization activity [74].Deciphering the exact mechanism underpinning the leishmanicidal action of isolated compounds fromConnarus seberosus,it was revealed that defects in the mitochondria and plasma membrane structure with the evidence of lipid accumulation were caused by 55 and 56 [75].Comparing 58 and its 3-O-methyl analog,59,to rosmarinic acid (based on the shared catechol nucleus),their potential mode of action is suggested as inhibition of reactive oxygen species [76,77].75 as a chemo-preventive agent acts by reducing inflammatory symptoms by suppressing NF-κB expression and other pro-inflammatory factors including iNOS,COX-2,TNF-α,IL-1β,and IL-6 [78].Compound 74 emulates an apoptosis induced suicidal mechanism which involves DNA fragmentation,inhibition of inflammation cytokines and the activation of caspases with downstream eff ects on gene transcriptional process [79].Structural similarities of anti-inflammatory coumarins with 74 precludes a similar mechanism of action [80].From the isolates ofArrabidaea brachypodaonly 67 altered organelle structure and function by attenuating cytoplasm puncturing and golgi apparatus swellings [81].

Table 2 Various classes of phenolic compounds with their IC50 exhibiting antileishmanial properties
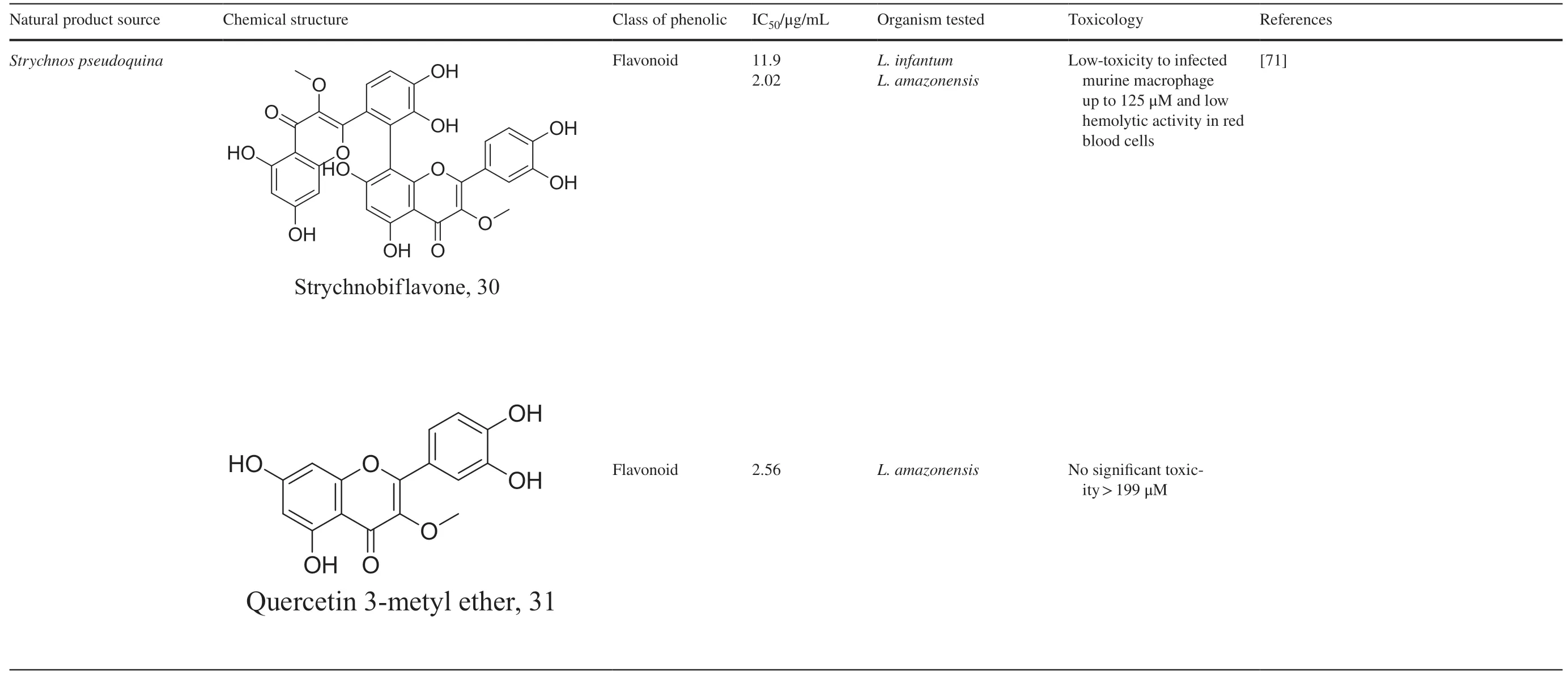
Table 2 (continued)

Table 2 (continued)

Table 2 (continued)

Table 2 (continued)

Table 2 (continued)
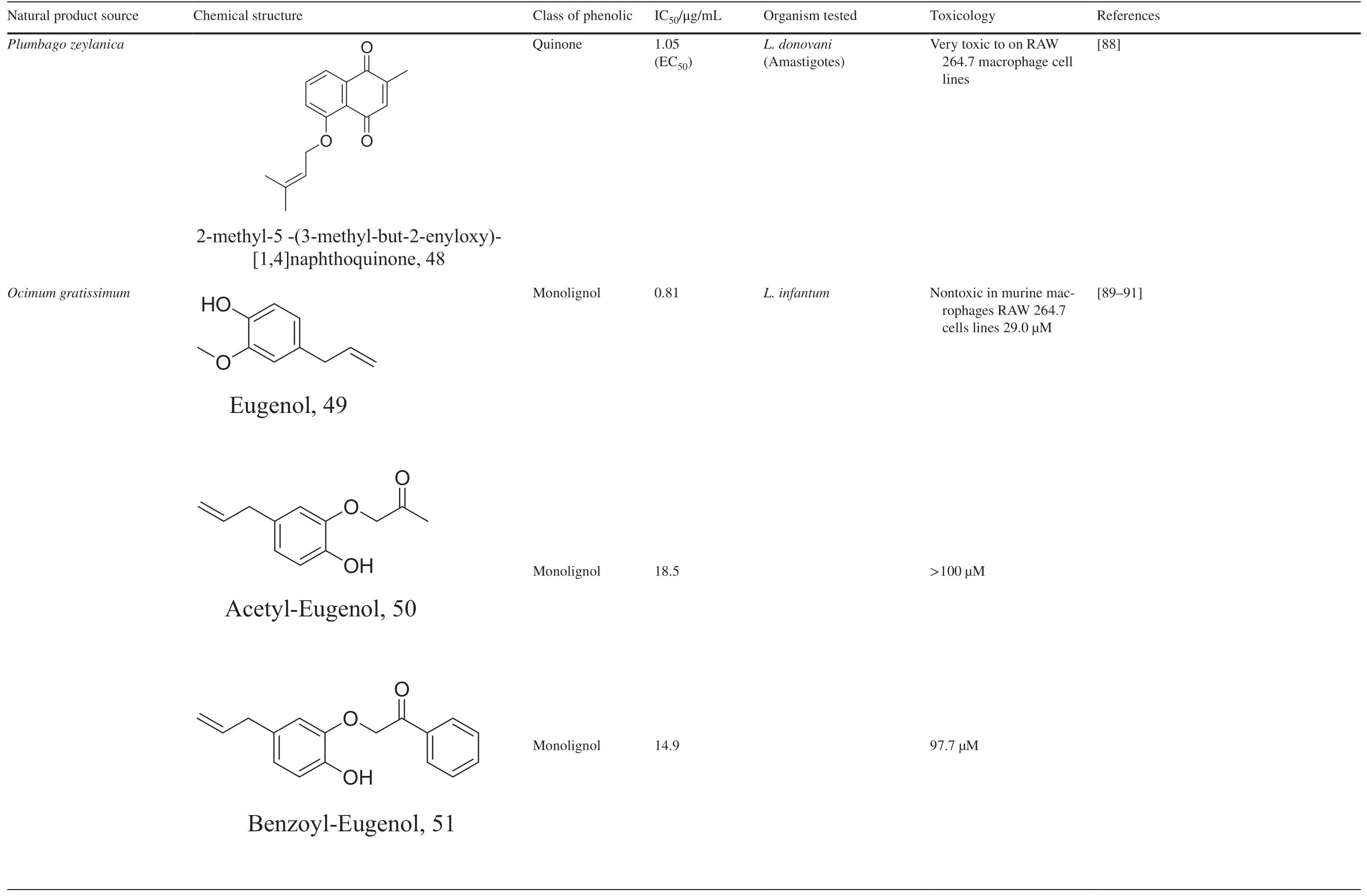
Table 2 (continued)

Table 2 (continued)

Table 2 (continued)

Table 2 (continued)

Table 2 (continued)

Table 2 (continued)

Table 2 (continued)

Table 2 (continued)

Table 2 (continued)

Table 2 (continued)
1.3.3 Terpenes and terpenoids
Another group of secondary metabolites with interesting anti-parasitic activities are terpenes.Ultrastructural changes of 79 in phenotypic screenings indicated mitochondrial blebs and lipid deformities [100,101].80 isolated from essential oils ofTetradenia ripariawere found to distort promastigote structure especially the fate of its chromatin followed by an apoptosis process which is suspected to be caused by caspase activation [102,103] (Tables 3 and 4).

Table 3 Various classes of terpenes and terpenoids with their IC50 exhibiting antileishmanial properties

Table 3 (continued)

Table 3 (continued)
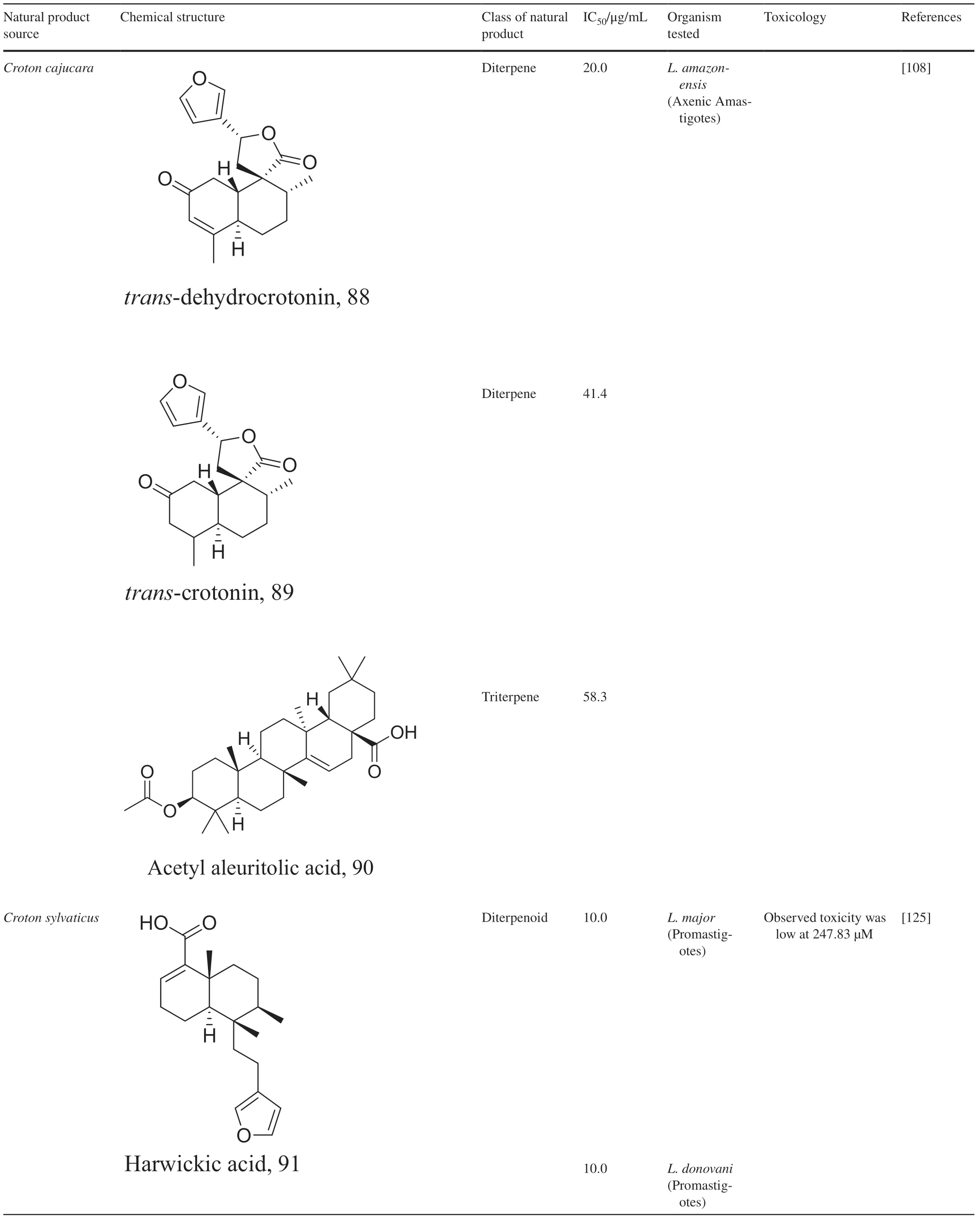
Table 3 (continued)
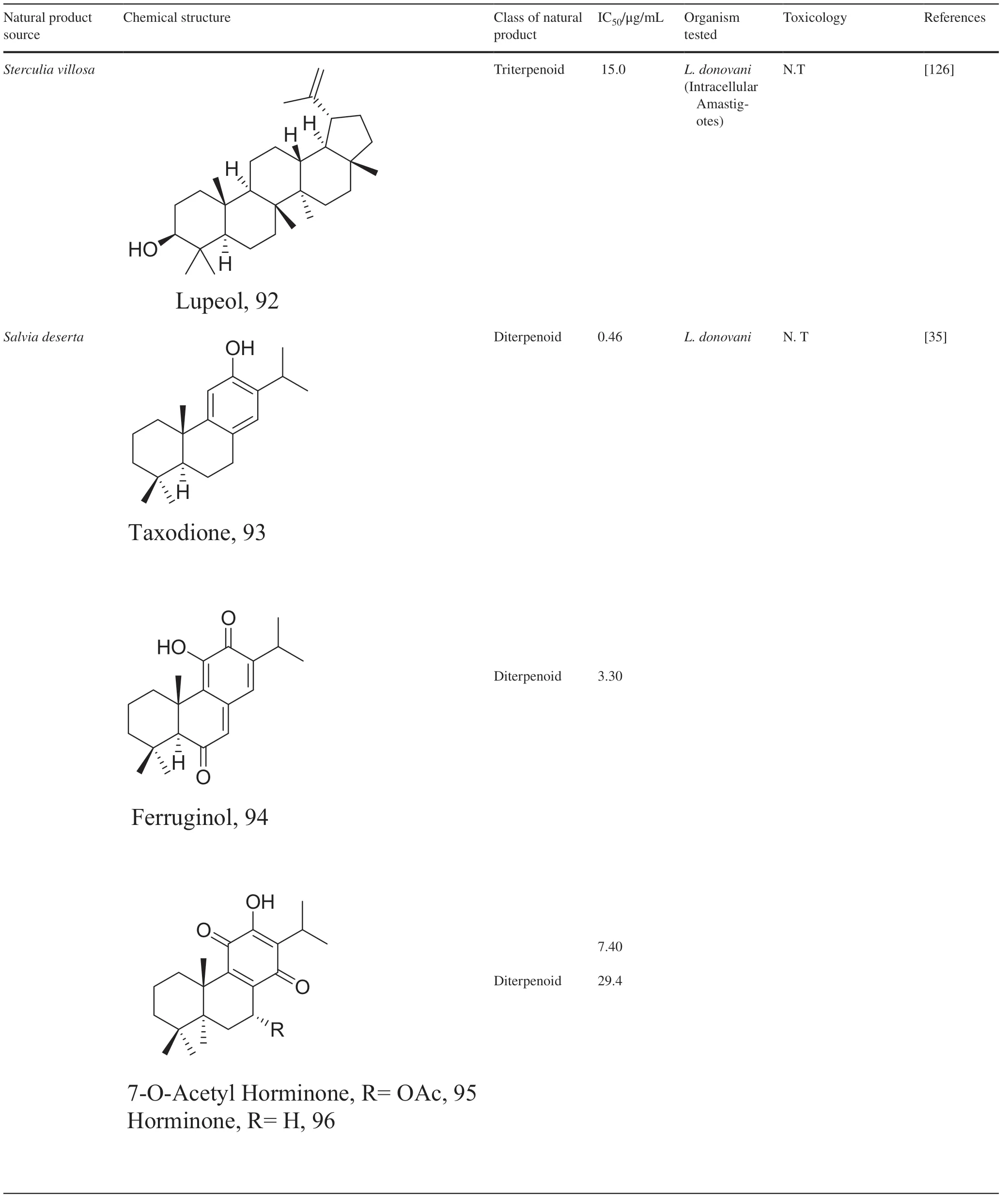
Table 3 (continued)

Table 3 (continued)

Table 3 (continued)

Table 3 (continued)

Table 3 (continued)

Table 3 (continued)

Table 3 (continued)

Table 3 (continued)
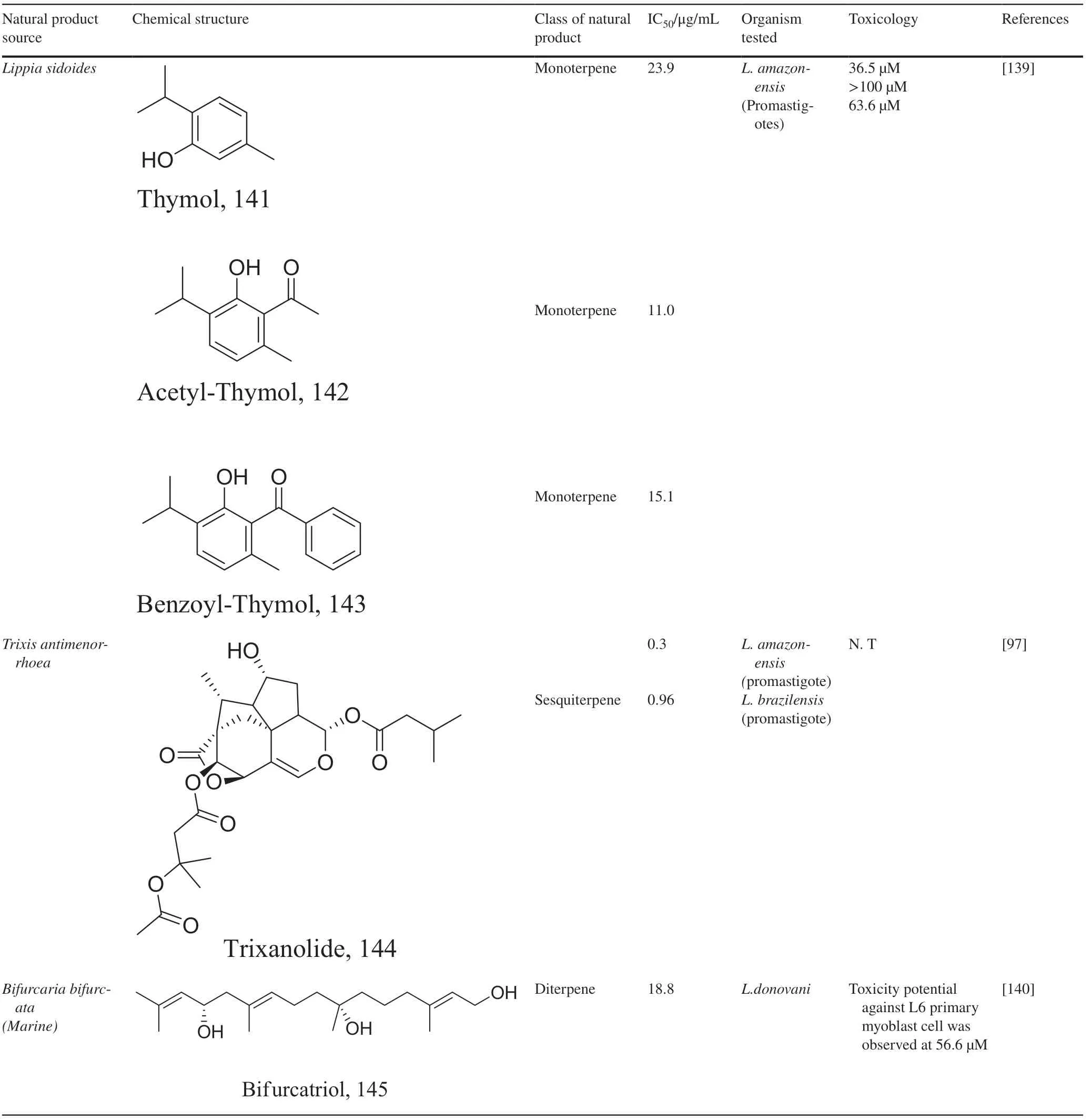
Table 3 (continued)

Table 3 (continued)
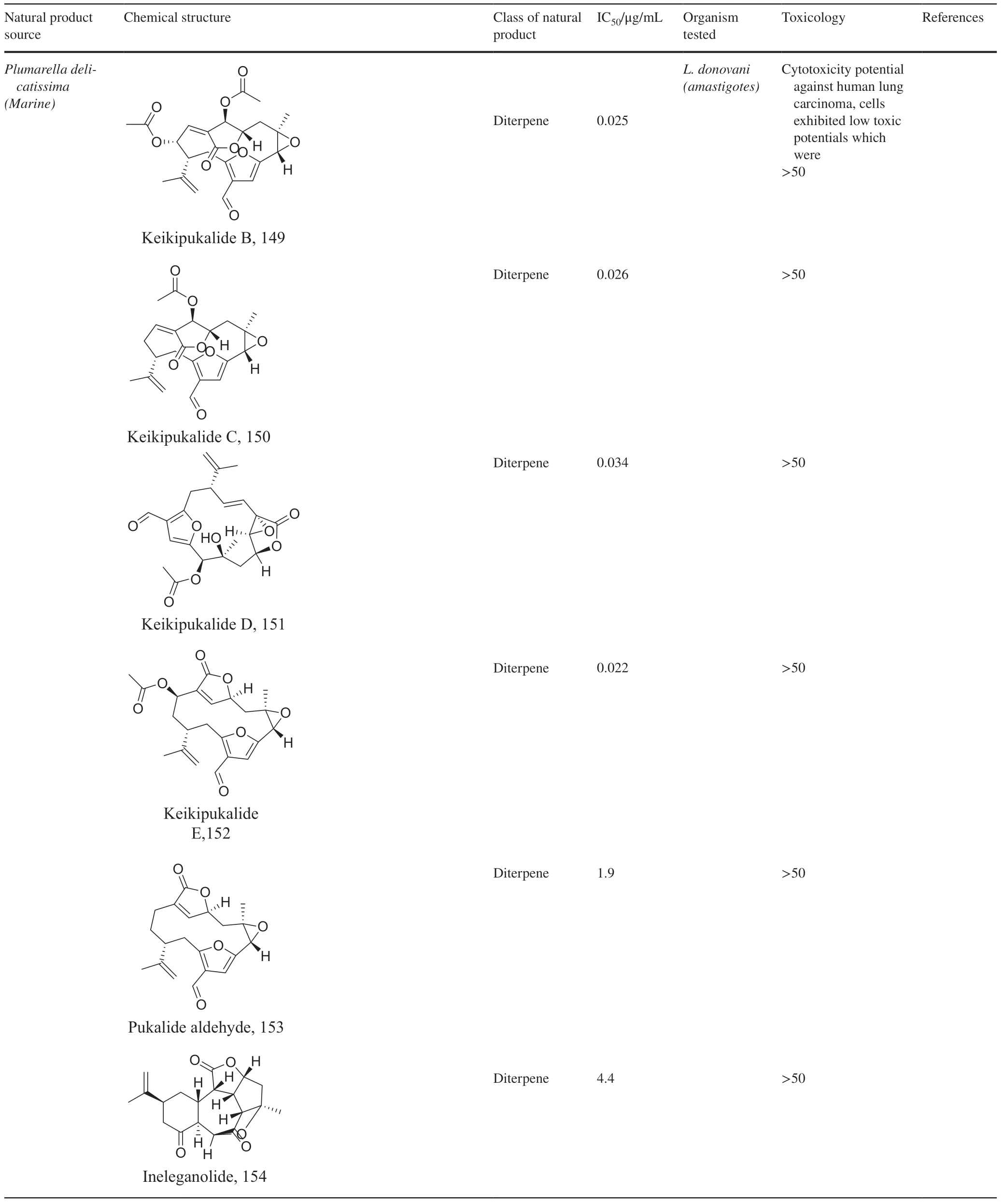
Table 3 (continued)

Table 3 (continued)

Natural product source Chemical structure Class of natural product IC50 /μg/mL Organism tested Toxicology References Dysidea avara(Marine)OH O Avarone,160 OH O Avarol,161 Sesquiterpene Sesquiterpene 28.21 20.28 7.64 7.42 7.08 3.19 L.infantum(Promastigotes)L.tropica(Promastigotes)L.infantum(Amastigote)L.infantum(Promastigotes)L.tropica(Promastigotes)L.infantum(Amastigote)Low toxicity against human microvascular endothelial cells and (human acute monocytic leukemia cells with CC50 62.19 and > 100 respectively.36.8 31.75[144]

Table 4 Various classes of steroids,fatty alcohol,lignan,and butanolide with their IC50 exhibiting antileishmanial properties

Table 4 (continued)

Table 4 (continued)

Table 4 (continued)

Table 4 (continued)

Table 4 (continued)
Halogenated terpenes 72 and 83 fromLaurencia dendroideawhich only diff er primarily in a double bond character also targets the same organelle via redox perturbation[104,105].The natural product 87 fromVanillosmopsis arboreashow promising activity through apoptosis induction characterized by mitochondrial dysfunction and oxidative stress [106].Similar mode of action was reported for 87 isolated fromTunisia chamomileessential oil againstL.amazonensisandL.infantum[107].Eff ects of clerodone terpenes,88,89 and 90 from the stem bark ofCroton cajucarahave been shown to obstruct ROS protection via trypanothione reductase inhibition [108].
Interest in marine natural products which led to the evaluation of marine terpenes like pentacyclic triterpene 92,which exhibited an anti-inflammatory action with enhanced levels of T cells and Th1 cytokines when compared to its control [109].
Elucidation of the exact mechanism of action of four triterpenes from the roots ofSalvia desertashowed that despite the strong antioxidant capacity of 93,it also kill parasites by inhibiting isopentenyl diphosphate condensation with the major target being farnesyl diphosphate synthase [110].Studies to also understand the molecular basics of 94 shows a similar action like 80,but fragmentation of DNA strands has been described for diterpene 95 and 96 [111,112].Inhibition of oxidative pathways particularly IFN-γ-related signaling by similar diterpenoid quinones isolated from the roots ofSalvia officinalishas also been shown to prevent disease proliferation and further protecting the host specie [113].Recent studies in estimating the role of the energy production in the form of ATP inLeishmaniawith acyl phloroglucinol derivatives has revealed 97 as a mitochondria complex II/III inhibitor [114].
Like terpenes which are formed by the head to tail condensation of isoprene units,terpenoids (terpenes with oxygen-containing functional group) also represent a unique group of natural products with high functionalization and promising pharmacological activity.Isolation of six germacranolides from the leaves and stems of theCalea specieshave shown promising activities againstL.donovaniandL.amazonensis[115,116].Among them morphological assessment studies with 100 and 111 indicated alterations in the nucleus and mitochondria describing an apoptosis like process through the mitosis motor downregulation pathway [115].Due to the similar core structure shared with germacra-1(10),11(13)-dien-12,6-olide a similar mechanism is envisaged for its counterpart 104 by aiding in generating ROS complementing the elucidated apoptosis process.The natural product 106 shares same structural core therefore may possess similar mode of action in addition to the inhibition of thiol-antioxidant enzymes [117].Interestingly,106 and its iso-conformer have also been disclosed to induce a proinflammatory inhibition via the NF-KB pathway [118].On the other hand,110 and 125 have also exerted multi-spectral activities including suppression of cell proliferation modulators and upregulation of microbicidal NO species [119].
1.3.4 Steroids
Steroids are a class of natural or synthetic organic compounds with three six membered rings fused with a five membered ring.Ergosterol,the main sterol inLeishmaniaparasite constitute a major component of the cell membrane and mitochondrion of the parasite which when inhibited leads to parasite death.164 extracted fromTrametes versicolormimicsLeishmaniaergosterol due to similarities in core structure but a break in oxygen-oxygen bond in ergosterol peroxide unleashes oxidation on lipids,proteins and nucleic acids of the parasite by free radical reaction leading to serious toxicity to theLeishmania parasite[145].Apart from the biological formation of bridge peroxides,the deleterious eff ects of other lanostane type steroids on membrane state and integrity causing parasite death has been reported[146,147].Also,anti-infective studies ofSassafras albidumand its bioactivity guided fractionation reported a sterol and fatty alcohol,162 and 163 respectively [36] as promising antileishmanial compounds.162 which diff ers from cholesterol at C24 position is believed to kill the parasite via an apoptosis mechanism involving DNA fragmentation,inhibition of inflammation cytokines and the activation of caspases [148,149].Evaluating the suicidal action of active isolates fromPentalinon andrieuxii,181 induced changes in immune responses particularly via necrosis and apoptosis characterized by increase in IL2 and IFN-γ which insinuates the control of pro-inflammatory cytokines by anti-inflammatory counterparts [150].182 halted the process of electron transport and ATP generation in the mitochondria [151].In addition,plasma membrane alterations with the administration of the other isolates depicts a sterol metabolism inhibition as a contributing factor to parasite death [151].
2 Conclusion
Though humans and natural products did not co-evolve,chemical prototypes from natural origins have numerous targets in both human and animal diseases.Their structural diversity,large hemical space and safety are intriguing characteristics that makes them very attractive.Diverse biomolecular functions including anti-leishmanial potentials are possessed by various plant families includingFabaceae,Annonaceae,Euphorbiaceae,Rutaceae,Myrsinaceae,Liliaceae,AraliaceaeandSimaroubaceae,as well as endophytes generaAlternaria,Arthrinium,Penicillium,Cochloibus,Fusarium,Colletotrichum,and Gibberella,and marine natural product possess.
Management of leishmaniasis is plagued with systemic toxicity,high cost of existing drugs,lengthy treatment periods,drug resistance and parasite diversity.Diff erent classes of natural products such as alkaloids,terpenes,terpenoids,and phenolics are examples of compounds evaluated towards the treatment of leishmaniasis.They exert their antileishmanial activities through calcium channel inhibitors,immunomodulatory through the enhancement of NO in macrophages,alterations in organelle membranes of the endoplasmic reticulum,respiration incapacitation and apoptosis.Other antileishmanial related mechanisms include cell membrane disruption via sterol biosynthesis inhibition,reactive oxygen species (ROS) generation,iron chelation,arginase inhibition,topoisomerase II intercalation,suppressing NF-κB expression and other pro-inflammatory,and trypanothione reductase inhibition.
Author contributionsPOS,RKA and SKK initiated the work,POS wrote the first draft supervised by SKK and POS All the authors contributed to the writing of the review,read and accepted the final draft article.
Declarations
Conflict of interestThe authors declare no conflict of interest.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License,which permits use,sharing,adaptation,distribution and reproduction in any medium or format,as long as you give appropriate credit to the original author(s) and the source,provide a link to the Creative Commons licence,and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence,unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use,you will need to obtain permission directly from the copyright holder.To view a copy of this licence,visit http:// creat iveco mmons.org/ licen ses/ by/4.0/ .
杂志排行
Natural Products and Bioprospecting的其它文章
- Termitomenins F and G,Two New Lignan Glucosides from Terminalia chebula var.tomentella (Kurz) C.B.Clarke
- Clerodane-type Diterpene Glycosides from Dicranopteris pedata
- Sesquiterpenoids and 2-(2-Phenylethyl)chromone Derivatives from the Resinous Heartwood of Aquilaria sinensis
- Traditional Uses and Pharmacologically Active Constituents of Dendrobium Plants for Dermatological Disorders:A Review
