Preparation and Mechanism Research of Hydrationheat-inhibiting Materials with Microcapsule Sustained-releasing Technology
2021-09-15JIAFujieYAOYanLIChangcheng
JIA Fujie, YAO Yan, LI Changcheng
(State Key Laboratory of Green Building Materials, China Building Materials Academy, Beijing 100024, China)
Abstract: Hydration-heat-inhibiting materials(HIM) with polysaccharide as core material was prepared using microcapsule sustained-releasing technology, through a centrifugal spray granulation process after melting together. The preparation process parameters of HIM were selected by the semi-adiabatic temperature rise test of cement paste. TAM air microcalorimeter was used to investigate the regulation performance of HIM on the hydration of cement. The influence of HIM on the microstructure of cement was investigated by XRD,SEM, and TG-DSC.The results showed that the most suitable wall material for HIM was polyethylene wax,the optimum polyethylene wax/polysaccharide mass ratio was 1, and the most effective particle size was 0.16-0.30 mm. Polysaccharide coated by polyethylene wax released slowly, and the peak heat release rate of cement could be reduced by 55.2% after continuous regulaion. The regulation period continued to 120 h. HIM mainly decreased the C3S reaction rate, which resulted in a 39.2% peak value reduction of hydration heat release rate.However, HIM had little regulation on C3A. The hydration heat release process of cement-based materials can be designed by adjusting the dosage of HIM.
Key words: microcapsule; sustained-release; hydration heat; inhibition
1 Introduction
Hydration process of cement can make the internal temperature of concrete rise as high as 40 - 60 ℃or even higher[1-2]. Crack is formed due to the thermal shrinkage in cooling stage under the constraint condition, which will endanger durability and service life of the structure. In order to solve thermal cracking, a lot of measures were used in the practical engineering, such as pipe-cooling system[3-8], pre-cooling aggregate and cool mixing water, heat preservation during curing[9],the increase of reinforcement bar[10], using low-heat cement as well as adding phase change materials[11-22]. All these measures lead to additional construction time and cost. Furthermore, some may have little effect or even have nagetive effects on the mechanical properties of concrete.
Polysaccharide is a kind of hydration heat inhibitor, which can change the heat release rate of cement and reduce the peak of concrete temperature. The cost of construction is low with polysaccharide. And it is easy to use[23-25]. Hydroxyl groups in polysaccharide can be adsorbed on the surface of cement particles to form an adsorption film, which hinders hydration of cement. Inhibiting effect of low dosage polysaccharide on hydration is not ideal enough. However, the setting time will become longer with the increase of dosage.In recent years, microcapsule sustained-release technology has been widely used in the fields of medicine,food, chemical industry,etc. Core materials are embedded or encapsulated in the wall materials, and begin to release when wall materials are opened under certain conditions. In this way release rate of core materials is delayed or decreased. Microcapsule technology was applicated in cement-based materials, mainly to achieve self-healing[26-28]. Some preparation methods were used likein situpolymerization[26], solvent evaporation method, the spray drying method and phase separation.Many functional core materials were encapsulated,such as the epoxy resin, double loop pentadiene[26-28]and sodium silicate.
Some wall materials were used concluding urea-formaldehyde resin[26], silicon dioxide inorganic shell[29], polystyrene[30]. Wall materials are opened when cement-based materials begin to crack, and the functional core material could be released to repair the structure. In order to enhance the inhibiting effect and reduce setting time,microcapsule sustained-release technology is used to delay the release rate of polysaccharide.
Regulation effect of HIM with different wall material/core material mass ratio and particle size on the hydration heat release of cement was investigated to obtain the most optimum preparation process parameters. Effect of HIM on cement hydration heat release was investigated by microcalorimeter. XRD, SEM and TG-DSC were used to investigate the microscopic characteristics of cement with HIM, and the regulation mechanism of cement hydration with HIM was expounded.
2 Experimental
2.1 Raw materials
Ordinary Portland cement with a surface area of 346 m2/kg and a density of 3.12 g/m3was used. The chemical compositions of cement are listed in Table 1.The phase compositions of cement are shown in Table 2.Polyethylene wax with 4 000 weight average molecular and 115 ℃ melting point was used; Fig.1 shows the GPC of polysaccharide. Molecular weight distribution is shown in Fig.2. Fully refined Paraffin wax of No.66 conforming to GB/T 446-2010 was used; Industrial stearic acid(1840 type) conforming to GB/T 9103-2013 was used.
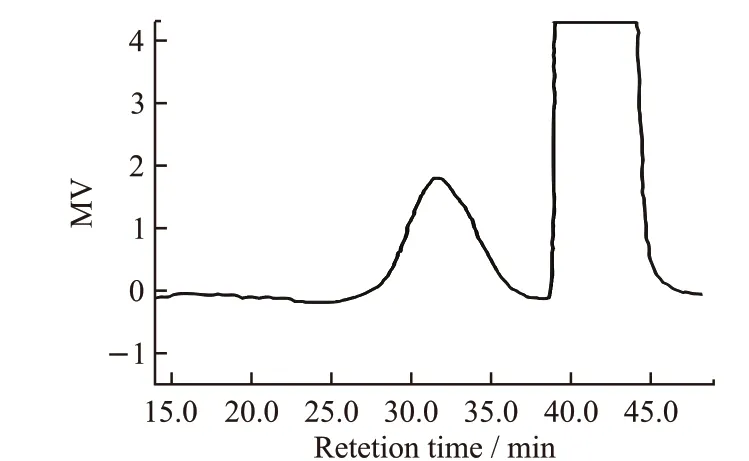
Fig.1 GPC of polysaccharide

Table 1 Chemical compositions of cement

Table 2 Phase compositions of cement
Fig.2 Molecular weight distribution
2.2 Preparation of HIM
HIM is prepared by centrifugal spray granulation with polysaccharide as the core material and with Polyethylene wax or Paraffin wax or stearic acid as wall material.
The wall material was placed in a reactor, heated to 120 ℃ until melted to liquid. Uniform liquid mixture was obtained after adding the polysaccharide in proportion through stirring. Temperature of the mixture could not exceed 130 ℃ for long time. HIM was prepared by centrifugal spray granulation. The particle size of HIM could be controlled according to the rotating speed of the centrifuge.
2.3 Test methods
2.3.1 Semi-adiabatic temperature rise test of cement paste
The semi-adiabatic temperature rise test of cement paste was carried out in a foam chamber with a water/binder mass ratio of 0.35 . Fig.3 shows the polystyrene foam box, with an external size of 210 mm×110 mm×130 mm, an internal size of 180 mm×80 mm×100 mm, and a wall thickness of 15 mm. The temperature sensor was at the center point of long side, as shown in Fig.3. Experiments were carried out at (20±2) ℃. Cement paste of (2 500.0±5.0) g was pured into the foam box.Then the foam box was sealed by the tape sealing.Test began and temperature was recorded. The test could be terminated when the temperature of cement paste droped to 22 ℃.
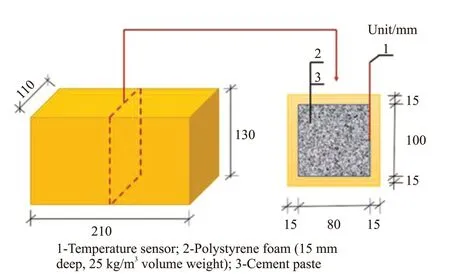
Fig.3 Semi-adiabatic temperature rise test device
2.3.2 Hydration heat
The heat release process of cement was measured by isothermal calorimeter (TA CO., TAM Air) with a sensitivity of 0.4 μJ and a baseline stability of ±0.08 μW/h. All experiments were kept at (20 ± 1) ℃, and the water/cement mass ratio (w/c) of the paste was set at 0.5 because of the good flowability of cement paste and the good dispersion of HIM. The paste was mixed for 2 min at 3 200 rpm before putting in calorimeter.
2.3.3 Microscopic characteristics
Phase analysis of cement was investigated by the D8 ADVANCE X-ray diffractometer (Bruker, Karlsruhe, Germany) with Cu-Kα radiation (k= 0.154 nm).The scanning range was 5°-80°with a scanning rate of 0.02°/s.
The hydrates morphologies of cement at 1, 3, 7 d were examined by Quanta250 Filed Emission Scanning Electron Microscope(FEI,Hillsboro,OA,USA).The vacuum ovendried sample was coated with 15 nm of gold to make it conductive before observation.
Thermal analyzer was used to acquire thermogravimetric(TG) and differential scanning calorimetry(DSC) curves of cement with and without HIM. The heating rate was 10 ℃/min under nitrogen gas flow,and the weight losses were recorded between room temperature and 1 000 ℃.
3 Results and discussion
3.1 Preparation process parameters
3.1.1 Wall materials
Fig.4 shows the semi-adiabatic temperature rise of cement with HIM prepared by different wall materials, taking polysaccharide as core material. The HIM dosage was 0.20% , particle size was between 0 and 0.63 mm. HIM prepared with three kinds of wall materials showed a good regulation effect on heat release. It can be found that the maximum temperature of cement adding HIM with stearic acid as wall material was reduced by 16 ℃. HIM with paraffin wax as wall material could decrease the hydration heat release of cement effectively. As a result, the semi-adiabatic temperature rise curve changed from single peak to multipeak, indicating that its regulation release ability is strong. However, there was a negative factor for HIM with paraffin wax as wall material. HIM will melt together in hot environment due to the low melting point,making it restricted to be used under hot environment.HIM with polyethylene wax as wall material could regulate heat release rate effectly. The semi-adiabatic temperature rise curve changed from single peak to double peak, and the maximum temperature decreased by 39 ℃. Moreover, HIM had good stability owing to the high melting point of polyethylene wax. Therefore,polyethylene wax was picked out as the most optimum wall material to prepare HIM.
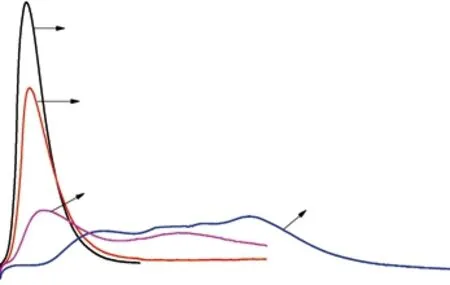
Fig.4 Semi-adiabatic temperature rise of cement paste
3.1.2 Wall material/core material mass ratio
Fig.5 shows the semi-adiabatic temperature rise of cement paste with polysaccharide and HIM. The polysaccharide dosage was 0.10%. The HIM dosage varied with the polysaccharide content in HIM, and the polysaccharide content kept at 0.10%. The semi-adiabatic temperature rise curve of cement paste mixed with polysaccharide was consistent with the curve of reference cement. The occurrence time of temperature peak was delayed by 17.3 h, and the maximum temperature was reduced by 15.2 ℃. It indicates that the concentration release of hydroxyl compounds in polysaccharides prolonged the hydration induction period of cement.And it could not effectively slow down the hydration acceleration period of cement ideally. Therefore, the polysaccharide did not have an enough effect on inhibiting the hydration process of cement.
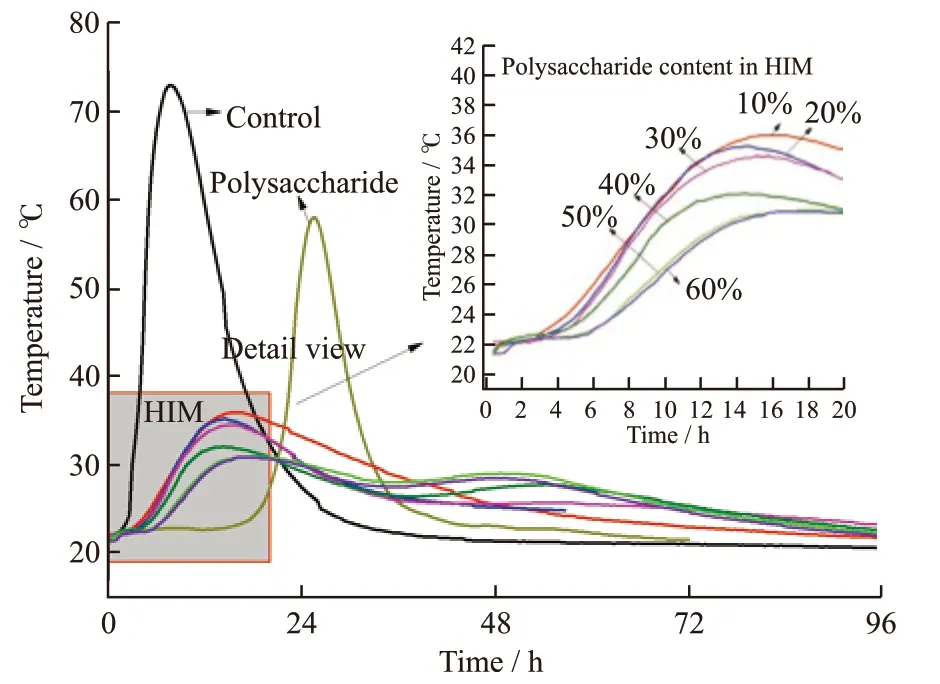
Fig.5 Semi-adiabatic temperature rise of cement paste
After HIM was added, the temperature rise of cement paste was effectively controlled. The semi-adiabatic temperature peak of cement paste changed from single peak to double peak. The heating stage and cooling stage was prolonged. Heat generation rate and heat dissipation rate of cement hydration tended to balance. With the mass fraction of polysaccharide increased from 10% to 60%, the semi-adiabatic temperature rise of cement paste decreased from 72.7 ℃to (30.7-35.8) ℃. The time of temperature peak was delayed by 6 - 8 h. Meanwhile it had no obvious effect on the setting time of cement. The first peak decreased and the second peak increased, when polysaccharide mass fraction reached 50%-60%. But the difference between them was not significant. Polyethylene wax can control the release rate of hydroxyl groups of the polysaccharide, making it slowly and continuously adsorbed on the surface of cement particles. The coating became weak with the increase of polysaccharide mass fraction. When polysaccharides mass fraction exceeded 60%, polyethylene wax and polysaccharides could not form a liquid mixture, and faied to form microcapsules.Therefore, considering the hydration control effect and preparation process, the mass fraction of polysaccharide is optimized to be 50%.
3.1.3 Particle size
Fig.6 shows the influence of particle size on semiadiabatic temperature rise of cement paste with HIM.The mass fraction of polysaccharide was 50%. HIM with particle size ranges of 0.08 - 0.16 mm, 0.16 - 0.30 mm and 0.30 - 0.63 mm were prepared and selected by adjusting the centrifuge speed. HIM with different particle sizes had good hydration heat regulation effect.With the decrease of particle size, the semi-adiabatic temperature rise of cement paste first decreased from 47.7 to 32.0 ℃ and then increased to 38.7 ℃. The occurrence time of the maximum temperature was delayed from 20.1 to 69.4 h. HIM with particle size ranging from 0.16 to 0.30 mm had the best regulation effect. The reason is that the larger the particle size was, the smaller the surface area was. At the same time, the thicker the wall material was, the slower the release rate of polysaccharide was. With the decrease of particle size, the release rate of polysaccharide was accelerated. The regulation ability of hydration heat was enhanced. However, when the particle size was too small (0.08 - 0.16 mm), the polysaccharide released too fast in the early stage and caused slow coagulation. The insufficient release in the later stage leading to a poor effect to inhibit hydration. All these lead to the increase of temperature in the later stage. Therefore, the suitable particle size of HIM was 0.16 - 0.30 mm.

Fig.6 Semi-adiabatic temperature rise of cement paste
Fig.7 shows the particle morphology of HIM by optical microscope. It can be seen that HIM prepared using centrifugal spray granulation appeared to be spherical with some defects on the surface. More defects were generated with mixing. Core material slowly dissolved out of the spherical granular body, as shown in Fig.8. The release progress of the core material from the wall material conforms to the opening-diffusion release mechanism. First,core material passes through the open part of the wall material through diffusion. Then core material enters in the external solution. The release speed is controlled by diffusion. The release rate of core material is determined by Fick’s first law, Eq.(1).It can be concluded that release rate of core material are greatly influenced by particle size. It is difficult for core material to release with thick wall.

Fig.7 Particle morphology of HIM
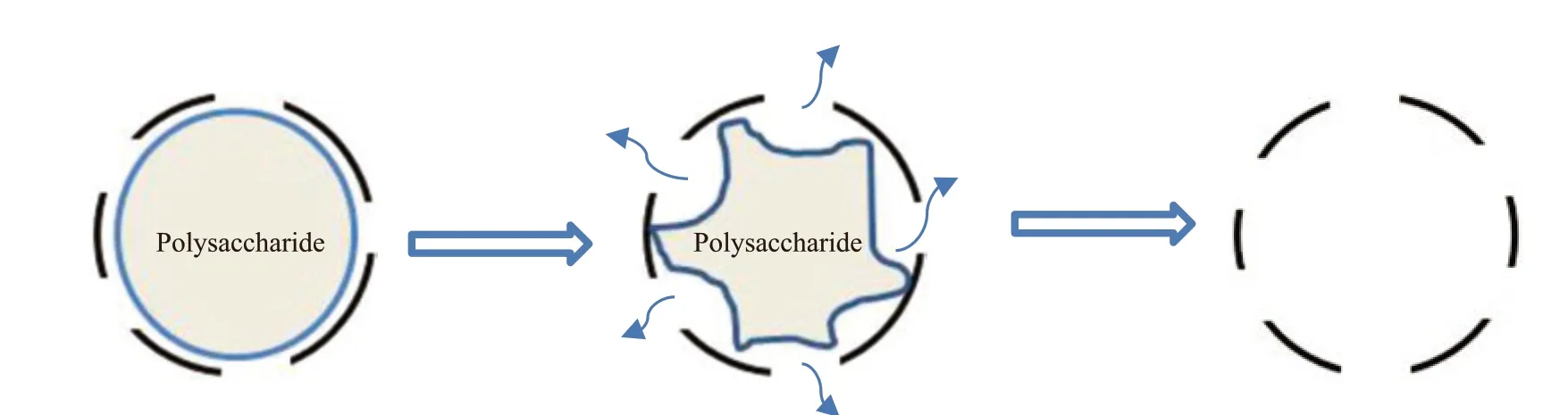
Fig.8 The release process of HIM

whereJtis release rate,Ais the open area of the microcapsules,Dis the diffusion coefficient of core material in the solution, ΔCis concentration difference,his the thickness of the wall.
Based on the above test results, it could be summarized that the best HIM was prepared with polysaccharide as the core material and polyethylene wax as the wall material. Wall material/core material mass ratio was 1, and the particle size was 0.16 - 0.30 mm.HIM prepared with the same preparation process was used during the subsequent tests.
3.2 Heat release process
Fig.9 shows the test results of heat release process of cement with different HIM dosage measured by TAM AIR microcalorimeter. HIM was prepared according to the optimized preparation parameters. It can be known that HIM showed a good effect to regulate hydration heat. The hydration heat release rate of cement decreased, and the ocurrence of the peak was delayed.The first peak and the second peak of heat release rate were gradually flat. When dosage of HIM increased from 0.10% to 0.35%, the peak heat release rate decreased by 13.4%, 28.9% and 55.2%, respectively.When dosage of HIM was 0.35%, the heat release rate from 36 to 74 h was almost a flat line. The cumulative heat at 24 h was decreased by 15.4%, 39.1% and 83%,respectively, compared with the control cement. The cumulative heat at 48 h was decreased by 9.1%, 17.4%and 33.3%, respectively. The cumulative heat at 72 h was similar to that at 48 h. The difference of cumulative heat decreases with the increase of age. HIM mainly regulated the heat release rate of cement, and has little effect on the total amount of hydration heat. The hydroxyl groups of the polysaccharide will adsorb on the surface of the cement particles, leading to inhibition of hydration. As the reaction proceeds, the alkalinity of the solution increases, the barrier layer is destroyed and the cement continues to hydrate[31]. Therefore, HIM mainly inhibits the hydration heat release rate, and has little effect on the cumulative heat release.The results of hydration heat test proved the value of sustained-release once again. By changing the dosage, the heat release process of cement can be designed, which leaded to reduction of thermal cracking.
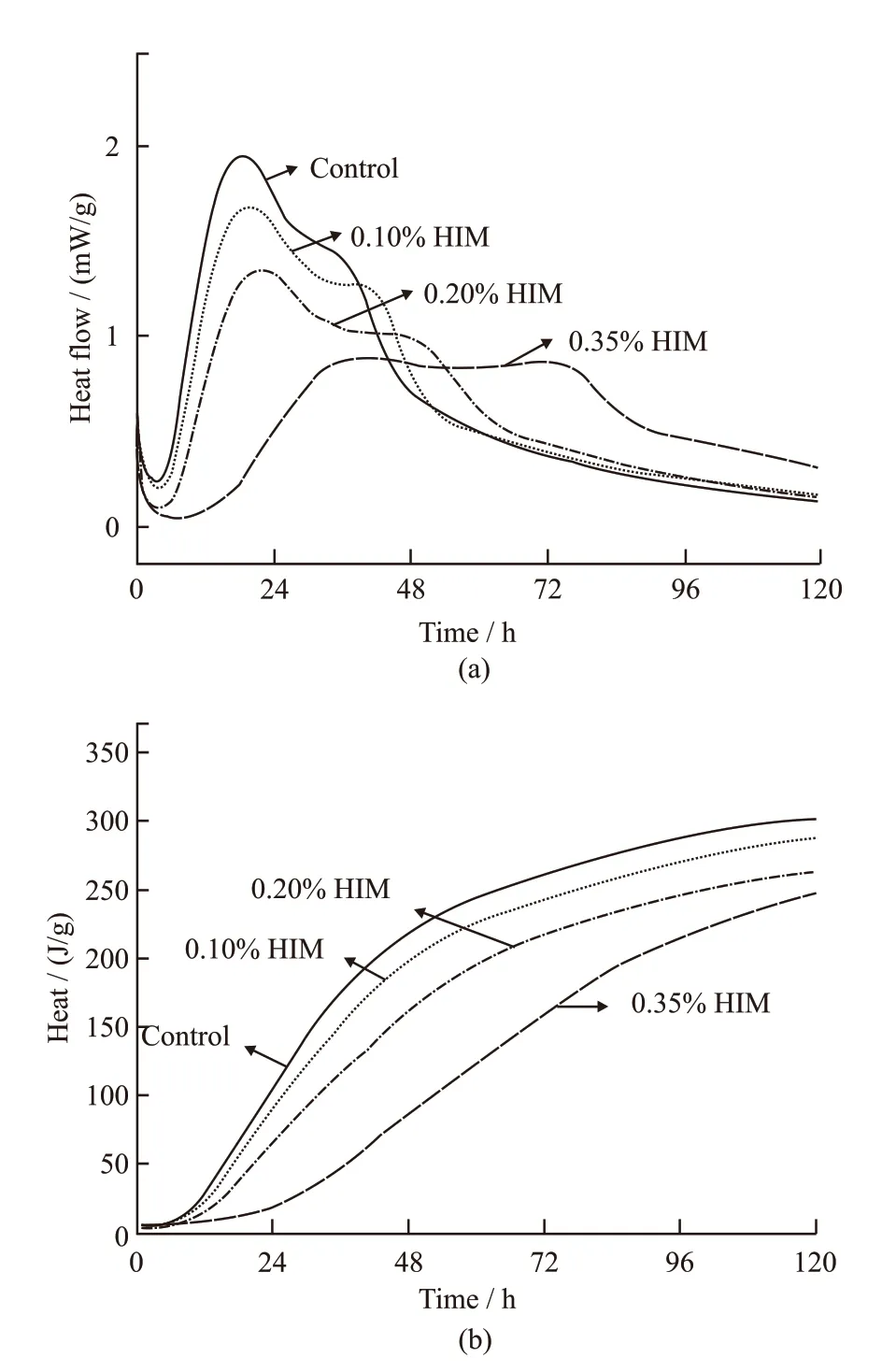
Fig.9 Calorimetric curve of cement paste with w/c 0.5 under the constant test temperature 20 ℃: (a) heat flow rate; (b)cumulative hydration heat of the cement pastes
3.3 Hydration mechanism
3.3.1 Influence of HIM on reaction of C3S and C3A
Fig.10 shows the influence of HIM on C3S reaction. The dosage of HIM was 0.20%. It can be found that HIM inhibited C3S reaction. The peak value of C3S reactioin rate decreased by 39.2%, and the occurrence of peak value was delayed by 4.5 h. The difference of cumulative heat release in the early age was large.It became smaller after 5 d hydration, and finally disappeared at 7 d. It indicated that HIM regulated the hydration process of C3S, slowed down the early hydration heat release rate.But HIM did not affect the total hydration heat.
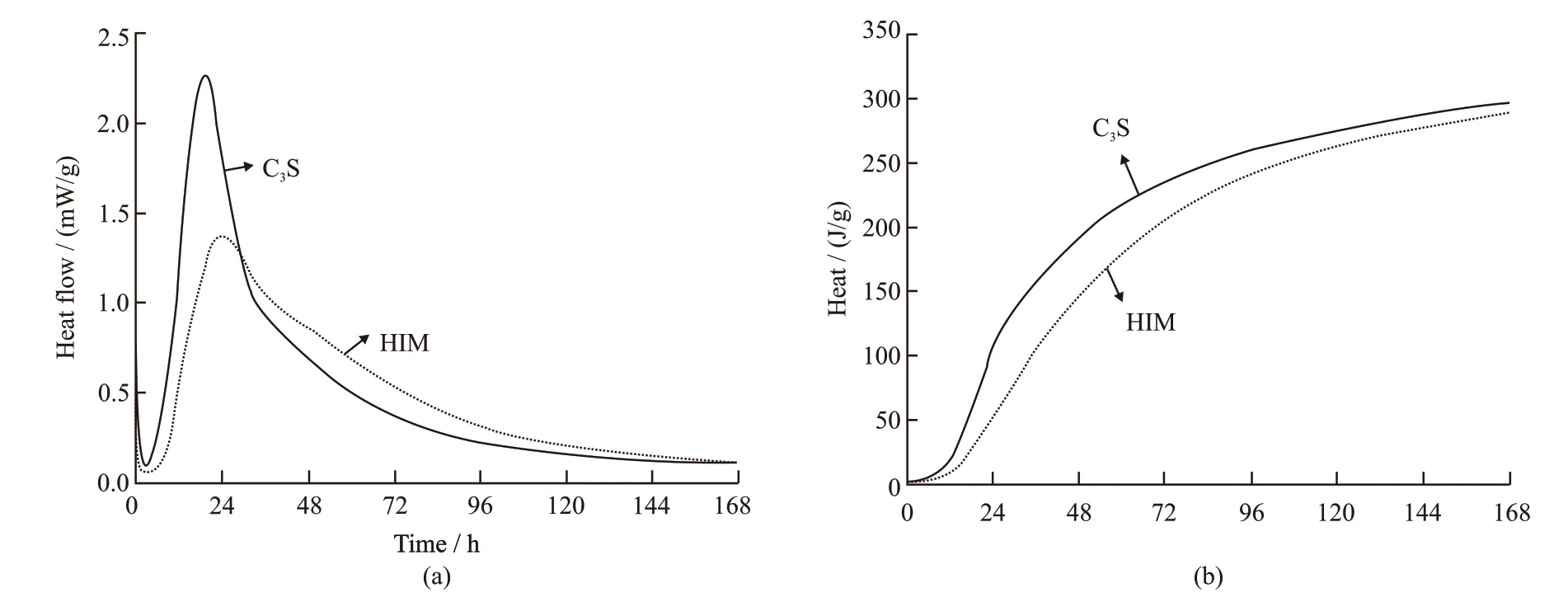
Fig.10 Calorimetric curve of C3S with w/c 0.5 under the constant test temperature 20 ℃: (a) heat flow rate; (b) cumulative hydration heat of the cement pastes
Fig.11 shows the influence of HIM on C3A reaction. The dosage of HIM was 0.20%. It can be observed that HIM had little effect on the regulation of C3A reaction, and the curves of hydration heat release rate and cumulative hydration heat tend to gather together. The reason is that the hydration reaction of C3A is rapid.The reaction of C3A finished when the polysaccharide in HIM had not been released and adsorbed on the surface of C3A. Comparing with Fig.10, it can be seen that HIM mainly inhibited C3S reaction, due to that the release rate of HIM matched the C3S reaction rate.
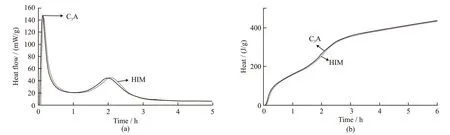
Fig.11 Calorimetric curve of C3A with w/c 0.5 under the constant test temperature 20 ℃: (a) heat flow rate; (b) cumulative hydration heat of the cement pastes
3.3.2 XRD
Fig.12 shows XRD patterns of hydration products adding with HIM and polysaccharide at different ages.The dosage of HIM was 0.20%, and the dosage of polysaccharide was 0.10%. The main crystal phases of cement were ettringite, Ca(OH)2and C3S. The addition of polysaccharide and HIM did not change the crystal phase of cement hydration products, but the quantity was slightly different.
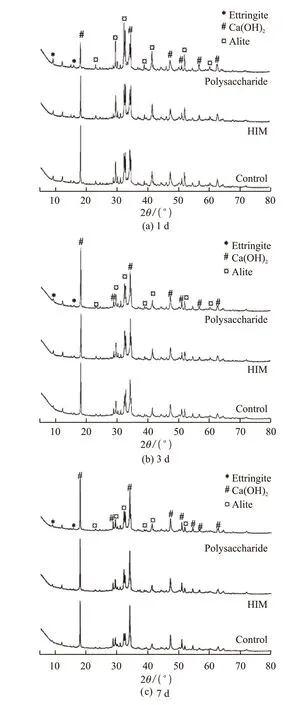
Fig.12 XRD patterns of cment paste with HIM and polysaccharide: (a)1 d; (b)3 d; (c)7 d
The diffraction peak of Ca(OH)2in reference cement was the strongest at 1d, and the diffraction peak of Ca(OH)2in hydration product of cement with polysaccharide was the lowest. The diffraction peak rule of C3S was opposite. Ettringite diffraction peak among the three showed the similarity. These results indicated that polysaccharide and HIM inhibited the formation of Ca(OH)2, and the inhibition effect of polysaccharide was stronger.
At 3 d, the diffraction peak of Ca(OH)2in cement with polysaccharide increased and was basically equal to that of the reference cement. The diffraction peak of Ca(OH)2in cement with HIM also increased, but was still the lowest. All the C3S diffraction peaks were weakened, and so were the ettringite peaks. This indicated that the inhibition effect of polysaccharides on Ca(OH)2production disappeared, while HIM still had a certain inhibitory effect, and its hydration regulation still played a role.
At 7 d, the peaks of Ca(OH)2, ettringite and C3S were almost the same, indicating that HIM had completed the inhibition and regulation of C3S reaction.The results of XRD showed that both polysaccharide and HIM could inhibit the hydration of silicate minerals. However, the polysaccharide could inhibit the hydration of silicate minerals for a short time, while HIM could realize slow release and continuous regulation due to microencapsulation technology. And it would not have influence on the composition and quantity of cement hydration products in the end.
3.3.3 TG-DSC
Fig.13 shows the TG-DSC curves of hydration products of cement with HIM and polysaccharide at different ages. The dosage of HIM was 0.20%, and the dosage of polysaccharide was 0.10%. Fig.14 shows the quantity of Ca(OH)2. As can be seen from Fig.13,compared with the control, the amount of Ca(OH)2in cement with polysaccharides and HIM at 1 d decreased by 25.3% and 6.8% respectively, indicating that both polysaccharides and HIM inhibited the hydration of silicate minerals in cement.The inhibitory effect of polysaccharides was significantly stronger than that of HIM.After 3 d hydration, the amount of Ca(OH)2in cement with polysaccharides and HIM increased, which was only 2.0% and 5.7% lower than the control. However,the amount of Ca(OH)2in cement with polysaccharide was higher than that of HIM, indicating that the inhibition effect of polysaccharides on hydration disappeared,while HIM continued to play a role in hydration. After 7 d hydration, the amount of Ca(OH)2in cement with polysaccharide and HIM was almost the same as the reference, indicating that the hydration degree of the three was the same, and the hydration regulation of HIM on cement was basically over. The results of TGDSC were consistent with the results of XRD analysis.The polysaccharide were released in a concentrated way to temporarily inhibit cement hydration and reduce the generation of early hydration products. Due to its microcapsule structure, HIM could continuously release polysaccharides to regulate cement hydration. TG-DSC results again proved the value of microcapsule sustained-release according to the amount of Ca(OH)2.
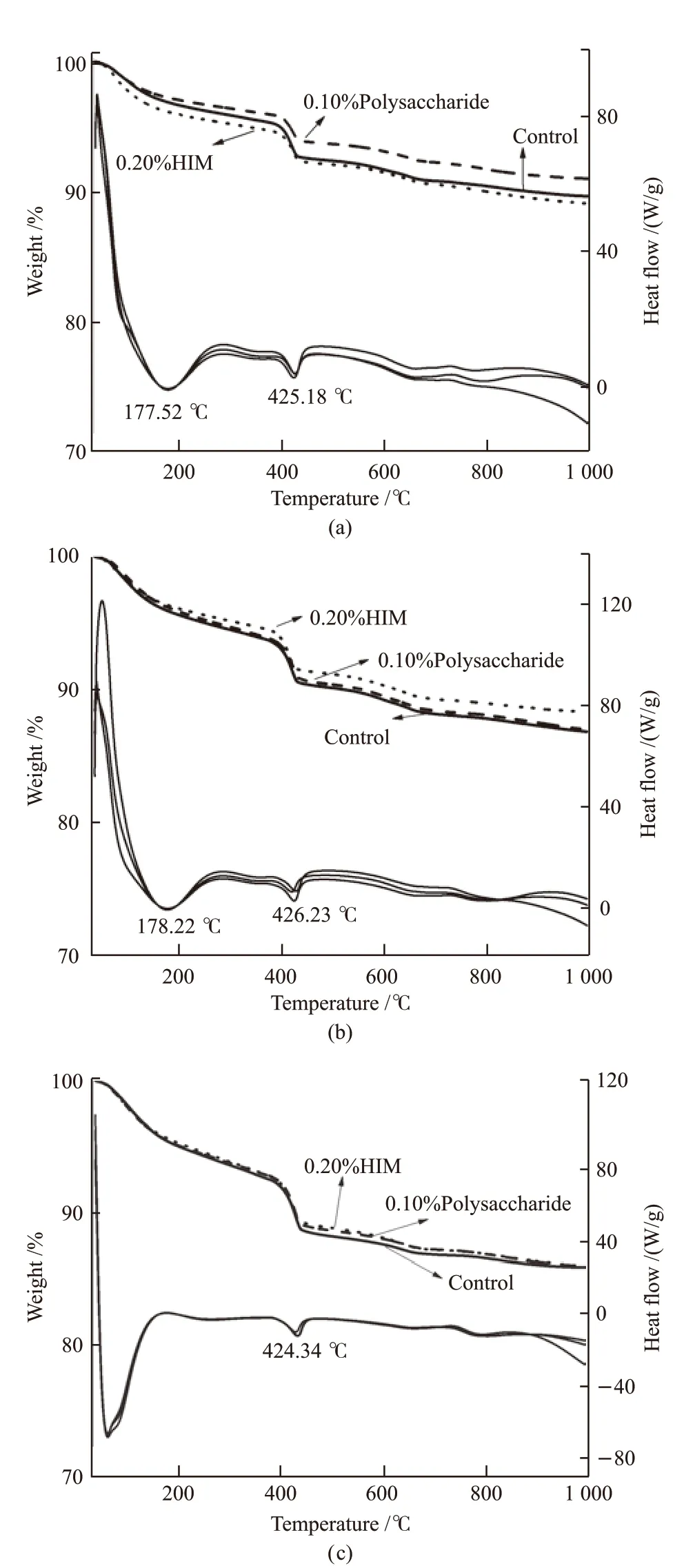
Fig.13 TG-DSC curves of cemet with HIM and polysaccharide:(a) 1 d; (b)3 d; (c)7 d
Fig.14 Quantity of Ca(OH)2
3.3.3 SEM
Fig.15 shows SEM images of hydration products of cement. The HIM dosage was 0.20%, and the polysaccharide dosage was 0.10%. It can be seen that polysaccharide and HIM did not change the morphology of cement hydration products, but only affected the generation time of hydration products. At 1 d, there were more needlestick ettringite in the hydration products.The difference was that the amount of hexagon-platelike Ca(OH)2in the hydration products of reference cement was the largest, and the amount of the cement with polysaccharide were the least. This indicated that both polysaccharides and HIM inhibited the hydration of silicate minerals, but polysaccharide had the strongest inhibitory effect. At 3 d, the amount of Ca(OH)2in hydration products of the three samples increased. The amount of cement with polysaccharide almost reached the reference, while the amount of Ca(OH)2in cement with HIM was relatively the least, indicating that the inhibition effect of HIM continued to play. At 7 d, there was no significant difference between ettringite and Ca(OH)2in hydration products of the three groups.SEM results confirmed XRD and TG-DSC again.
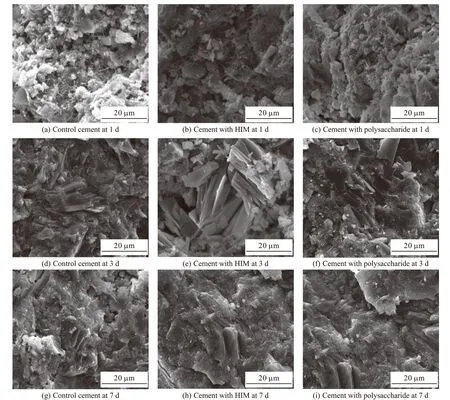
Fig.15 SEM images of hydration products of cement
4 Conclusions
a) Hydration-heat-inhibiting materials(HIM) with polysaccharide as core material was prepared using microcapsule sustained-releasing technology, through a centrifugal spray granulation process after melting together. The results showed that the most suitable wall material for HIM was polyethylene wax, the optimum polyethylene wax/polysaccharide mass ratio was 1, and the most effective particle size was 0.16 - 0.30 mm.
b) After HIM was added, the heat release rate slowed down in the induction and acceleration stages.Continuous release of polysaccharides inhibited the hydration reaction, which made the heat release rate and heat dissipation rate tend to balance, in which way realized the control of cement hydration.
c) The hydration heat release rate of cement was regulated by HIM. However, HIM did not affect the cumulative heat. The mechanism of hydration heat regulation is to regulate the hydration process of C3S. HIM slowed down the hydration from 1 to 3 d, but the effect disappeared at 7 d. HIM could not regulate the hydration process of C3A because the sustained releasing rate of HIM did not match the hydration rate of C3A.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Mechanical Properties and Microstructure of Al2O3/SiC Composite Ceramics for Solar Heat Absorber
- Effect of Friction Stir Welding on Bulk Metallic Glasses
- Effects of Lay-up Types of Out-of-autoclave Prepregs on Preparation Quality of L-shape Composite Laminates
- Hypereutectic Al-Si Matrix Composites Prepared by In Situ Fe2O3/Al System
- Preparation of Heavyweight Ultra-high Performance Concrete Using Barite Sand and Titanium-rich Heavy Slag Sand
- Effects of Shale and CaO Incorporation on Mechanical Properties and Autogenous Deformation of Early-age Concrete
