废水处理中异化硝酸盐还原为铵的研究进展
2021-09-13万雨轩王鑫
万雨轩 王鑫

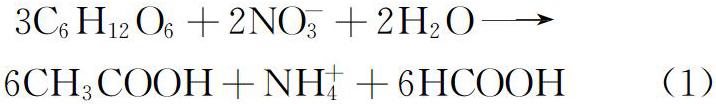
摘 要:废水处理过程中涉及多种氮转化途径,其中,异化硝酸盐还原为铵(Dissimilatory Nitrate Reduction to Ammonium,DNRA)能够将NO-3/NO-2转化为NH+4,是氮素转化的重要一环。概述了DNRA过程的两步反应机理以及涉及的微生物,着重讨论了废水处理中影响DNRA过程的潜在因素,包括溶解氧、碳源种类、氮源种类、碳氮比、温度、pH值以及废水成分等,总结分析了各种因素如何调控DNRA与反硝化过程对硝态氮的竞争,并对废水处理中DNRA过程的两种主要分析手段进行了介绍。综述了DNRA过程在废水处理中的发生机制及其贡献,对未来DNRA过程的深入研究及废水中氮的去除或回收具有重要意义。
关键词:异化硝酸盐还原;废水处理;氮回收;同位素示踪
中图分类号:X703.1 文献标志码:A 文章编号:2096-6717(2021)06-0134-11
Abstract: There are many ways of nitrogen transformation in wastewater treatment, among which dissimilatory nitrate reduction to ammonium (DNRA) convert NO-3/NO-2 into NH+4, which is an important part of nitrogen transformation.This paper reviews the two-step reaction mechanism of DNRA process and microorganisms involved. In addition, the potential factors affecting the DNRA process in wastewater treatment, such as dissolved oxygen, types of carbon or nitrogen sources, C/N ratio, temperature, pH and special substances contained in wastewater,are emphatically discussed to explore how to regulate the competition between DNRA and denitrification fornitrate, and this paper introduces two main analytical methods for DNRA process in wastewater treatment.This paper confirms the occurrence and contribution of DNRA process in wastewater treatment, and the in-depth study of DNRA is of great significance for the removal or recovery of nitrogen in wastewater.
Keywords: dissimilatory nitrate reduction; wastewater treatment; nitrogen recovery; isotope tracing
氮在自然界中至關重要,是生物合成关键细胞成分所必需的重要营养元素,其可用性取决于微生物间进行的各种氮转化反应。近年来,人们对微生物的氮转化过程进行了深入研究,将其归纳为7个过程,如图1所示。其中,异化硝酸盐还原为铵(Dissimilatory Nitrate Reduction to Ammonium, DNRA)的过程是由微生物介导,将NO-3和NO-2直接还原为NH+4的酶促氧化还原反应。早在1938年,DNRA过程就被证明可以发生在常见的土壤细菌中,如Clostridium welchii[1]。1975年,Stanford等通过15N同位素示踪技术进一步证实了DNRA过程的存在[2]。然而,由于技术上的限制,之后的大多数研究仍然认为反硝化过程是进行硝酸盐还原的主要途径,而DNRA过程对硝酸盐还原的影响较小。直到1988年,Tiedje等提出由于NH+4的流动性比NO-3小,NH+4更容易被保留在环境中[3]。反硝化过程生成N2和N2O造成氮损失,增加温室气体排放,而通过DNRA过程生成NH+4能够为生态系统保留活性氮[3],因此,DNRA在不同生态系统中的重要性逐渐被意识到。
DNRA过程已经被证明能够在各种海洋、河口和土壤生态系统中发生[4-7]。而作为全球最大的人工生态系统,污水处理厂(WWTP)涉及硝化、反硝化、厌氧氨氧化和DNRA等过程,在全球氮循环中发挥着重要作用[8]。目前,大多数污水处理厂主要应用反硝化过程将N以N2的形式去除,同时产生N2O温室气体[9]。虽然厌氧氨氧化细菌在污水处理厂中也广泛存在,但认为它们对氮转化的贡献率不大[10]。废水处理中的反硝化和厌氧氨氧化过程已经得到了广泛研究,如影响因素、相关微生物和脱氮效率等[11-13]。与之相反,针对废水处理中DNRA过程的研究相对较少。在DNRA过程中,N的最终产物为NH+4,能够保留活性N促进污水处理厂副产品的产生,增加收益。同时,DNRA过程只产生氨氮,减少了温室气体N2O的排放。因此,废水处理中DNRA过程的发生对废水中氮的转化,甚至全球氮循环平衡都起着至关重要的作用。
为了更深层次地理解DNRA过程以及其在氮循环中的重要作用,笔者总结了DNRA过程反应机理,功能微生物及其生理、遗传调控。对废水处理中可能影响DNRA过程的因素进行了详细探讨,并介绍了用于评估DNRA过程的两种方法。
1 DNRA的反应过程
1.1 DNRA反应机理
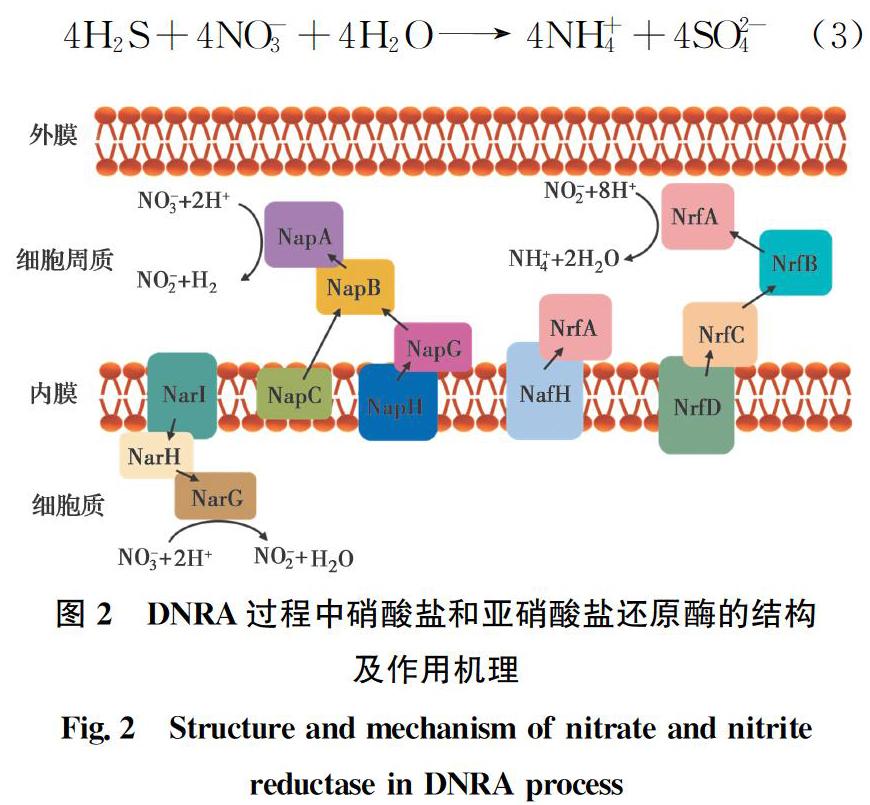
DNRA过程分为两步(图2),第1步是硝酸盐还原为亚硝酸盐,主要是由膜结合硝酸盐还原酶(Nar)或周质硝酸盐还原酶(Nap)催化,其中,还原硝酸盐的亚基NarG和NapA以醌为电子供体产生亚硝酸盐[14]。第2步是亚硝酸盐转化为铵,由周质五血红素细胞色素c亚硝酸盐还原酶(NrfA)催化,而不形成中间产物。NrfA是可溶的周质蛋白,分别通过细胞色素NrfHA复合物或者NrfBCD复合物从醌获得电子(图2)。此外,研究发现,NrfA蛋白质的几种结构可将NO-2还原为NO,NO再被还原为NH+4和N2O(图1虚线),这被认为是硝酸盐氨化的额外途径[15]。
与反硝化过程中的硝酸盐呼吸过程不同,DNRA过程根据细菌种类、生长底物和能量获取方式的不同可分为两种:呼吸型和发酵型[16]。两种DNRA方式中,NO-3到NO-2的还原过程始终是呼吸型的,其分类取决于NO-2到NH+4的还原类型。大多数DNRA过程是发酵型,细菌以有机物作为电子供体,通过底物水平磷酸化立即产生能量。以葡萄糖为例,糖酵解过程中产生的NADH在硝态氮还原过程中被氧化,生成发酵产物甲酸和乙酸,同时产生NH+4(式1)[17]。呼吸型DNRA一般指自养型DNRA,通常指以S2-作为电子供体,还原硝态氮为氨氮,S2-最终产物是单质S或者SO-4(式(2)、式(3))[18]。
1.2 DNRA过程相关微生物
随着DNRA过程研究的日益深入,越来越多的菌株被证明具有将NO-3/ NO-2还原成NH+4的能力。根据电子供体的不同,DNRA菌可分为自养菌和异养菌两类。大部分DNRA菌是异养型,需要有机碳源作为电子供体;少数DNRA菌是自养型,例如化能自养微生物Thiobacillus denitrificans、Desulfovibrio desulfuricans和D. propionicus等。根据呼吸类型也可将DNRA细菌分为好氧菌、微好氧菌、兼性厌氧菌以及严格厌氧菌。相对于O2为电子受体(好氧呼吸)而言,微生物以NO-3作为电子受体进行缺氧呼吸的产能效率非常低,因此,DNRA细菌多为兼性厌氧菌和严格厌氧菌。
多种参与氮转化的细菌在污水处理系统中共存,其中,DNRA细菌群落广泛存在。最近,Wang等[19]对中国不同地区的污水处理厂的8个处理单元的微生物群落进行分析发现,DNRA细菌群落中Nitrospira丰度最高,其次是Brocadia,Anaeromyxobacter和Geothrix。城市污水处理厂A2/O工艺系统的厌氧池中存在较高的有机物浓度和一定量的硝酸盐,具备了适合DNRA细菌生存的条件。刘芹等[20]在A2/O工艺处理系统中鉴定出进行DNRA的菌属主要为Thauera、Hydrogenophaga和Geobacter。但目前针对污水处理厂中DNRA相关微生物的种类、丰度以及与其他微生物群落的种间机制的深入研究还相对较少。
1.3 废水处理中的DNRA过程
通过对传统市政污水处理厂的DNRA过程进行评估,发现DNRA过程在6个不同规模污水处理厂的全部处理单元中广泛存在,但对N转化的贡献并不显著[19]。各国已经开始实施污水处理厂升级,使用额外的氧化、吸附和过滤技术可能会导致DNRA细菌数量的增多。北京污水处理厂升级改造后,DNRA细菌与反硝化细菌之间的比率从1.10显著增加至1.93,DNRA过程贡献率增大[21]。此外,季节变化及地理位置差异也会影响DNRA过程在废水处理中的贡献。在季风气候期,印度炼油厂废水经过处理后,氨氮含量与冬季相比增加了9倍,DNRA细菌的丰度增加了3倍,DNRA对硝酸盐转化的贡献率超过反硝化过程[22]。
废水处理厂采用厌氧氨氧化细菌脱氮时,DNRA过程是维持厌氧氨氧化过程稳定进行的关键步骤[11]。例如,采用同步硝化、厌氧氨氧化和反硝化工艺(SNAD)处理垃圾渗滤液时,DNRA细菌催化还原硝酸盐,与氨氧化细菌和厌氧氨氧化细菌协同作用实现氮的去除[23]。使用气升式反应器去除低碳氮比废水中的氮,也是基于厌氧氨氧化、同步硝化反硝化和DNRA的共同作用[24]。因此,即使DNRA过程在废水处理中的贡献率不高,但其作用不容忽视。
2 废水处理中DNRA过程的潜在影響因素
废水处理过程中涉及多个氮素转化反应,其中DNRA与反硝化过程是还原硝态氮的两个竞争途径。两者都是以有机物或无机物为碳源,在低氧条件下发生,大部分情况下存在此消彼长的关系。因此,针对DNRA过程潜在影响因素的讨论,主要从影响DNRA和反硝化过程之间竞争的因素进行分析。
2.1 溶解氧
溶解在水中的分子态氧称为溶解氧,水中溶解氧的含量与空气中氧的分压、水的温度都有密切关系。污水处理厂通过改变水中溶解氧浓度实现不同的好氧和厌氧生物工艺,进而转化或去除废水中的氮。已有研究表明[25-26],溶解氧的变化会影响生物膜反应器、生物接触氧化池和生物流化床等的脱氮效果。理论上,在厌氧条件下,每摩尔NO-3在DNRA过程中传递的电子(8e-)比反硝化过程(5e-)多,而厌氧条件下终端电子受体短缺,细菌更倾向于使用DNRA而非反硝化作为获取能量的途径[27-32]。因此,DNRA过程倾向于在强还原条件下发生。已有研究表明[33-34],在强还原条件下,DNRA过程的活性较高。在周期性缺氧的河口,DNRA过程起主导作用,缺氧持续一段时间后,反硝化过程几乎完全消失[35]。但对6个市政污水处理厂所有处理单元的微生物群落进行调查显示,DNRA细菌在好氧区域仍然存在[19]。在实验室中对氧气如何调控DNRA和反硝化过程之间的竞争进行探究,发现反硝化过程在低于1 μmol/L O2的条件下优于DNRA,而在更高的O2水平下,DNRA过程占据优势[36]。推测出现这一现象的原因是DNRA对氧化还原条件变化的敏感性低于反硝化过程,对O2的敏感性较低[37]。在氧气控制实验中,污水处理厂溶解氧含量会影响DNRA与反硝化过程对硝态氮的竞争,调整溶解氧周期性变化可能对DNRA过程更有利。
2.2 碳源种类
绝大多数污水处理厂都以微生物为处理污水的核心,在这种处理方式下,微生物本身的生长需求是污水厂首要解决的问题。因此,污水处理厂通常人工投加甲醇、乙酸和葡萄糖等简单的有机化合物维持微生物的生长,进而保证脱氮过程顺利进行。DNRA细菌可利用多种有机物作为碳源,碳源的化学性质是决定DNRA和反硝化过程竞争的另一个关键因素。一些研究表明,葡萄糖的添加会刺激DNRA过程,而其他碳源,如稻草、甘油、甲醇和琥珀酸盐不促进DNRA,Yin等[38]将该现象归因于上述碳源是发酵的不良产物。当使用发酵碳源作为电子供体时,发酵过程会产生大量还原剂,有利于DNRA过程[30]。除了针对发酵型碳源的研究,乙酸盐作为呼吸型DNRA的底物时,其速率低于发酵型DNRA,而同作为呼吸型DNRA底物的丙酸盐,DNRA速率低于乙酸盐[30]。此外,研究人员发现[39],与反硝化过程相比,天然有机碳源对DNRA过程的促进作用更大。因此,污水处理厂中可投加发酵型碳源葡萄糖或者天然有机碳源促进DNRA过程。Carlson等[40]探究了94种碳源对硝态氮还原终产物的影响,发现同一碳源对不同微生物DNRA过程的影响并不相同。因此,碳源的选择还需要结合污水处理厂的微生物种类进行具体分析。
2.3 氮源种类
除了碳源的化学性质,氮源的种类对DNRA过程也十分重要,NO-3和NO-2的供应是决定硝酸盐异化还原途径的一个重要因素。Kraft等[27]认为驱动DNRA和反硝化细菌之间竞争的是NO-3和NO-2的供应,并在恒化器中对此进行了验证。当提供足够NO-3作为电子受体时,DNRA过程是主要的还原途径;当NO-2作为生物反应器中的末端电子受体时,反硝化作用普遍存在。进一步研究证明,DNRA细菌对硝酸盐的亲和力强,硝酸盐可能是更有利于DNRA细菌生长的氮源[41]。而Yoon等[28]则认为决定两个过程竞争的是NO-2相对于NO-3的比例。高的NO-2/NO-3比例有利于DNRA过程,低比例有利于反硝化,而在没有NO-3的情况下,NO-2的浓度并不影响两个过程的竞争。目前,对于氮源的种类如何影响DNRA和反硝化过程的竞争还存在争议,硝态氮种类是否会成为废水处理中DNRA过程的潜在影响因素还需要进一步的研究。
2.4 碳氮比
碳源和氮源分别是DNRA过程的电子供體和受体,两者的比例对硝酸盐异化还原途径的选择具有重要影响,即碳氮比能调节DNRA和反硝化过程对环境中硝态氮的竞争。最初,Tiedje[3]和Smith[42]的研究相继证明了碳氮比在分配DNRA和反硝化之间的重要性。基于此,在实验室进行了大量分批培养和恒化器实验,探究DNRA在高碳氮比下占优的潜在机制。使用恒化器对来自海洋环境中的混合菌群进行培养,发现在缺乏硝酸盐的条件下DNRA过程更活跃,而碳源供应不足时,硝态氮主要通过反硝化过程去除[27]。为了进一步阐明驱动DNRA和反硝化之间生态平衡的确切机制,Van den Berg等[31]使用乙酸盐作为电子供体对反硝化细菌和DNRA细菌之间的微生物竞争进行了探究。碳源供应不足时(<1.86 mol C/mol N),反硝化细菌占主导地位,恒化器系统中检测不到DNRA过程;提高进水中乙酸钠的含量(>1.86 mol C/mol N),DNRA细菌逐渐取代反硝化细菌,超过70%的NO-3还原为NH+4。在高碳氮比的条件下,NO-3供应不足,DNRA细菌对NO-3的底物亲和力更大,在与反硝化细菌的竞争中占据优势。碳氮比的影响在电化学系统处理硝酸盐废水的研究中也得到了进一步证明,DNRA过程在高碳氮比下占据优势,可将44%的NO-3转化为NH4+[43]。不同地区由于饮食习惯和工业类型的差异,导致污水处理厂进水的碳氮比并不相同,DNRA过程也会受到不同程度的影响,控制高碳氮比是促进DNRA过程发生的重要手段。
2.5 温度和pH
温度对于微生物的生命活动起决定性的影响,而由微生物介导的DNRA过程在温度较高的自然区域反应更剧烈,例如亚热带河口、海岸和海洋。在大多数海岸生态系统中,DNRA过程的占比和速率随季节变化,夏季高温增加了沉积物耗氧量,创造了更多的还原性条件,有利于DNRA过程[44]。然而,受季节变化和地理纬度位置的影响,污水处理厂中水温很难保持恒定,这会导致活性污泥中细菌群落发生变化,从而影响处理效果[45]。污水处理中,改变温度可调控硝态氮通过DNRA过程生成氨根,从而实现资源循环利用。Lai等[46]针对DNRA过程如何受温度调控进行了进一步探究,发现随着温度从10 ℃提高到40 ℃,DNRA过程显著增强。与反硝化过程相比,两者的速率随温度升高均增大,但DNRA速率的增量更大[44]。
pH值的变化会影响亚硝酸盐还原酶的活性,进而对DNRA和反硝化的机理过程产生影响[47]。反硝化过程的最适pH值范围为6~8,在此范围内,反硝化速率随pH值的增大先增大后减小[48]。而DNRA过程的最适pH值范围为5~9,与反硝化过程相比,中性及偏碱性的环境能够增强DNRA过程对硝态氮的竞争力[34, 49-50]。在中性和碱性水稻土中,DNRA过程是主要的氮转化途径,而在酸性水稻土中,这一过程可忽略不计[51]。根据DNRA反应式(1),DNRA过程是一个产碱的过程,因此pH值和DNRA过程相互影响,相互制约。
实际污水处理过程中,适当提高水温、维持中性、偏碱性的环境更有利于DNRA过程。
2.6 废水中的其他元素
废水中的水质复杂,不同地区的废水水质往往存在很大差异,而废水中含有的其他元素也会影响DNRA过程,例如二价铁离子和硫化物。在澳大利亚亚拉河口证实了存在Fe2+驱动的DNRA过程(式(4)),添加高含量的Fe2+时会大大抑制反硝化作用,促进DNRA过程[4, 52-53]。Roberts等发现[54],在有氧条件下,沉积物孔隙水中Fe2+含量的增多会提高DNRA过程的比例,当沉积物孔隙水中的Fe2+含量达到峰值(>400 μmol/L)时,硝酸盐还原逐渐从反硝化向DNRA转变。在血清瓶实验中,高含量的Fe2+使DNRA速率增加了一倍,而反硝化速率却下降[55]。这一现象可能是由于高含量的Fe2+通过破坏细胞内电子传输抑制了反硝化作用,从而促进DNRA过程[52, 56]。在东非卡布诺湾的含铁水柱中发现了不同的现象,添加Fe2 +可以不同程度地增强反硝化和DNRA过程,前者比后者高3.3倍,然而,由于技术限制,目前还无法建立确切机制来描述这一过程[57]。此外,最近研究发现[58-59],电缆细菌可以通过溶解FeS来改变周围沉积物中的Fe2 +浓度,间接影响反硝化和DNRA过程。Fe2 +可以改变硝酸根还原路径,废水中含有高含量的Fe2+时,DNRA过程可能更占优势。
污水中缺乏有机物作为碳源时,硫化物可以作为无机碳源,为反硝化和DNRA过程提供能源。硫化物也是决定呼吸DNRA和反硝化之间竞争的关键因素。最初研究表明[60],缓慢氧化的FeS更有利于反硝化过程,而迅速氧化的H2S产生大量S2-,有利于DNRA过程。游离硫化物(S2-)可以抑制反硝化作用中的N2O或NO还原为N2的过程,从而积累亚硝酸盐,为DNRA和硫化物代谢提供电子供体[61]。进一步研究发现[62-63],游离硫化物与硝酸根的比值高会导致DNRA过程占优,低比例有利于反硝化。当比值大于1.3 mol S/ mol N时,硝酸盐易通过DNRA途径还原为氨,硫化物也更偏向于被氧化为硫酸盐。在波罗的海中也观察到了同样趋势,S2-浓度达到40 μmol/L会刺激反硝化作用,更高的浓度则有利于DNRA过程[64-65]。
3 DNRA过程测定方法
目前,针对DNRA过程的测定通常可以采用两种方法:一是通过nrfA基因对DNRA微生物的数量和功能基因的丰度进行定量;二是采用15N同位素示踪技术确定DNRA过程的总转化率和速率,进而评估DNRA的重要性。
硝酸盐还原酶在DNRA和反硝化途径中都很常见,而nrfA基因编码的周质五血红素细胞色素c亚硝酸盐还原酶能够将DNRA和反硝化过程区分开[27, 66-67]。nrfA基因在进行DNRA过程的不同细菌中被发现,包括变形菌门、拟杆菌门、厚壁菌门和浮霉菌门等[16, 68]。因此,nrfA基因可以作为DNRA的功能基因,通过PCR定量其基因丰度是评估DNRA潜力的重要方法[69-70]。Li等[71]通过nrfA功能基因定量对中国富营养化程度不同的浅水湖泊的微生物群落进行分析发现,DNRA细菌的丰度和群落结构可能是湖泊富营养化的重要调节剂。Wang等[19]确定污水处理厂8个处理单元中Nitrospira是主要的DNRA菌属,所采用的方法也是对nrfA基因进行定量。通过基因定量除了可以确定污水处理厂中DNRA相关微生物的分布,还可以估计DNRA过程的活性。研究发现[70, 72-73],在不同生態系统中DNRA过程的活性与nrfA基因的丰度之间存在显著联系,例如河口、河流、海岸和稻田生态系统等。Shu等[8, 13]通过nrfA基因定量先后证明了厌氧氨氧化、反硝化和DNRA在废水处理过程中共存;其次,nrfA基因具有较高的丰度,表明废水处理中DNRA过程不可忽视。
DNRA微生物的数量和功能基因的丰度不一定能完全反映DNRA的活性,还需要研究总转化率和速率来评估DNRA的重要性。1992年,Ambus等[74]第1次针对河岸沼泽提出了土壤总DNRA率的测量,添加15NO-3作为示踪剂后,计算NH+4中15N的富集来分析DNRA转化率,之后Silver等[75]、Huygens等[76]据此提出了分析方程用于研究各种生态系统中的DNRA。有研究认为[77-78],数值模拟可以成为对NO-3所有代谢途径进行分析的替代方法,同时提出了包含DNRA的15N示踪模型。近几年,15N同位素示踪技术成为研究生态系统中氮的最终归宿或者量化氮转化率的主要手段[79-81]。针对土壤生态系统,通过15N同位素示踪技术发现土壤水分增加会导致DNRA过程在NO-3还原中的占比增加,达到92.9%[82]。Pan等[83]结合功能基因定量、高通量分析和网络分析等多种手段,研究了华北4种肥沃农田的硝酸盐还原过程,DNRA是主导过程,占比达到84%±14%。15N同位素示踪技术也被用来评估水生生态系统DNRA的转化率和速率。Li等[84]测定闽江口的DNRA速率范围在0.45~2.92 nmol/(g·h)之间,进一步预测了亚热带河口DNRA过程的大小和空间分布。DNRA是湖泊沉积物中重要的硝酸盐还原途径,对中国12个浅水湖泊沉积物的DNRA速率和转化率进行定量,发现碳氮比和nrfA基因丰度是影响DNRA过程的非生物和生物因素[73]。在上海国家湿地公园,利用该技术首次同时测定了反硝化作用、厌氧氨氧化和DNRA的速率,以此评估DNRA在人工湿地系统中的作用[7]。Wang等[19]利用15N同位素示踪对废水处理中的DNRA过程进行评估,发现DNRA潜在速率低于反硝化,但高于厌氧氨氧化。15N同位素示踪技术帮助量化DNRA过程的速率和贡献率,但其测量方法的不完善仍然制约有关DNRA过程的研究。优化同位素测定仪器是量化DNRA过程速率的关键,通过优化可以准确快速测定15N标记的产物(29N2、30N2)进而评估DNRA过程。开发数学模型同时计算多个转化过程(反硝化、厌氧氨氧化和DNRA等)的速率,并用实测数据对数学模型进行验证,对于准确评估DNRA的贡献率至关重要。
4 结论与展望
在自然生态系统中,由于DNRA过程不仅能够减少温室气体的排放,还可以将较难利用的NO-3-N转化为更容易被微生物利用的NH+4-N,因此,其在氮循环中的作用得到了越来越多的关注和认可。但目前关于DNRA过程的研究大多数集中在土壤系统中,在水体系统中的研究则相对较少。在自然水体中,DNRA过程虽然不能够将总氮降低到一定的水平,但可以通过与反硝化过程竞争,从而减少N2O温室气体的排放。在人工水体(例如污水处理厂)中,DNRA过程可以将废水中的NO-3降低到排放标准,同时产生的含有NH+4的废水可以通过二次处理加工成为营养肥料,实现废水的资源化。
DNRA与反硝化作为两个竞争性的硝酸盐还原过程,在废水处理过程中同时存在。了解影响两个过程之间竞争的因素,对于更好预测DNRA过程在废水处理中的发生和贡献率具有重要意义。溶解氧、碳源种类、氮源种类、碳氮比、温度、pH值以及废水组分等都会不同程度地影响DNRA过程,调控其与反硝化过程之间的竞争。通过优化废水处理中的运行参数,可以使废水达标排放,同时回收资源,降低运行成本,例如,在高碳氮比的条件下,DNRA优于反硝化过程。实际应用中为了确定各参数的影响,还需要通过nrfA功能基因定量和15N同位素示踪技术来评估DNRA的活性并量化DNRA的速率和贡献率。
參考文献:
[1]WOODS D D. The reduction of nitrate to ammonia by Clostridium welchii[J]. The Biochemical Journal, 1938, 32(11): 2000-2012.
[2]STANFORD G, LEGG J O, DZIENIA S, et al. Denitrification and associated nitrogen transformations in soils[J]. Soil Science, 1975, 120(2): 147-152.
[3]TIEDJE J M. Ecology of denitrification and dissimilatory nitrate reduction to ammonium[J]. Environmental Microbiology of Anaerobes, 1988(April): 179-244.
[4]KESSLER A J, ROBERTS K L, BISSETT A, et al. Biogeochemical controls on the relative importance of denitrification and dissimilatory nitrate reduction to ammonium in estuaries[J]. Global Biogeochemical Cycles, 2018, 32(7): 1045-1057.
[5]WANG S Y, PI Y X, SONG Y P, et al. Hotspot of dissimilatory nitrate reduction to ammonium (DNRA) process in freshwater sediments of riparian zones[J]. Water Research, 2020, 173: 115539.
[6]LUVIZOTTO D M, ARAUJO J E, DE CSSIA P SILVA M, et al. The rates and players of denitrification, dissimilatory nitrate reduction to ammonia (DNRA) and anaerobic ammonia oxidation (anammox) in mangrove soils[J]. Anais Da Academia Brasileira De Ciências, 2019, 91(Sup1): 14.
[7]ZHAO Y Y, BU C N, YANG H L, et al. Survey of dissimilatory nitrate reduction to ammonium microbial community at national wetland of Shanghai, China[J]. Chemosphere, 2020, 250: 126195.
[8]SHU D T, HE Y L, YUE H, et al. Metagenomic and quantitative insights into microbial communities and functional genes of nitrogen and iron cycling in twelve wastewater treatment systems[J]. Chemical Engineering Journal, 2016, 290: 21-30.
[9]MCCARTY P L. What is the best biological process for nitrogen removal:When and why?[J]. Environmental Science & Technology, 2018, 52(7): 3835-3841.
[10]WANG S Y, PENG Y Z, MA B, et al. Anaerobic ammonium oxidation in traditional municipal wastewater treatment plants with low-strength ammonium loading: Widespread but overlooked[J]. Water Research, 2015, 84: 66-75.
[11]WANG Q K, DING C, TAO G H, et al. Analysis of enhanced nitrogen removal mechanisms in a validation wastewater treatment plant containing anammox bacteria[J]. Applied Microbiology and Biotechnology, 2019, 103(3): 1255-1265.
[12]YUAN Q, WANG H Y, CHU Z S, et al. Influence of C/N ratio on MBBR denitrification for advanced nitrogen removal of wastewater treatment plant effluent[J]. Desalination and Water Treatment, 2017, 66: 158-165.
[13]SHU D T, HE Y L, YUE H, et al. Microbial structures and community functions of anaerobic sludge in six full-scale wastewater treatment plants as revealed by 454 high-throughput pyrosequencing[J]. Bioresource Technology, 2015, 186: 163-172.
[14]SIMON J, KLOTZ M G. Diversity and evolution of bioenergetic systems involved in microbial nitrogen compound transformations[J]. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 2013, 1827(2): 114-135.
[15]KERN M, SIMON J. Electron transport chains and bioenergetics of respiratory nitrogen metabolism in Wolinella succinogenes and other Epsilonproteobacteria[J]. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 2009, 1787(6): 646-656.
[16]MOHAN S B, SCHMID M, JETTEN M, et al. Detection and widespread distribution of the nrfA gene encoding nitrite reduction to ammonia, a short circuit in the biological nitrogen cycle that competes with denitrification[J]. FEMS Microbiology Ecology, 2004, 49(3): 433-443.
[17]卜翠娜. 異化硝酸盐还原菌(DNRA)的环境分布及富集培养研究[D]. 济南: 山东大学, 2018.
BU C N. Study on environmental distribution and enrichment culture of dissimilatory nitrate reduction to ammonium(DNRA)bacteria[D]. Jinan: Shandong University, 2018. (in Chinese)
[18]LU W W, ZHANG H L, SHI W M. Dissimilatory nitrate reduction to ammonium in an anaerobic agricultural soil as affected by glucose and free sulfide[J]. European Journal of Soil Biology, 2013, 58: 98-104.
[19]WANG S Y, LIU C L, WANG X X, et al. Dissimilatory nitrate reduction to ammonium (DNRA) in traditional municipal wastewater treatment plants in China: Widespread but low contribution[J]. Water Research, 2020, 179: 115877.
[20]刘芹, 彭党聪. 城市污水生物脱氮系统中DNRA的检测与分析[J]. 中国给水排水, 2019, 35(19): 1-6.
LIU Q, PENG D C. Detection and genetic analysis of dissimilatory nitrate reduction to ammonium in biological nitrogen removal system for municipal sewage[J]. China Water & Wastewater, 2019, 35(19): 1-6. (in Chinese)
[21]WANG Q J, LIANG J S, ZHAO C, et al. Wastewater treatment plant upgrade induces the receiving river retaining bioavailable nitrogen sources[J]. Environmental Pollution, 2020, 263: 114478.
[22]SOARES-CASTRO P, YADAV T C, VIGGOR S, et al. Seasonal bacterial community dynamics in a crude oil refinery wastewater treatment plant[J]. Applied Microbiology and Biotechnology, 2019, 103(21/22): 9131-9141.
[23]WANG Y M, LIN Z Y, HE L, et al. Simultaneous partial nitrification, anammox and denitrification (SNAD) process for nitrogen and refractory organic compounds removal from mature landfill leachate: Performance and metagenome-based microbial ecology[J]. Bioresource Technology, 2019, 294: 122166.
[24]MIRGHORAYSHI M, ZINATIZADEH A A, VAN LOOSDRECHT M. Evaluating the process performance and potential of a high-rate single airlift bioreactor for simultaneous carbon and nitrogen removal through coupling different pathways from a nitrogen-rich wastewater[J]. Bioresource Technology, 2018, 260: 44-52.
[25]ZIELINSKA M, BERNAT K, CYDZIK-KWIATKOWSKA A, et al. Nitrogen removal from wastewater and bacterial diversity in activated sludge at different COD/N ratios and dissolved oxygen concentrations[J]. Journal of Environmental Sciences, 2012, 24(6): 990-998.
[26]许德超, 朱婷婷, 阳立平, 等. 溶解氧对生物接触氧化+生物流化床联合脱氮效果的影响[J]. 环境污染与防治, 2020, 42(12): 1557-1562.
XU D C, ZHU T T, YANG L P, et al. Effects of dissolved oxygen on nitrogen removal by a combination of biological contact oxidation and biological fluidized bed[J]. Environmental Pollution & Control, 2020, 42(12): 1557-1562. (in Chinese)
[27]KRAFT B, TEGETMEYER H E, SHARMA R, et al. The environmental controls that govern the end product of bacterial nitrate respiration[J]. Science, 2014, 345(6197): 676-679.
[28]YOON S, CRUZ-GARCA C, SANFORD R, et al. Denitrification versus respiratory ammonification: environmental controls of two competing dissimilatory NO-3/NO-2 reduction pathways in Shewanella loihica strain PV-4[J]. The ISME Jounal, 2015, 9(5): 1093-1104.
[29]VAN DEN BERG E M, VAN DONGEN U, ABBAS B, et al. Enrichment of DNRA bacteria in a continuous culture[J]. The ISME Journal, 2015, 9(10): 2153-2161.
[30]VAN DEN BERG E M, ELISRIO M P, KUENEN J G, et al. Fermentative bacteria influence the competition between denitrifiers and DNRA bacteria[J]. Frontiers in Microbiology, 2017, 8: 1684.
[31]VAN DEN BERG E M, BOLEIJ M, KUENEN J G, et al. DNRA and denitrification coexist over a broad range of acetate/N-NO-3 ratios, in a chemostat enrichment culture[J]. Frontiers in Microbiology, 2016, 7: 1842.
[32]PANDEY A, SUTER H, HE J Z, et al. Dissimilatory nitrate reduction to ammonium dominates nitrate reduction in long-term low nitrogen fertilized rice paddies[J]. Soil Biology and Biochemistry, 2019, 131: 149-156.
[33]PATRICK W H, WILLIAMS B G, MORAGHAN J T. A simple system for controlling redox potential and pH in soil suspensions[J]. Soil Science Society of America Journal, 1973, 37(2): 331-332.
[34]陳韬, 李剑沣, 邹子介, 等. 氧化还原电位和pH对生物滞留系统硝酸盐异化还原为氨作用的影响[J]. 科学技术与工程, 2018, 18(4): 368-373.
CHEN T, LI J F, ZOU Z J, et al. Effects of redox potential and pH on the effect of dissimilatory nitrate reduction to ammonium in bioretention system[J]. Science Technology and Engineering, 2018, 18(4): 368-373. (in Chinese)
[35]JNTTI H, AALTO S L, PAERL H W. Effects of ferrous iron and hydrogen sulfide on nitrate reduction in the sediments of an estuary experiencing hypoxia[J]. Estuaries and Coasts, 2021, 44(1): 1-12.
[36]COJEAN A N Y, ZOPFI J, GERSTER A, et al. Direct O2 control on the partitioning between denitrification and dissimilatory nitrate reduction to ammonium in lake sediments[J]. Biogeosciences, 2019, 16(23): 4705-4718.
[37]PETT-RIDGE J, SILVER W L, FIRESTONE M K. Redox fluctuations frame microbial community impacts on N-cycling rates in a humid tropical forest soil[J]. Biogeochemistry, 2006, 81(1): 95-110.
[38]YIN S X, CHEN D, CHEN L M, et al. Dissimilatory nitrate reduction to ammonium and responsible microorganisms in two Chinese and Australian paddy soils[J]. Soil Biology and Biochemistry, 2002, 34(8): 1131-1137.
[39]RAHMAN M M, ROBERTS K L, GRACE M R, et al. Role of organic carbon, nitrate and ferrous iron on the partitioning between denitrification and DNRA in constructed stormwater urban wetlands[J]. Science of the Total Environment, 2019, 666: 608-617.
[40]CARLSON H K, LUI L M, PRICE M N, et al. Selective carbon sources influence the end products of microbial nitrate respiration[J]. The ISME Journal, 2020, 14(8): 2034-2045.
[41]DONG L F, SOBEY M N, SMITH C J, et al. Dissimilatory reduction of nitrate to ammonium, not denitrification or anammox, dominates benthic nitrate reduction in tropical estuaries[J]. Limnology and Oceanography, 2011, 56(1): 279-291.
[42]SMITH M S. Dissimilatory reduction of NO-2 to NH+4 and N2O by a soil Citrobacter sp[J]. Applied and Environmental Microbiology, 1982, 43(4): 854-860.
[43]WAN Y X, HUANG Z L, ZHOU L A, et al. Bioelectrochemical ammoniation coupled with microbial electrolysis for nitrogen recovery from nitrate in wastewater[J]. Environmental Science & Technology, 2020, 54(5): 3002-3011.
[44]RAHMAN M, GRACE M R, ROBERTS K L, et al. Effect of temperature and drying-rewetting of sediments on the partitioning between denitrification and DNRA in constructed urban stormwater wetlands[J]. Ecological Engineering, 2019, 140: 105586.
[45]贺赟, 李魁晓, 王佳伟, 等. 不同季节城市污水处理厂微生物群落特性[J]. 环境科学, 2021, 42(3): 1488-1495.
HE Y, LI K X, WANG J W, et al. Microbial community structure of waste water treatment plants in different seasons[J]. Environmental Science, 2021, 42(3): 1488-1495. (in Chinese)
[46]LAI T V, RYDER M H, RATHJEN J R, et al. Dissimilatory nitrate reduction to ammonium increased with rising temperature[J]. Biology and Fertility of Soils, 2021, 57(3): 363-372.
[47]殷士學. 淹水土壤中硝态氮异化还原成铵过程的研究[D]. 南京: 南京农业大学, 2000.
YIN S X.Dissimilatory nitrate reduction to ammonium in submerged soils[D]. Nanjing: Nanjing Agricultural University, 2000. (in Chinese)
[48]李權斌, 荣宏伟, 张朝升, 等. pH对生物膜同步硝化反硝化脱氮及其N2O产量的影响[J]. 水处理技术, 2016, 42(1): 121-124,135.
LI Q B, RONG H W, ZHANG C S, et al. Effects of pH on SND and the N2O production in biofilm reactor[J]. Technology of Water Treatment, 2016, 42(1): 121-124,135. (in Chinese)
[49]韦宗敏, 黄少斌, 蒋然. 碳源对微生物硝酸盐异化还原成铵过程的影响[J]. 工业安全与环保, 2012, 38(9): 4-7,14.
WEI Z M, HUANG S B, JIANG R. Effect of carbon on dissimilatory nitrate reduction to ammonium process[J]. Industrial Safety and Environmental Protection, 2012, 38(9): 4-7,14. (in Chinese)
[50]FERNANDES S O, BONIN P C, MICHOTEY V D, et al. Nitrogen-limited mangrove ecosystems conserve N through dissimilatory nitrate reduction to ammonium[J]. Scientific Reports, 2012, 2: 419.
[51]ZHANG J B, LAN T, MLLER C, et al. Dissimilatory nitrate reduction to ammonium (DNRA) plays an important role in soil nitrogen conservation in neutral and alkaline but not acidic rice soil[J]. Journal of Soils and Sediments, 2015, 15(3): 523-531.
[52]ROBERTSON E K, THAMDRUP B. The fate of nitrogen is linked to iron(II) availability in a freshwater lake sediment[J]. Geochimica et Cosmochimica Acta, 2017, 205: 84-99.
[53]COJEAN A N Y, LEHMANN M F, ROBERTSON E K, et al. Controls of H2S, Fe2+, and Mn2+ on microbial NO-3-reducing processes in sediments of an eutrophic lake[J]. Frontiers in Microbiology, 2020, 11: 1158.
[54]ROBERTS K L, KESSLER A J, GRACE M R, et al. Increased rates of dissimilatory nitrate reduction to ammonium (DNRA) under oxic conditions in a periodically hypoxic estuary[J]. Geochimica et Cosmochimica Acta, 2014, 133: 313-324.
[55]ROBERTSON E K, ROBERTS K L, BURDORF L D W, et al. Dissimilatory nitrate reduction to ammonium coupled to Fe(II) oxidation in sediments of a periodically hypoxic estuary[J]. Limnology and Oceanography, 2016, 61(1): 365-381.
[56]CARLSON H K, CLARK I C, MELNYK R A, et al. Toward a mechanistic understanding of anaerobic nitrate-dependent iron oxidation: balancing electron uptake and detoxification[J]. Frontiers in Microbiology, 2012, 3: 57.
[57]MICHIELS C C, DARCHAMBEAU F, ROLAND F A E, et al. Iron-dependent nitrogen cycling in a ferruginous lake and the nutrient status of Proterozoic oceans[J]. Nature Geoscience, 2017, 10(3): 217-221.
[58]RAO A M F, MALKIN S Y, HIDALGO-MARTINEZ S, et al. The impact of electrogenic sulfide oxidation on elemental cycling and solute fluxes in coastal sediment[J]. Geochimica et Cosmochimica Acta, 2016, 172: 265-286.
[59]KESSLER A J, WAWRYK M, MARZOCCHI U, et al. Cable bacteria promote DNRA through iron sulfide dissolution[J]. Limnology and Oceanography, 2019, 64(3): 1228-1238.
[60]BRUNET R C, GARCIA-GIL L J. Sulfide-induced dissimilatory nitrate reduction to ammonia in anaerobic freshwater sediments[J]. FEMS Microbiology Ecology, 1996, 21(2): 131-138.
[61]AELION C M, WARTTINGER U. Low sulfide concentrations affect nitrate transformations in freshwater and saline coastal retention pond sediments[J]. Soil Biology and Biochemistry, 2009, 41(4): 735-741.
[62]YIN Z X, XIE L, ZHOU Q. Effects of sulfide on the integration of denitrification with anaerobic digestion[J]. Journal of Bioscience and Bioengineering, 2015, 120(4): 426-431.
[63]POKORNA D, ZABRANSKA J. Sulfur-oxidizing bacteria in environmental technology[J]. Biotechnology Advances, 2015, 33(6): 1246-1259.
[64]DALSGAARD T, DE BRABANDERE L, HALL P O J. Denitrification in the water column of the central Baltic Sea[J]. Geochimica et Cosmochimica Acta, 2013, 106: 247-260.
[65]BONAGLIA S, KLAWONN I, DE BRABANDERE L, et al. Denitrification and DNRA at the Baltic Sea oxic-anoxic interface: Substrate spectrum and kinetics[J]. Limnology and Oceanography, 2016, 61(5): 1900-1915.
[66]WANG X G, TAMIEV D, ALAGURAJAN J, et al. The role of the NADH-dependent nitrite reductase, Nir, from Escherichia coli in fermentative ammonification[J]. Archives of Microbiology, 2019, 201(4): 519-530.
[67]KIM H, PARK D, YOON S. pH control enables simultaneous enhancement of nitrogen retention and N2O reduction in Shewanella loihica strain PV-4[J]. Frontiers in Microbiology, 2017, 8: 1820.
[68]GILES M, MORLEY N, BAGGS E M, et al. Soil nitrate reducing processes-drivers, mechanisms for spatial variation, and significance for nitrous oxide production[J]. Frontiers in Microbiology, 2012, 3: 407.
[69]YIN G, HOU L, LIU M, et al. DNRA in intertidal sediments of the Yangtze Estuary[J]. Journal of Geophysical Research: Biogeosciences, 2017, 122(8): 1988-1998.
[70]LI X F, SARDANS J, GARGALLO-GARRIGA A, et al. Nitrogen reduction processes in paddy soils across climatic gradients: Key controlling factors and environmental implications[J]. Geoderma, 2020, 368: 114275.
[71]LI X W, SONG C L, ZHOU Z J, et al. Comparison of community and function of dissimilatory nitrate reduction to ammonium (DNRA) bacteria in Chinese shallow lakes with different eutrophication degrees[J]. Water, 2020, 12(1): 174.
[72]LI X F, GAO D Z, HOU L J, et al. Soil substrates rather than gene abundance dominate DNRA capacity in the Spartina alterniflora ecotones of estuarine and intertidal wetlands[J]. Plant and Soil, 2019, 436(1/2): 123-140.
[73]PANG Y M, JI G D. Biotic factors drive distinct DNRA potential rates and contributions in typical Chinese shallow lake sediments[J]. Environmental Pollution, 2019, 254: 112903.
[74]AMBUS P, MOSIER A, CHRISTENSEN S. Nitrogen turnover rates in a riparian Fen determined by 15N dilution[J]. Biology and Fertility of Soils, 1992, 14(4): 230-236.
[75]SILVER W L, HERMAN D J, FIRESTONE M K. Dissimilatory nitrate reduction to ammonium in upland tropical forest soils[J]. Ecology, 2001, 82(9): 2410-2416.
[76]HUYGENS D, BOECKX P, TEMPLER P, et al. Mechanisms for retention of bioavailable nitrogen in volcanic rainforest soils[J]. Nature Geoscience, 2008, 1(8): 543-548.
[77]RTTING T, MLLER C. 15N tracing models with a Monte Carlo optimization procedure provide new insights on gross N transformations in soils[J]. Soil Biology and Biochemistry, 2007, 39(9): 2351-2361.
[78]RTTING T, HUYGENS D, STAELENS J, et al. Advances in 15N-tracing experiments: New labelling and data analysis approaches[J]. Biochemical Society Transactions, 2011, 39(1): 279-283.
[79]WANG J, ZHANG J B, MLLER C, et al. Evaluation of the mixing of sands into soils on nitrification potential from different land-use systems[J]. European Journal of Soil Biology, 2017, 81: 25-30.
[80]LIU X, HAN J G, MA Z W, et al. Effect of Carbon source on dissimilatory nitrate reduction to ammonium in Costal Wetland sediments[J]. Journal of Soil Science and Plant Nutrition, 2016, 16(2): 337-349.
[81]PANDEY A, SUTER H, HE J Z, et al. Dissimilatory nitrate ammonification and N2 fixation helps maintain nitrogen nutrition in resource-limited rice paddies[J]. Biology and Fertility of Soils, 2021, 57(1): 107-115.
[82]CHEN Z M, DING W X, XU Y H, et al. Importance of heterotrophic nitrification and dissimilatory nitrate reduction to ammonium in a cropland soil: Evidences from a 15N tracing study to literature synthesis[J]. Soil Biology and Biochemistry, 2015, 91: 65-75.
[83]PAN H W, QIN Y, WANG Y T, et al. Dissimilatory nitrate/nitrite reduction to ammonium (DNRA) pathway dominates nitrate reduction processes in rhizosphere and non-rhizosphere of four fertilized farmland soil[J]. Environmental Research, 2020, 186: 109612.
[84]LI X F, QIAN W, HOU L J, et al. Soil organic carbon controls dissimilatory nitrate reduction to ammonium along a freshwater-oligohaline gradient of Min River Estuary, Southeast China[J]. Marine Pollution Bulletin, 2020, 160: 111696.
(編辑 胡玲)
