A retrospective observational study on maintenance and complications of totally implantable venous access ports in 563 patients:Prolonged versus short flushing intervals
2021-09-10YuejiaoZhangRuiyiZhaoNanJiangYunShiQianmiWangYeSheng
Yuejiao Zhang,Ruiyi Zhao,Nan Jiang,Yun Shi,Qianmi Wang,Ye Sheng
Nursing Department,The Second Affiliated Hospital,Zhejiang University School of Medicine,Hangzhou,China
Keywords:Central venous access devices Complications Flushing interval Outpatients
ABSTRACT Objectives: To assess whether the extension of the flushing interval will increase risks of complications associated with totally implantable venous access port (TIVAP) in the off-treatment period.Methods: A retrospective single-center observational study was performed.Patients with a TIVAP in the off-treatment period that underwent regular flushing in our clinic were included.Data concerning patients and their TIVAPs were recorded.Patient baseline characteristics and TIVAP-related complications were analyzed.Continuous variables were analyzed by ANOVA or the Kruskal-Wallis H test.To compare the occurrence of TIVAP-related complications,the chi-square test was used;if needed,Fisher’s exact test was used.Results: Totally 607 patients were reviewed,and 563 patients were finally included.Thirteen complications were recorded,including 11 cases of catheter occlusion(1.95%),one case of port cannula rotation(0.18%),and one case of catheter tip malposition (0.18%).No device-related infection or venous thrombosis was recorded.Among these patients,the average flushing interval was 35.27±13.09 days.Patients were divided into three groups according to the flushing interval:every 28 days or less (Group 1, n=133);every 29-44 days(Group 2,n=350);and every 45 days or more(Group 3,n=80).No significant difference in catheter-related complications was found among the three groups (P >0.05).Conclusions: In the TIVAP off-treatment period,patients without any history of TIVAP-related complications during approximately one year can attempt to prolong the flushing interval to more than 4 weeks;we further suggest that 5-6 weeks may be an appropriate option for these patients.
What is known?
• Totally implantable venous access port (TIVAP) is widely used for chemotherapy in cancer patients.The manufacturer recommends a 4-week flushing interval for TIVAPs with openended catheters but no specific port status.
• The flushing interval for totally implantable venous access ports(TIVAPs) during the nontreatment period is unknown.
What is new?
• The retrospective survey found that patients self-extended the flushing interval for TIVAPs during the off-treatment period.
• The survey found that it did not increase risks of TIVAP-related complications in oncology patients to prolong the flushing interval of TIVAPs not in use to more than 4 weeks.
1.Introduction
Oncology patients receiving chemotherapy need a central vascular device due to the severe irritancy of the medicine to the vasculature and the frequency of infusions.Peripherally inserted central catheter (PICC) and totally implanted venous access port(TIVAP) are the most common choices.In recent years,the use of TIVAPs,which are designed to permit repeated injections,infusions,and,optionally,blood sampling,has become increasingly frequent in oncology settings [1,2].The TIVAP is a device located entirely under the dermis that contains a subdermal reservoir accessed through the dermis using a needle and a catheter that extends to the central vein [3-5].It has a better cosmetic appearance and less influence on the patient’s daily life and produces higher patient satisfaction than other options[6].However,TIVAPs have early and late complications.Late port complications mainly include infection,venous thrombosis,catheter occlusion and malfunction [7].Several studies have demonstrated that catheterrelated infection and thrombotic events may result in catheter removal in 10% of patients [8-11].Thrombotic occlusion may also result in catheter removal.To maintain normal function,ports need frequent flushing.Whereas cancer patients may relapse after completing chemotherapy,some choose to reserve the port in case a second round of chemotherapy is needed [12].Patients who do not use the port have to undergo regular port flushing,which not only increases their medical and logistic expenses but also causes inconvenience,especially for elderly patients who may need someone to accompany them to the flushing sessions [13].Therefore,the duration of the flushing interval is very important.However,the best practice for port maintenance,including flushing protocols,flushing content,and the flushing interval,is undefined.Flushing content includes heparin or saline at different volumes as well as different concentrations of heparin,with different regulations at different institutions.For the flushing interval,most manufacturers indicate that flushing must be performed every 4 weeks when the device is not in use(i.e.,during the off-treatment period)[14].Meanwhile,several studies have shown that extended flushing intervals are safe [15,16].In fact,in our outpatient setting,we found that some patients could not follow the 4-week interval strictly after the completion of treatment,but complications rarely occurred.This retrospective study was conducted to assess whether an extension of the flushing interval would increase risks of TIVAP-related complications.
2.Method
2.1.Study design and setting
This is a single-center retrospective observational study.Patients who did not use the TIVAP during its off-treatment period were included in this study.The study was approved by the ethics board of The Second Affiliated Hospital of Zhejiang University School of Medicine.
2.2.Subjects and sampling
2.2.1.Patient selection
Some cancer patients chose to maintain implantation of the TIVAP after finishing chemotherapy,and thus regular flushing was needed.We selected patients who had finished chemotherapy before July 2019 and underwent regular TIVAP flushing in our outpatient setting.The inclusion criteria were:1) cancer patients with open-ended single lumen TIVAP catheters;2) nonuse of the TIVAP for more than one month;3) received at least twice consecutive regular flushing in our outpatient setting;and 4)without TIVAP-related complications.The exclusion criteria included patients who had relapsed or were undergoing chemotherapy or infusion using the TIVAP.We recorded all information about the patient and his/her port when the patient first visited our outpatient setting.Patients were included in the study when they underwent the second port flushing in our outpatient setting,allowing calculation as one flushing interval.We counted one port flushing interval as one observation.
2.2.2.Data collection
Data from August 2019 to December 2020 in our outpatient setting were collected and analyzed.Information was collected from the medical records,including baseline patient characteristics,duration of retention of each TIVAP,maintenance interval and TIVAP-related complications.Concerning catheter-related occlusions,we classified them as complete and incomplete occlusions.Complete occlusion was defined as high resistance to flushing and the inability to draw blood,whereas incomplete occlusion was defined as no or low resistance to flushing and the inability to draw blood.The TIVAP-related complication rate was calculated as the number of patients with complications/total number of patients.
2.2.3.Flushing procedures
When patients visited our outpatient setting,they were cared for by experienced nurses.The flushing protocol was the same for all patients.The materials included sterile gloves,a sterile surgical orifice towel,a Huber needle,20 ml normal saline,3 ml heparin sodium (100 U/ml),a 10 ml injection syringe,alcohol and 2%chlorhexidine gluconate in alcohol solution,sterile gauze and transparent dressing.The procedure was performed in five steps in strict accordance with standard aseptic precautions.Step 1:Assessment of the port and disinfection of the skin;Step 2:Draping of the orifice towel and insertion of the Huber needle;Step 3:Withdrawal and disposal of 2 ml of blood;Step 4:Flushing with 20 ml of normal saline using the push-pause technique and positive pressure locking with 3 ml heparin sodium;Step 5:Withdrawal of the Huber needle,compression of the insertion site with sterile gauze and application of the transparent dressing.Patients were informed to maintain the dressing for at least 24 h.
2.3.Statistical analysis
Patients were divided into three groups:Group 1,with a short flushing interval (≤28 days);Group 2,with a medium flushing interval(29-44 days);Group 3,with a long flushing interval(≥45 days).Continuous variables with a normal distribution are described as the mean and standard deviation and were analyzed by ANOVA.Other variables are described as medians and quartiles and were analyzed by the Kruskal-WallisHtest.Categorical data(gender,marital status,education level,medical insurance,TIVAP insertion site) are described as frequencies and percentages and were analyzed with the chi-square test or Fisher’s exact test.To compare the occurrence of TIVAP-related complications,the chisquare test was used;if needed,Fisher’s exact test was used.Pvalues <0.05 were considered to be statistically significant.All analyses were performed with IBM SPSS software version 24.
3.Results
3.1.Patient characteristics
The medical records of 607 patients were reviewed.Among these patients,41 received port infusions during our observation period,and 3 patients’flushing intervals were uncertain,including a patient who underwent his first port flushing more than 17 months after he finished his final round of chemotherapy.Finally,a total of 563 cancer patients who had completed their chemotherapy using a TIVAP before July 2019 and had started surveillance were included.Totally,1,427 times were followed up for those patients.The characteristics of the 563 patients are shown in Table 1.The mean age of these patients was 58.02 years (SD=11.15).The flushing intervals(Mean±SD)were 26.99±1.99,34.13±3.80 and 62.25 ± 23.53 days in the three groups.No significant difference was found for gender,age,marital status,education level,medicalinsurance or catheter site among the three groups.The median indwelling duration was 14 months in Group 1,while 16 months in Group 2 and 13.5 months in Group 3.The indwelling duration of the TIVAP is shown in Table 1 and Fig.1.During our observation,some patients underwent port maintenance in accordance with a certain flushing interval,and some did not.They were divided into three groups accordingly to their port flushing interval.The specific observation frequencies for the three groups are listed in Table 2.

Table 1Patient characteristics (n=563).
3.2.Incidence of TIVAP-related complication
Three kinds of complications were found in 13 patients,namely,catheter occlusion,internal jugular vein malposition and port casing rotation,among which catheter occlusion was the most frequent and could be found in every group.All catheter occlusions returned to patency after treatment of urokinase thrombolysis.Two patients with port casing rotation and catheter tip malposition chose to have their ports removed.No patient developed an infection during the whole observation period.The details of the complications are listed in Table 3.The total incidence of TIVAPrelated complications was 2.26%,1.76% and 5.00% in the three groups.No significant difference was found (P=0.28) among the three groups(Table 4).

Fig.1.Indwelling duration of the TIVAP for the three groups
3.3.Incidence of TIVAP catheter occlusion
In Group 2,we found one patient with port casing rotation,leading to complete catheter occlusion.In Group 3,one patient had catheter tip flotation to the internal jugular vein,resulting in incomplete catheter occlusion.Secondary occlusion was avoided in the two cases.Thus,the incidence of catheter occlusion in the three groups was 2.26% (3/133),1.43% (5/350),and 3.75% (3/80),respectively.The incidences of incomplete and complete occlusion were not significantly different among the three groups (Table 4).

Table 2Specific follow-up frequencies for the three groups.

Table 3Characteristics of the 13 patients with TIVAP-related complications.
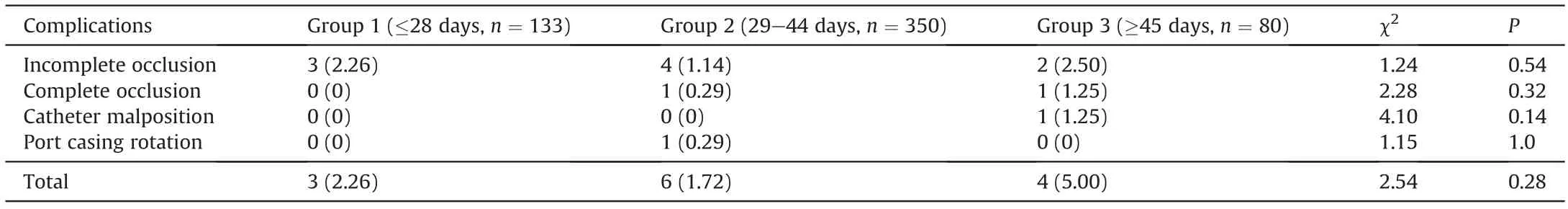
Table 4TIVAP-related complication rates in three groups.
4.Discussion
Regular maintenance is important to maintain the proper functioning of the TIVAP[17-19].To date,infusion therapy practice standards do not indicate an exact flushing interval for TIVAPs[20],especially for ports that are not in use.According to the instructions written by the manufacturers,a flushing interval of 4 weeks is recommended for open-ended port catheters,regardless of the specifics of the status of the TIVAP (in-use or off-use) or the flush and lock solution.However,this recommendation was not strictly followed.We found that a large number of patients prolonged the flushing interval for different reasons when their devices were not in use.A total of 76.38%(430/563)of patients did not follow the 4-week flushing interval strictly in our study,and 62.2%(350/563)of patients extended the flushing interval to 5-6 weeks without an increase in complications (P=0.28).This phenomenon has attracted attention in recent years,and several studies have reported finding a proper flushing interval.A prospective nonrandomized study by Jorge AD enrolled oncology patients with ports upon completion of systemic therapy and found that extending port flushing to every 3 months was relatively safe[15].However,as it did not make comparisons with shorter intervals,this report was unable to provide strong evidence of this safety.Another study conducted by Rasero L divided patients with portsinto a short-term group (<45 days) and a long-term group (>45 days) according to the flushing interval,and as a result,found no significant difference in catheter occlusion incidence [16].Unfortunately,the sample size was relatively small(less than 90 patients totally).It is even more unfortunate that the recommendation of a 4-week interval was ignored and not used as a cutoff point for the grouping,which is somewhat controversial.
Regarding TIVAP catheter occlusion,Milani A’s study showed a significant relationship with the frequency of port flushing [14].However,in their study,patients were receiving infusions using the TIVAP;in contrast,all our patients’ports were not in use,and most patients had maintained their ports for 1-2 years.In our study,4 patients in Group 3(flushing interval of at least 45 days)developed TIVAP-related complications,including 3 catheter occlusions.There seemed to be a higher incidence of catheter occlusion in Group 3(3.75%)than in Group 1(2.26%)and group 2(1.43%),but none of the differences was statistically significant (P=0.37).
Recently,a meta-analysis showed that a port flushing interval longer than 4 weeks is safe and that extending the flushing interval to 8 weeks might not increase the incidence of total complications and catheter occlusions [21],which is partly consistent with our study.Fortunately,our research also revealed the safety of exceeding the 4-week flushing interval.With sufficient evidence,it is obvious that a longer port flushing interval leads to a reduction in healthcare costs and an improvement in patient quality of life.According to related studies and our research,the flushing interval could be flexible rather than rigid.Although we still cannot draw a definite conclusion regarding the exact flushing interval for nonuse TIVAPs,the results provide support to extending the flushing interval for these ports beyond 4 weeks and remind other researchers to perform prospective studies.Finally,we recommend 5-6 weeks as a relatively safe flushing interval for patients with ports not in use for approximately one year and without any complication history.
Our study has some limitations as a retrospective study,and the sample size of this study was relatively small.Prospective studies usually have fewer potential sources of bias and confounding than retrospective studies.We plan to perform prospective studies based on the results of this retrospective study in the future.
5.Conclusion
In conclusion,during the TIVAP off-treatment period,patients without any history of TIVAP-related complications for approximately one year or more could attempt to prolong the flushing interval beyond 4 weeks,and 5-6 weeks may be an appropriate option for these patients.However,once a complication occurs,the flushing interval within 4 weeks should be strictly obeyed.Prospective studies are needed to obtain stronger evidence.
Funding
None.
CRediT authorship contribution statement
Yuejiao Zhang:Conceptualization,Writing-Original draft preparation,Writing -Review &Editing.Ruiyi Zhao:Conceptualization,Supervision,Methodology,Resources,Writing -Review &Editing.Nan Jiang:Conceptualization,Data curation,Formal analysis.Yun Shi:Conceptualization,Investigation,Formal analysis.Qianmi Wang:Investigation,Project administration,Formal analysis,Data curation.Ye Sheng:Investigation,Validation,Methodology,Formal analysis.
Declaration of competing interest
The authors have declared no conflict of interest.
Acknowledgement
Our deep gratitude to statistician Zexin Chen for instructing us on data statistics and supervising the statistical method of this study.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijnss.2021.05.005.
杂志排行
International Journal of Nursing Sciences的其它文章
- A family nurse-led intervention for reducing health services’utilization in individuals with chronic diseases:The ADVICE pilot study
- Social practices of nurse care coordination using sensor technologies-Challenges with an alert system adoption in assisted living communities for older adults
- Challenges faced by community health nurses to achieve universal health coverage in Myanmar:A mixed methods study
- Retrospective analysis of exercise capacity in patients with coronary artery disease after percutaneous coronary intervention or coronary artery bypass graft
- The impacts of organizational culture and neoliberal ideology on the continued existence of incivility and bullying in healthcare institutions:A discussion paper
- A qualitative study of nurses’ experiences of self-care counseling in migrant patients with heart failure
