Up-Scalable Fabrication of SnO2 with Multifunctional Interface for High Performance Perovskite Solar Modules
2021-09-03GuoqingTongLuisOnoYuqiangLiuHuiZhangTongleBuYabingQi
Guoqing Tong, Luis K. Ono, Yuqiang Liu, Hui Zhang, Tongle Bu, Yabing Qi✉
ABSTRACT Tin dioxide (SnO2) has been demonstrated as one of the promising electron transport layers for highefficiency perovskite solar cells (PSCs). However, scalable fabrication of SnO2 films with uniform coverage, desirable thickness and a low defect density in perovskite solar modules (PSMs) is still challenging. Here, we report preparation of high-quality large-area SnO2 films by chemical bath deposition (CBD) with the addition of KMnO4. The strong oxidizing nature of KMnO4 promotes the conversion from Sn(II) to Sn(VI), leading to reduced trap defects and a higher carrier mobility of SnO2. In addition, K ions diffuse into the perovskite film resulting in larger grain sizes, passivated grain boundaries, and reduced hysteresis of PSCs. Furthermore,Mn ion doping improves both the crystallinity and the phase stability of the perovskite film. Such a multifunctional interface engineering strategy enabled us to achieve a power conversion efficiency (PCE) of 21.70% with less hysteresis for lab-scale PSCs. Using this method,we also fabricated 5 × 5 and 10 × 10 cm2 PSMs, which showed PCEs of 15.62% and 11.80% (active area PCEs are 17.26% and 13.72%),respectively. For the encapsulated 5 × 5 cm2 PSM, we obtained a T80 operation lifetime (the lifespan during which the solar module PCE drops to 80% of its initial value) exceeding 1000 h in ambient condition.
KEYWORDS Perovskites; Solar modules; Operational stability; Interface passivation; SnO2
1 Introduction
The attributes of high performance, simple fabrication process and low-cost make perovskite solar cells (PSCs) a good candidate in the field of energy conversion [1-7]. The record powder conversion efficiency (PCE) of 25.5% for lab-scale PSCs now is comparable with the commercial photovoltaics[8, 9]. Up to now, the high performance of PSCs is mainly based on a planar or mesoporous structure [10-14]. In these architectures, the electron transport layer (ETL) as the bottom layer is the key in the device fabrication toward high efficiency and long-term stability [15]. Tin dioxide (SnO2)has been demonstrated as an efficient ETL candidate in the case of PSCs due to its excellent mobility, high light transmittance and suitable energy level alignments with perovskites [16-19]. It can be easily coated on the planar and/or tandem structure by spin-coating [12, 20, 21], chemical bath deposition (CBD) [22, 23], sputtering deposition [24] and atomic layer deposition (ALD) [25-27].
High-quality and advanced fabrication techniques of SnO2films not only help achieve a high efficiency in labscale, but also promote the development of perovskite solar modules (PSMs) [28]. To date, the record mini-module efficiency of PSMs is over 18.6% with a module area of ~ 30 cm2[29]. However, the stability still lags behind because of poor interface and fragile perovskite materials [20]. Therefore, high efficiency and long-term stability of PSMs not only need a high-quality perovskite active layer, but also require the preparation of ETLs and interface modification [20, 30, 31]. Better uniformity across the large area, simple fabrication process and low-cost/temperature of ETLs are imperative for the upscalable fabrication of PSMs [32]. However, when upscaling PSMs the risk to form voids/pinholes inevitably increases in the conventional spin-coating process because of the thickness fluctuation in the ultra-thin SnO2layer [33]. Also, the mesoporous layer as ETL adds additional complexity in the module fabrication and increases the series resistance simultaneously [34, 35]. Thus, a compact, high crystalline and high mobility ETLs are important for module fabrication. Sputtering deposition has been commonly used to deposit uniform SnO2thin films across a large area at low temperature; however, the low crystallization and the large density of defects lead to poor carrier extraction [24]. The relatively high-cost of ALD makes it less desirable for mass production [17]. Although conventional CBD is a low-cost process compatible with upscalable deposition,the need of a seed layer and additional surface passivation make the deposition process complex [22]. Recently,CBD was demonstrated as an efficient way to produce high-quality SnO2by precisely controlling the thickness,which led to a record output efficiency of 25.2% in labscale [36]. However, large area SnO2deposition is still rarely reported using the CBD process (Table S1) because additional surface treatments are needed to passivate the oxygen vacancies and surface hydroxyl groups in the SnO2film [20, 37-40], which not only increases the nonradiative recombination, but also accelerates the degradation of the devices[40, 41]. It is worth noting that the conventional surface passivation of SnO2layer is mainly based on spincoating techniques or additional spin-coating process [20,37, 39-43], which makes them challenging for large-scale fabrication. Therefore, it is vital to achieve high-quality SnO2thin films and proper SnO2/perovskite interfaces by a more facile approach.
In this work, we prepared the SnO2thin layers on both lab-scale and upscalable substrates by introducing the potassium permanganate (KMnO4) into the precursor solution in the CBD method (abbreviated as SnO2/K-ETL;SnO2without KMnO4is abbreviated as SnO2-ETL). Strong oxidant of KMnO4not only facilitates the complete oxidation of the SnCl2precursor solution, but also provides additional treatment by K and Mn ions. K ions can diffuse into perovskites to enlarge grain size and passivate the grain boundaries (GBs) simultaneously. Unintentional Mn doping not only improves the crystallinity of perovskite films, but also enhances the phase stability of perovskites.Moreover, the presence of K ions can effectively reduce the hysteresis of PSCs/PSMs. This multifunctional interface engineering strategy led to a champion power conversion efficiency (PCE) of 21.70% for the SnO2/K-based PSCs (active area = 0.09 cm2). Furthermore, the large area PSMs of 5 × 5 and 10 × 10 cm2were fabricated to generate the module PCEs of 15.62% (active area PCE = 17.26%)and 11.80% (active area PCE = 13.72%), respectively. AT80lifetime (the lifespan during which the solar module PCE drops to 80% of its initial value) over 1000 h was demonstrated on the 5 × 5 cm2PSM under continuous AM 1.5G light illumination at a fixed bias in ambient condition(relative humidity RH ~ 55%, 25 °C).
2 Experimental Section
2.1 Materials
Formamidinium iodide (FAI), methylammonium iodide(MAI), methylammonium bromide (MABr) and methylammonium chloride (MACl) were purchased from Greatcell solar (Australia). PbI2(99.99%) was purchased from TCI. 2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenylamine)9,9′-spirobifluorene (Spiro-OMeTAD) was purchased from Xi’an Polymer Light Technology (China).Dimethylformamide (DMF), dimethyl sulfoxide (DMSO),isopropanol (IPA), chlorobenzene (CB), and hydrochloric acid (HCl, 36.4%) were purchased from Wako (Japan).4-tert-butylpyridine (99.9%), acetonitrile (99.9%),SnCl2·2H2O (99.99%), urea and mercaptoacetic acid (98%)were purchased from Sigma-Aldrich. Potassium permanganate (KMnO4, 99.3%) was purchased from Nacalai Tesque. All reagents were used as received without further purification.
2.2 SnO2 Thin Film
The SnO2thin film was deposited by CBD method. Specifically, 5 g urea was dissolved into 400 mL distilled water in a glassware. After stirring for 5 min, 100 µL mercaptoacetic acid, 5 mL HCl and 1.1 g SnCl2·2H2O were sequentially dropped into solution and kept stirring for another 5 min.The solution needs to be diluted four times before use. The cleaned fluorine-doped tin oxide (FTO) substrates were placed vertically in the glassware that contained SnCl2precursor solution. Then, the glassware was placed in an oven with a temperature of 95 °C. After 3 h heating, the different amounts of KMnO4were added into glassware. After 10 min continuous heating, the glassware was taken out followed by cleaning FTO substrates. Finally, the as-prepared FTO/SnO2substrates were annealed at 180 °C for 1 h in ambient.
2.3 Devices Fabrication
FTO substrates (1.5 × 1.5 cm2) were sequentially washed by the detergent, distilled water and IPA for 30 min,respectively. Then, the FTO substrates were dried by N2flow and treated by using UV-ozone for one hour. After deposition of SnO2by CBD method, the perovskite film(Cs0.05FA0.85MA0.1PbI2.85Br0.15) was fabricated by two-step spin-coating. First, 1.35 M PbI2and 0.0675 M CsI were dissolved in mixed solution (1.9 mL DMF and 0.1 mL DMSO) and stirred at 70 °C for 30 min. The PbI2precursor solution was coated on FTO/SnO2substrates by spincoating (3000 rpm, 30 s). Second, the mixed FAI/MAI/MABr/MACl precursor solution (FAI 1000 mg; MAI 110 g;MABr 110 mg; MACl 110 mg, dissolved in 15 mL IPA) was spin-coated (3000 rpm, 30 s) on the top of the FTO/SnO2/PbI2. To obtain the perovskite layer, the as-prepared films were annealed at 150 °C for 15 min in ambient environment (RH ~ 20%, 20 °C). 100 μL of spiro-OMeTAD solution(spiro-OMeTAD solution was prepared as reported in our previous works [20, 44].) was spin-coated on the perovskite film at 3500 rpm for 30 s. Finally, 100 nm gold electrode was coated by thermal evaporation under a high vacuum pressure of 1 × 10−5Torr. Except for the gold evaporation,all the processes were performed in an ambient environment with a relative humidity (RH) of ~ 20%.
2.4 PSM Fabrication
The FTO substrates of 5 × 5 and 10 × 10 cm2PSMs were cleaned by the detergent, distilled water and IPA for 30 min, respectively. The SnO2film, perovskite layer, spiro-OMeTAD film and gold electrode were deposited by CBD,spin-coating and thermal evaporation method, respectively,as we discussed before. For the 5 × 5 cm2PSM, the FTO substrate contains 7 sub-cells with a defined size of 6.65 mm by 49 mm. Similarly, 14 sub-cells with a defined size of 6.65 mm by 99 mm were made in 10 × 10 cm2PSM. The gap between each FTO pattern is named as P1 with a width of 50 μm. After deposition of SnO2film, perovskite layer and spiro-OMeTAD, another line defined as P2 was carried out by laser scribing via a CO2laser (output intensity is ~ 5.6 W) to expose the bottom FTO electrodes in order to construct the series connections between the sub-cells in the PSMs. Finally, P3 line was performed by mechanical scribing to sperate each sub-cell after gold evaporation. The laser scribing process and thermal evaporation were conducted in an ambient environment with a RH of ~ 55%. All the other processes were performed in a dry condition (RH ~ 20%).
2.5 Photovoltaic Characterization
TheJ-Vcurves of the lab-scale PSCs (1.5 × 1.5 cm2)and PSMs (5 × 5 cm2) are carried out under AM 1.5 G,100 mW cm−2by using a solar simulator (Newport Oriel Sol 1A, Xenon-lamp, USHIO, UXL-150SO) and Keithley 2420 source meter. TheJ-Vcurves of 10 × 10 cm2PSMs were measured under AM 1.5 G, 100 mW cm−2by using a solar simulator with an output beam of 8 × 8 inches (Newport Oriel Sol 3A, Xenon-lamp, 94083A). All the solar simulators are calibrated by using a KG3 reference Si-cell(Enlitech, Oriel Instruments Model Number 90026564,2 × 2 cm2) before test. The lab-scale PSCs were measured under reverse scan (1.3 to −0.1 V) and forward scan (−0.1 to 1.3 V) with a scan rate of 0.13 V s−1. Similarly, the J-V scan range of PSMs are from 12 to −0.2 V (forward: −0.2 to 12 V) and 16.0 to −0.2 V (forward: −0.2 to 16 V) for 5 × 5 cm2PSMs and 10 × 10 cm2PSMs, respectively. The scan rate for PSMs is 0.25 V s−1. No preconditioning protocol was used before the characterization in this work. The EQE spectra of lab-scale PSCs were characterized from 300 to 850 nm using Oriel IQE 200. The lab-scale PSCs(1.5 × 1.5 cm2) were tested by using a metal shadow mask with an aperture area of 0.09 cm2and the designated area of 22.4 cm2for 5 × 5 cm2PSMs and 91.8 cm2for 10 × 10 cm2PSMs were defined by a corresponding metal mask.
2.6 Characterization
XRD measurements (from 10° to 60°) were investigated by using a Bruker D8 Discover instrument (Bruker AXS GmbH, Karlsruhe, Germany) equipped with Cu wavelengthI= 1.54 Å X-ray source operated at 1600 W and Goebel mirror. The scan interval is 0.02° per step. The top-view and cross section SEM images were investigated by using a scanning electron microscope (Helios NanoLab G3 UC,FEI). Surface investigations were performed by ultraviolet photoemission spectroscopy (UPS) andX-ray photoelectron spectroscopy (XPS). AnX-ray photoelectron spectrometer (XPS-AXIS Ultra HAS, Kratos) equipped with monochromatic Al-Kα = 1486.6 eV and nonmonochromatic He-I = 21.22 eV sources was used for measurements. For sputtering in secondary ion mass spectroscopy (SIMS), a 3 keV Ar+primary beam with a current of 10 mA and a diameter of 100 µm was used. The steady-state photoluminescence (PL) spectra were obtained by using a JASCO FP-8500 spectrometer. The time-resolved photoluminescence (TRPL) measurements were carried out by using time-correlated, single-photon counting technique (Hamamatsu, C10627) equipped with a femtosecond mode-locked Ti:sapphire laser (SpectraPhysics, MAITAI XF-IMW). The wavelength of excitation is 405 nm with an intensity of 1.3 mW. The space-charge limited current (SCLC) test was performed in dark condition by using a Keithley 2420 source meter (from −0.1 to 2.25 V). The electrochemical impedance spectroscopy (EIS) measurement data were acquired from the Autolab electrochemical working station with an amplitude of 5 mV at a fixed voltage of 1.0 V under one sun illumination (AM 1.5 irradiation). The frequency range is from 1 MHz to 100 Hz.
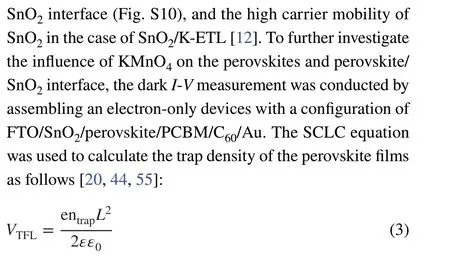
3 Results and Discussion
Figure 1 illustrates the fabrication process of SnO2-ETL films in the case of PSM by the CBD method. For SnO2/K-ETL film, urea, SnCl2·2H2O, mercaptoacetic acid and hydrochloric acid (HCl) were first dissolved in deionized water as the precursor solution (Step ①). The glassware containing precursor solution and FTO substrates was heated to 95 °C and kept for 3 h in the oven (Step ②). Then, the KMnO4was added into the glassware (Step ③). After 10 min continuous heating, the samples were taken out followed by post-annealing at 180 °C (1 h) on a hotplate (Step ④)(More details can be found in the experimental procedures).For SnO2-ETL film, the process only includes the step ①,② and ④. It is noted that the acidic environment (Fig. S1)in the precursor solution makes the following well-known chemical reaction possible, when the KMnO4was added into precursor solution.
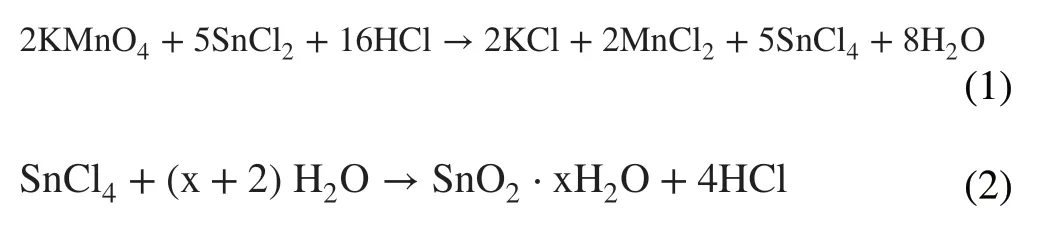
The products of KCl and MnCl2generated from the above chemical reaction can be deposited on the surface of SnO2film in step ③ process.
To identify our hypothesis,X-ray photoelectron spectroscopy (XPS) was employed to characterize the surface property changes of SnO2-ETLs with/without KMnO4additive.The typical peaks of K 2p and Mn 2p orbitals appeared in the SnO2/K-ETL samples confirming that potassium and manganese are successfully deposited on the surface of SnO2layer.The binding energy of 642.0 eV for Mn 2p3/2is assigned to MnCl2in SnO2/K-ETL film [45]. These results are well consistent with our hypothesis in Eq. 1. The two spin-orbit splitting peaks of Sn 3d in the SnO2-ETL in Fig. 2c shift from 495.1 and 486.8 eV to high binding energies of 495.5 and 487.1 eV (SnO2/K-ETL), which indicates more Sn (II)conversion to Sn (IV) [46, 47]. In parallel, the Cl ions are inevitably adsorbed on the top of SnO2film because of the precursor solution containing SnCl2and HCl. However, the increased intensity of Cl 2p in Fig. S2 suggests that large amounts of Cl ions as the products are deposited on the surface in the case of SnO2/K-ETL film due to the formation of KCl and MnCl2, which is consistent with our hypothesis in Eqs. 1 and 2.
To further evaluate the quality of SnO2-ETLs, the spacecharge limited current (SCLC) was conducted to evaluate the carrier mobility in the case of SnO2and SnO2/K-ETLs based on a sandwich structure of FTO/SnO2/Au (More details are found in experimental procedures) [39]. As seen in Fig. S3,the carrier mobility of SnO2is enhanced from SnO2-ETL(1.62 × 10−3cm2V−1S−1) to SnO2/K-ETL (3.91 × 10−3cm2V−1S−1) after KMnO4incorporation.
The electronic structures of SnO2-ETLs were also determined by using the ultraviolet photoemission spectroscopy(UPS) measurement as seen in Fig. 2d. The ionization energy (IE) values of samples can be extracted by the formula of IE = 21.22−Ecutoff+ Eonset[44, 48]. The corresponding values of SnO2-ETL and SnO2/K-ETL films are 8.49 and 8.12 eV, respectively, which suggests the presence of potassium and manganese ions affects the energy level of SnO2films. In combination with the optical bandgap of SnO2films(Fig. S4), the corresponding electron affinity (EA) values of SnO2films with/without KMnO4are 4.12 and 4.53 eV,respectively.

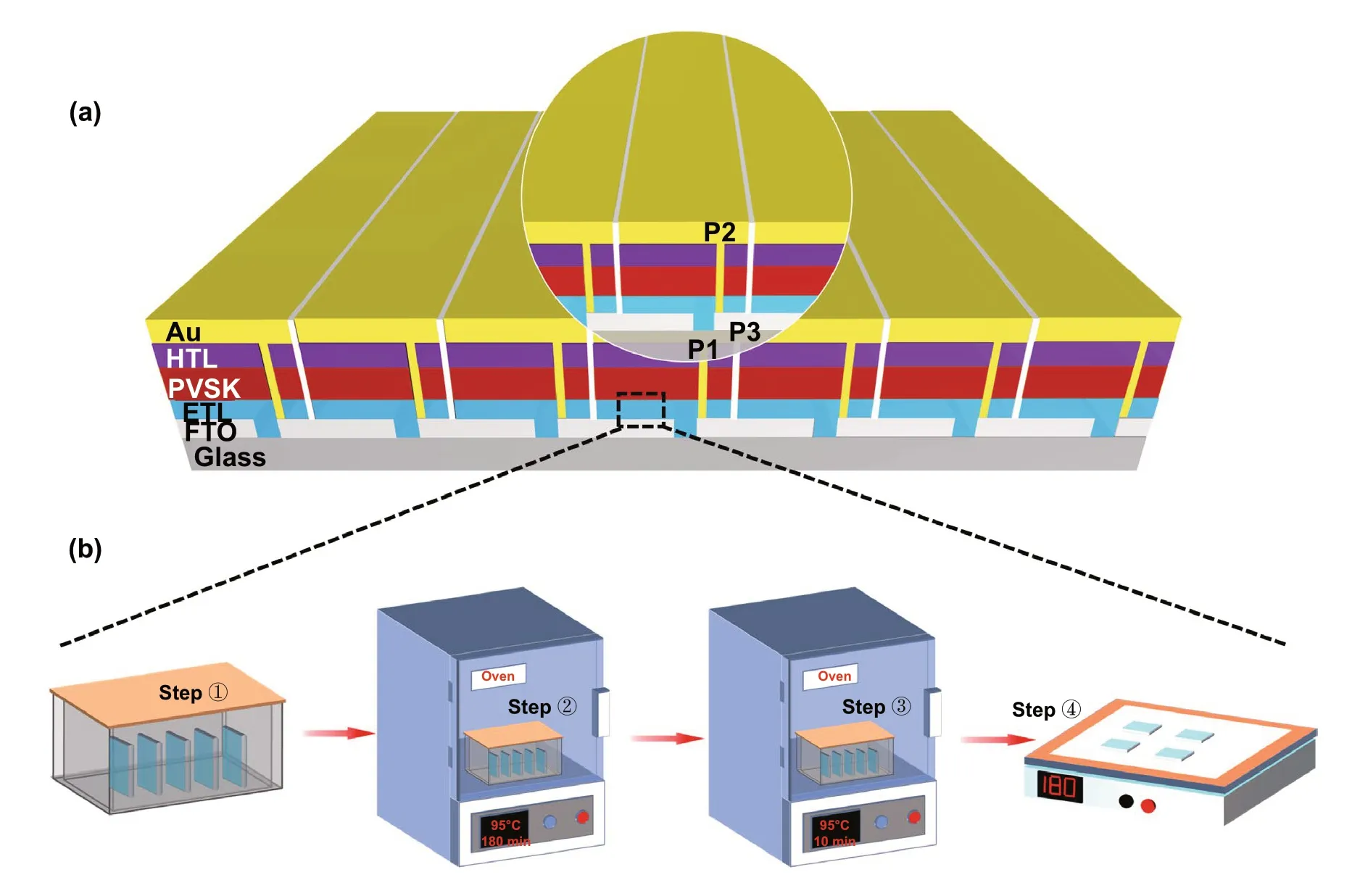
Fig. 1 a Illustration of perovskite solar module architecture (ETL/HTL is electron/hole transport layer. PVSK is the perovskite layer.). b Schematic illustration of the SnO2 films fabricated by chemical bath deposition
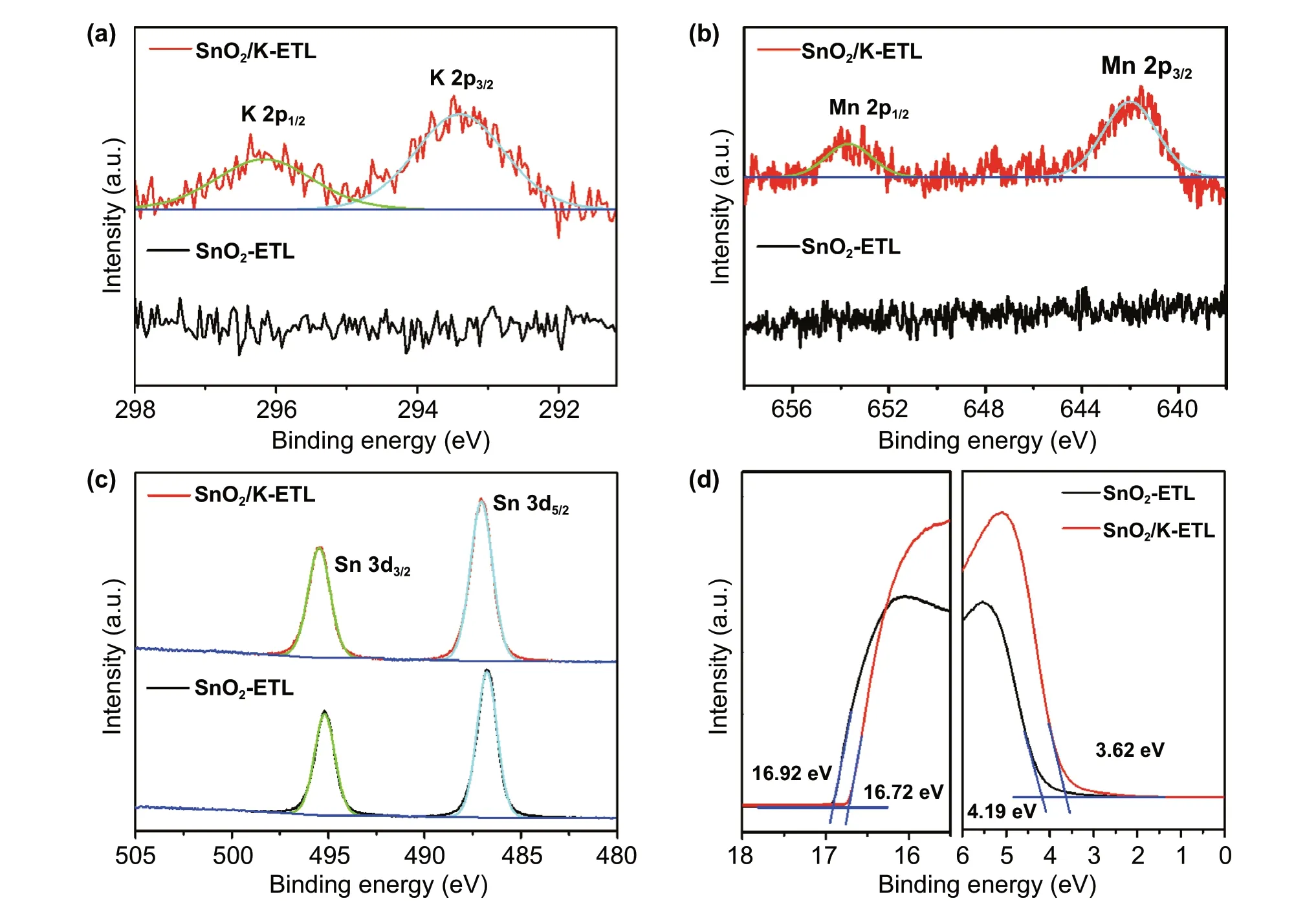
Fig. 2 Characterization of SnO2 and SnO2/K Films. XPS spectra of the SnO2-ETL and SnO2/K-ETL films. a K 2p, b Mn 2p (B) and c Sn 3d. d UPS spectra acquired on the SnO2-ETL and SnO2/K-ETL films
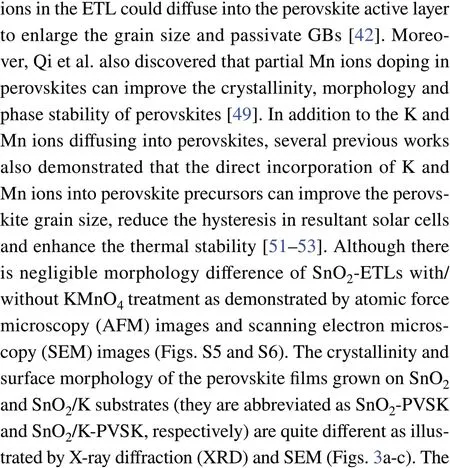
whereVTFLrepresents the onset voltage of the trap filled limit.ε0andεare the constant of vacuum permittivity in free space and the relative dielectric constant of perovskite. e is electric charge.Lis the thickness of the perovskite film (Fig. S11). The reduced trap defects of SnO2/K-PVSK(2.94 × 1015cm−3) compared with SnO2-PVSK(3.76 × 1015cm−3) suggest the presence of K and Mn ions can effectively improve the crystallinity of perovskite film and reduce the defects.
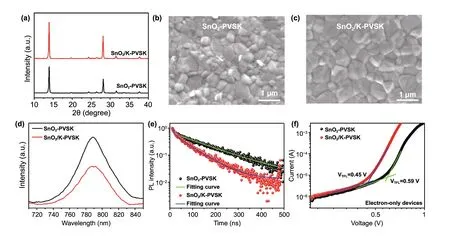
Fig. 3 Characterization of Perovskite Films Based on SnO2 and SnO2/K Substrates. a X-ray diffraction patterns of the perovskite films deposited on SnO2 and SnO2/K substrates. Top view of SEM images of the perovskite films deposited on b SnO2 substrates and c SnO2/K substrates. d PL spectra and e TRPL curves of the perovskite films deposited on SnO2 and SnO2/K substrates. f Dark I-V curves of the perovskite films based on electron-only devices
Figure 4a illustrates the structure of planar PSCs based on SnO2and SnO2/K (they are abbreviated as SnO2-PSC and SnO2/K-PSC, respectively). Typical cross section SEM images of devices in Figs. 4b, c and S12 show uniform and compact perovskite layers. A relatively low PCEs of 20.09%and 17.05% for SnO2-PSC under reverse scan (RS) and forward scan (FS) exhibited a large hysteresis. As the amount of KMnO4is increased, the photovoltaic performance of devices increases as shown in Fig. S13 and Table S3. The champion PCE is achieved at a concentration of 8 mM,beyond which the performance starts to decrease. Moreover, the hysteresis (hysteresis index (HI) = PCERS/PCEFS)[56] is also improved with the introduction of KMnO4. The HI decreases from 1.18 for 0 mM KMnO4to 1.05 for 8 mM KMnO4. Figure 4d shows the photovoltaic performance of the best-performing SnO2-PSCs and SnO2/K-PSCs. Compared to SnO2based device that shows a large hysteresis, the reduced hysteresis after KMnO4treatment is observed with the PCERSof 21.70% and PCEFSof 20.58%. The corresponding integrated Jscfrom EQE are 22.69 and 23.11 mA cm−2,respectively (Fig. 4e), which are in agreement with Jscextracted fromJ − Vcurves, with a discrepancy below 2%.It is worth noting that the enhanced EQE in the wavelength region of 700 ~ 800 nm in the case of the SnO2/K-based PSC is ascribed to the reduced trap density at the surface of the perovskite film and faster carrier extraction at the perovskite/HTL interface by the incorporation of K and Mn ions into the perovskites [44]. Additionally, both SnO2and SnO2/K-based PSCs exhibit stable output (under initial maximum power point (MPP) voltage) with PCE of 18.67%and 20.58%, respectively (Fig. 4f).
Statistical analyses of the photovoltaic parameters reveal that the performance is reproducible with minimal variations and low HI of 1.05 for SnO2/K-PSC compared to SnO2based devices with large hysteresis (HI = 1.40). (Figs. 4g,S14 and Table S4). It is well known that hysteresis is mainly originated from: [52, 57] 1) the ion migration in perovskites;2) charge trapping/de-trapping in deep trap sites originated from defects; 3) ferroelectric polarization of perovskites.The same composition of perovskite used in the PSCs suggests that the main influence comes from issues 1) and 2). Large amounts of defects at ETL/perovskite interface serve as the recombination centers in the devices, which highly increase the nonradiative recombination, leading to a large hysteresis [57]. In parallel, the iodide ions migrating through GBs are accumulated at electrode and interface of ETL/perovskite, which increases the capacitance in the devices, resulting in a large hysteresis simultaneously [57,58]. However, the reduced interfacial trap-assisted recombination at ETL/perovskite by decorating the interface with the KMnO4treatment and the inhibition of the ion migration by potassium doping can effectively eliminate the hysteresis[57, 59]. Our results are consistent with previous findings that the presence of potassium halide layer could reduce the ionic migration, leading to a reduced hysteresis [50, 57]. In addition, the enlarged perovskite grain size and enhanced crystallinity of the perovskite film in the case of SnO2/K sample can also reduce the hysteresis because of less GBs and defects [60, 61].
Besides the hysteresis, we also evaluated the light-intensity-dependent Vocof PSCs under increasing illumination intensities, which reflects the trap-assisted recombination[39]. As seen in Fig. 4h, the SnO2/K-PSC with a small slope value (1.37 kT q−1) compared to the SnO2based device (1.90 kT q−1) indicates less trap-assisted recombination, which agrees well with TRPL results. Furthermore, the electrochemical impedance spectroscopy (EIS)measurements were performed under AM 1.5 G light illumination at a bias voltage of 1.0 V. By fitting the equivalent circuit diagram as seen in Fig. 4i, the closer sheet resistance (Rs) of two devices suggests the same configuration in devices (Table S5). In contrast, the smaller semicircle defined as charge transport resistance (Rct) of SnO2/K-PSC (22.44 Ω) compared with SnO2-PSC (62.57 Ω) reveals the fastest carrier transport, which is ascribed to less trap density and well energy level alignment at the interface of SnO2/perovskite. In addition to the efficiency,the stability of devices is also an important part to evaluate the performance of devices. We first check the phase stability of perovskite that was stored in ambient air in a dry room with a relative humidity of ~ 20% without any encapsulation for 5 months. For both devices, the high intensity of the PbI2peak at 12.6° is observed after storage for 5 months, which indicates that part of perovskite active materials has decomposed to PbI2(Fig. S15). But when compared to the SnO2-PSC, the SnO2/K-PSC shows a substantially stronger perovskite peak at ~ 14°, suggesting that degradation of perovskite in SnO2/K-PSC is slower than that in SnO2-PSC. In addition, we also evaluated the storage stability of the resultant solar cell devices.The average efficiency of SnO2/K-PSC is 18.8%, which is much higher than SnO2-PSC with a PCE of 17.4% (Fig.S16 and Table S6). On the basis of the above analyses, we conclude that SnO2/K-ETL can improve the phase stability of perovskite.
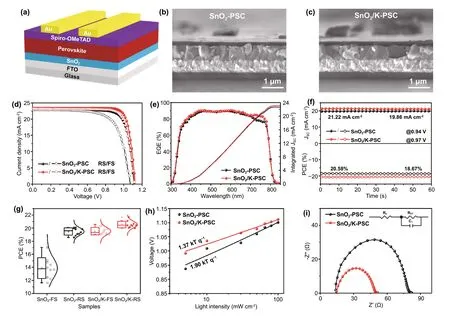
Fig. 4 Characterization and Performance of Lab-Scale Perovskite Solar Cells. a Schematic drawing showing the perovskite solar cell device structure. Cross section SEM images of the PSCs based on b SnO2-ETLs and c SnO2/K-ETLs. d J-V curves, e EQE spectra and f the steady-state power output performance of PSCs based on SnO2 and SnO2/K-ETLs. g Statistical distribution of the solar cell performances. h The dependence of open-circuit voltage on light intensity for the PSCs based on SnO2 and SnO2/K-ETLs. i Nyquist plots of the PSCs based on SnO2 and SnO2/K-ETLs. The inset shows the equivalent circuit diagram
To highlight the benefits of this approach for fabricating SnO2layer, we also demonstrated its feasibility to be up-scalable (Figs. 5c (inset) and S17). Statistical analyses of photovoltaics performance based on 5 × 5 cm2PSMs(10 devices) with a designed area of 22.4 cm2(Fig. S18a)showed an average PCE of 11.97 ± 1.16% and 13.62 ± 0.87%for SnO2-PSMs and SnO2/K-PSMs, respectively (Fig. S19,Tables S7-S8). The champion module PCEs of 15.62% with aVocof 7.59 V, aJscof 2.95 mA cm−2and a FF of 0.699 under reverse scan and 14.25% with aVocof 7.17 V, aJscof 2.98 mA cm−2and a FF of 0.697 under forward scan for SnO2/K-PSM show a reduced hysteresis (HI = 1.10)compared with SnO2-PSM showing a large hysteresis(HI = 1.95) with module PCEs of 14.58% and 7.49% under RS and FS, respectively (Fig. 5a, Table S9). It is worth to note that the active area PCE for the champion devices is up to 17.26% with a geometric fill factor (GFF) = 90.5%(Fig. S18b), which is higher than previous related reports(Table S10). Furthermore, 10 × 10 cm2PSMs with a designed area of 91.8 cm2by employing SnO2/K as ETL were fabricated with an average PCE of 10.06 ± 0.99%(Fig. S20 and Table S11). The best module generates a PCE of 11.80% (Vocof 13.16 V,Jscof 1.38 mA cm−2andFFof 0.648) under reverse scan and 10.17% (Vocof 12.65 V,Jscof 1.40 mA cm−2andFFof 0.574) under forward scan(Fig. 5b). The corresponding active area PCE is over 13.72%with a GFF of 86.0% (Fig. S21).
In addition, the operation stability of 5 × 5 cm2PSM (with UV-curing polymer encapsulation) in Figs. 5c and S22 was evaluated under continuous AM 1.5G light illumination at a fixed bias that is determined by the initial maximum power point fromJ-Vcurves in ambient condition (RH ~ 55%,25 °C). A fast decay in the performance of SnO2-PSM is observed, resulting in a poor operational stability with a T80lifetime (the lifespan of the solar module PCE drops to 80% of its initial performance) of 471 h. In contrast, the SnO2/K-PSM exhibits an excellent operational performance with aT80lifetime of 1006 h. This outstanding stability is ascribed to the better contact of SnO2/perovskite interface and high crystallinity of the perovskite layer. Two decay processes, including a fast exponential decay regime followed by a subsequent slower linear one, are often discussed as commonly observed profiles in operational stability of PSCs [56, 62]. The exponential decay corresponds to a fast burn-in process associated with migration of cation vacancies, which induces the formation of an additional Debye layer at the interface between ETL and perovskite, inhibiting carrier extraction [62, 63]. The reduced burn-in region in the exponential decay profile in the case of SnO2/K-PSM indicates the ETL/perovskite interface is well passivated by KMnO4treatment, reducing the accumulation of ionic defects and vacancies at the interfaces. However, severe iodide ions migration in the SnO2-PSM can accelerate the degradation of perovskite and the formation of recombination centers, which results in a high recombination and efficiency loss, leading to a fast decay and a poor stability [57, 58, 64]. Moreover, Mn doping also improves the stability of perovskite in the case of SnO2/K-PSMs in the permanent degradation regime (linear decay). The volumetric ratio between BX6(B= Pb2+,X= I, Br) octahedra and cations will determine the phase stability of perovskites [49,65]. The presence of Mn2+ion has a smaller radius of 0.97 Å in comparison with Pb2+ion (1.33 Å), which substantially induces the contraction of the BX6octahedral volume, leading to a better holding of the BX6octahedra by the mixed cation in the perovskite structure[49]. This volume contraction would possibly improve the stability of mixed cation,which enhances the operational stability of SnO2/K-PSM.
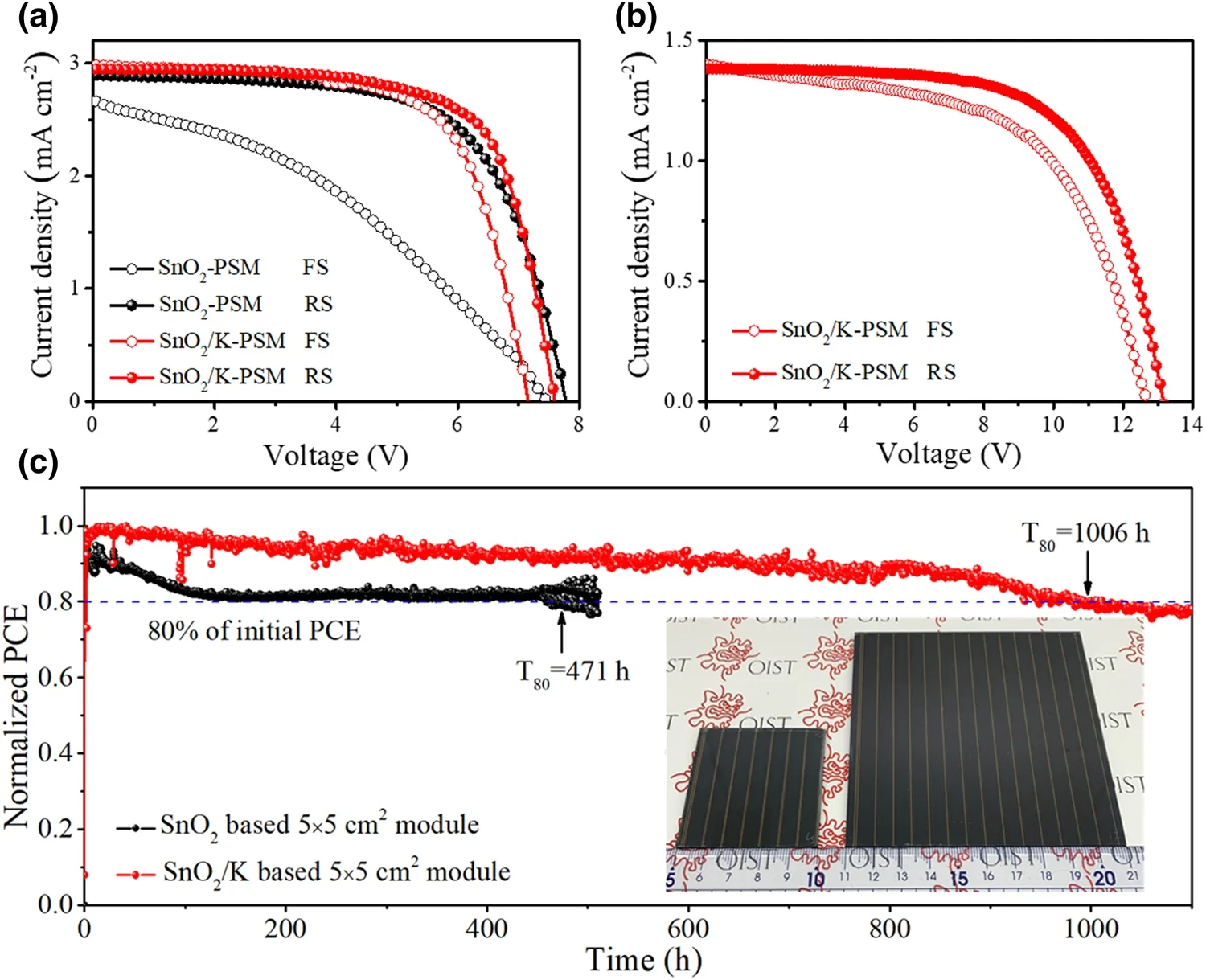
Fig. 5 Performance of Perovskite Solar Cell Modules. a J-V curves of 5 × 5 cm2 PSMs based on SnO2 with/without KMnO4 passivation under forward and reverse scan. b J-V curves of 10 × 10 cm2 PSMs based on SnO2/K under forward and reverse scan. c Operational stability of 5 × 5 cm2 PSMs based on SnO2/K with encapsulation under a steady applied voltage and constant illumination (AM 1.5G, 100 mW cm−2), inset:photographs of 5 × 5 cm2 and 10 × 10 cm2 PSMs
4 Conclusion
In summary, we developed a facile CBD method to prepare the SnO2layer in large area by introducing KMnO4as an additive. High oxidant property of KMnO4not only promotes the formation of Sn (IV), but also provides additional potassium and manganese ions. The presence of potassium can effectively reduce the hysteresis and enlarge perovskite grains. Meanwhile, manganese doping not only improves the crystallinity of perovskite grains, but also enhances the phase stability of perovskites. A champion PCE of 21.70% with a reduced hysteresis was obtained for labscale devices by this multifunctional interface engineering strategy. Importantly, this precursor solution passivation in CBD method was demonstrated as a compatible technique for upscalable fabrication of solar modules. The best module efficiency of 15.62% (active area PCE = 17.26%) and 11.80% (active area PCE = 13.72%) were achieved for 5 × 5 and 10 × 10 cm2PSMs, respectively. The continuous operation stability of the SnO2/K-based PSM showed a T80operation lifetime exceeding 1000 h in ambient condition (relative humidity 55%, 25 °C) for the encapsulated 5 × 5 cm2SnO2/K-based PSM.
AcknowledgementsThis work was supported by funding from the Energy Materials and Surface Sciences Unit of the Okinawa Institute of Science and Technology Graduate University, the OIST R&D Cluster Research Program, the OIST Proof of Concept (POC)Program, and JST A-STEP Grant Number JPMJTM20HS, Japan.We thank the OIST Micro/Nanofabrication Section and Imaging Section for the support.
Author ContributionsY.B.Q. supervised the project. Y.B.Q. and G.T. conceived the idea and designed the experiments. G.T. fabricated the perovskite solar cells and solar modules and performed the characterizations, except for those mentioned below. L.K.O.performed UPS, XPS and SIMS measurements on SnO2, perovskite films and helped encapsulate PSMs. Y.L. helped perform the PL and TRPL measurements on perovskite films. H.Z. helped conduct the EIS measurements on the solar cell devices. T.B. helped deposit SnO2films. All authors contributed to writing the paper.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,adaptation, distribution and reproduction in any medium or format,as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http:// creat iveco mmons. org/ licen ses/ by/4. 0/.
Supplementary InformationThe online version contains supplementary material available at https:// doi. org/ 10. 1007/s40820- 021- 00675-7.
杂志排行
Nano-Micro Letters的其它文章
- Boron Nanosheet-Supported Rh Catalysts for Hydrogen Evolution: A New Territory for the Strong Metal-Support Interaction Effect
- Porous Carbon Architecture Assembled by Cross-Linked Carbon Leaves with Implanted Atomic Cobalt for High-Performance Li-S Batteries
- The Main Progress of Perovskite Solar Cells in 2020-2021
- Interface Engineered Microcellular Magnetic Conductive Polyurethane Nanocomposite Foams for Electromagnetic Interference Shielding
- A Review on Metal- and Metal Oxide-Based Nanozymes: Properties, Mechanisms,and Applications
- Flexible and Waterproof 2D/1D/0D Construction of MXene-Based Nanocomposites for Electromagnetic Wave Absorption, EMI Shielding, and Photothermal Conversion
