Environmental Drivers of Temporal and Spatial Fluctuations of Mesozooplankton Community in Daya Bay,Northern South China Sea
2021-09-01LIKaizhiMAJieHUANGLiangminTANYehuiandSONGXingyu
LI Kaizhi, MA Jie, HUANG Liangmin, TAN Yehui,4), and SONG Xingyu,5),*
1) Key Laboratory of Tropical Marine Bio-Resources and Ecology (LMB), Guangdong Provincial Key Laboratory of Applied Marine Biology (LMB), South China Sea Institute of Oceanology, Chinese Academy of Sciences,Guangzhou 510301, China
2) Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), Guangzhou 511458, China
3) School of Life Sciences, East China Normal University, Shanghai 200241, China
4) University of Chinese Academy of Sciences, Beijing 100049, China
5) Nansha Marine Ecological and Environmental Research Station, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou 510301, China
Abstract The response of zooplankton to the ecological environment in Daya Bay is unclear under the influence of both climate changes and anthropogenic activities on a seasonal to inter-annual scale. Based on monthly surveys and historical data, we found the zooplankton community had changed temporally and spatially. A total of 134 species was recorded during the study, and copepods dominated numerically in terms of diversity and abundance. Both copepods and cladocerans were the main contributors to zooplankton abundance. The community structure of zooplankton was temporally classified into the warm and cold groups, and spatially into the three groups located in the marine cage-culture area (MCCA), the outflow of nuclear power plants (ONPP) and unpolluted waters(UPW). The zooplankton was characterized by low biomass (dry weight), high diversity and abundance in the warm period in contrast to that in the cold period. Compared with the other two groups, the MCCA group of zooplankton showed high abundance, low diversity and biomass. Variations in dominant species were closely related to temperature, salinity and chlorophyll a concentration.Species diversity and dry weight decreased in comparison with 30 years ago, while zooplankton abundance increased. The seasonal variation in zooplankton was affected mainly by temperature that was controlled by monsoon, while the spatial difference in the community structure was probably due to eutrophication in the MCCA and thermal water discharge from ONPP. The zooplankton community is undergoing great changes with the tendency of miniaturization and gelatinization in recent 30 years in Daya Bay.
Key words zooplankton; monsoon; eutrophication; thermal water discharge; Daya Bay; South China Sea
1 Introduction
Coastal zones are of importance to the land-sea interface areas, and are quite sensitive to the perturbation by the intensive human activities and climate change. The bay is a typical coastal region. Due to its special natural environment and economic value, the bay is more affected by human activities and more complex and fragile than open seas. Bays are subject to various types of anthropogenic activities, such as domestic sewage, industrial waste, harbors, aquaculture and thermal water discharge. Nutrient input caused by human activities is a key factor affecting the ecological environment of the embayment (Jickells, 1998). Increased inputs of nutrients over the last decades have resulted in eutrophication in bays around the world (Howarthet al., 2002; Boesch,2009; Ferreiraet al., 2011; Cloernet al., 2020). These nutrients are taken up by phytoplankton, which often results in harmful algal blooms in highly eutrophic waters(Masó and Garcés, 2006; Ferreiraet al., 2011). The species and size composition of the planktonic community has been altered with the proportion of small mesozooplankton and jellyfish increasing in heavily eutrophic bays (Uye, 1994; Purcellet al., 2007; Sunet al., 2011).An increase in the abundance of certain zooplankton taxa and a decrease in zooplankton community diversity have been found in eutrophicated semi-closed bays (Thompsonet al., 2007; Biancalana and Torres, 2011). The trophic transfer efficiency from nutrients to zooplankton and to planktivorous fish may be reduced or hindered in eutrophic waters (Schmokeret al., 2016). Another environmental issue in bays is the potential thermal stress to aquatic organisms living in the surrounding waters associated with the operation of nuclear power plants (Shiahet al., 2006). The plankton community may be smaller in size to tolerate the thermal addition (Jianget al., 2009).
Daya Bay is a semi-enclosed bay on the continental shelf of the northern South China Sea. It is characterized by a mild subtropical climate with the northeast monsoon prevailing from October to April and the southwest monsoon from May to September (Xu, 1989). Daya Bay and the surrounding area have been considered as a natural nursery, feeding and breeding ground for 150 more fishery species (Wanget al., 2010), and also listed as a key economic development area for the development of various types of industries along the shore (Wanget al., 2011).In the last 30 years, the nutrient structure has become eutrophic mainly as a result of the rapid expansion of marine culture and waste drainage from land (Wuet al.,2010, 2017). Two nuclear power stations, the Daya Bay Nuclear Power Plant (DNPP) and the Ling’ao Nuclear Power Plant (LNPP), have been operating since 1994 and 2003, respectively. The thermal effluent has substantially influenced the ecosystem in Daya Bay (Tanget al., 2003).Changes in the ecological environment have resulted in changes in phytoplankton. The growth of small diatoms has accelerated and these have become predominant in the enriched aquaculture areas in the west waters of the Daya Bay (Wanget al., 2009; Wuet al., 2017), while a marked shift from diatoms to dinoflagellates has occurred in the waters adjacent to the DNPP and LNPP due to the power plant thermal discharge when the temperature increases to a threshold level of 35℃ (Liet al., 2011). The effects of human activities on phytoplankton communities,caused mainly by eutrophication and thermal water discharge in Daya Bay, result in decreased diversity and a miniaturization trend (Wanget al., 2009; Liet al., 2011;Wuet al., 2017).
Zooplankton dynamics are influenced mainly by marine environmental factors, and their variations can reflect ambient and even global environmental changes (Edwards and Richardson, 2004). The zooplankton has been surveyed since the 1980s in Daya Bay, as a background investigation of the environment and resources (Xu,1989). Zooplankton showed high species diversity and their distribution was closely related to the water masses and currents before the development of the nuclear power plants (Lianet al., 1990). Seasonal changes in the zooplankton community featured increasing medusa abundance and wet biomass in the marine culture region of Daya Bay (Lianet al., 2011; Duet al., 2013). Some previous studies focused on the short-term or seasonal variation in zooplankton (Liet al., 2014), but the annual or inter-annual variations of mesozooplankton in response to high environmental perturbations remain poorly documented.
The objective of the present study is to attempt to understand the annual variations in the zooplankton community and to evaluate their temporal and spatial fluctuations associated with natural environmental variables and anthropogenic perturbations based on monthly surveys in Daya Bay. We hypothesized that the zooplankton community has changed due to the changing environment over the last 30 years. To test this hypothesis, species diversity and abundance found in this study were compared with the results surveyed in 1986 - 1987 based on the same sampling and analysis methods. It was expected that this study would provide insight into the processes and underlying mechanisms of environmental drivers affecting planktonic community structure and function in the embayment.
2 Materials and Methods
2.1 Study Area
Daya Bay with an area of 600 km2, is located between 114°30´ and 114°50´E and 22°30´ and 22°50´N (Fig.1).Dapeng’ao Bay in the southwest section and Aotou Bay in the northwest section are busy sea areas which are greatly affected by human activities. DNPP and LNPP are located in the western section. No major rivers discharge into the bay, and most of its water originates from the South China Sea. Currents in the bay are mostly controlled by tides (Xu, 1989). The surface waters remain for approximately 3.2 d, and the water exchange rate is slow,which takes 15 d to update once. A wide range of water temperature changes occur due to the influence of the Asia East monsoon in Daya Bay, and its salinity is lower than that of the outer seas affected by coastal currents all year round. Its water depth ranges from 6 to 16 m, with an average of 10 m. Ecological monitoring has been carried out at 12 fixed stations (solid circles in Fig.1) by scientists from the Marine Biology Research Station at Daya Bay, Chinese Academy of Sciences during early January,April, July and October since the 1990s, including the temperature, salinity, chlorophylla(Chla), nutrients,phytoplankton, zooplankton and benthos (Wanget al.,2008).
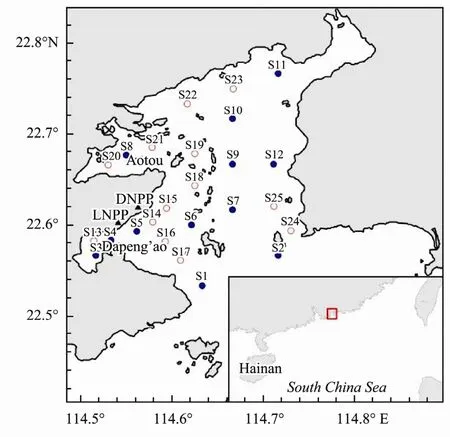
Fig.1 Location of Daya Bay in the northern South China Sea (square shown in the large map), and the sampling sites in Daya Bay from May 2013 to April 2014.
2.2 Field Sampling
Sampling was conducted monthly from May 2013 to April 2014 at 25 stations (Fig.1). Zooplankton collections were obtained by vertical hauls of a plankton net (mouth diameter: 30 cm, mesh size: 160 μm) twice from 1 m above the bottom to the surface at each station. One sample was for species identification and the other for dry weight measurement. Samples for identification were immediately preserved in 5% formaldehyde. Samples for dry weight were filtered onto a mesh (20 μm), and then stored in liquid nitrogen.
Temperature and salinity were measuredin situusing a YSI 6600 multi-parameter water quality monitor. For the determination of surface chlorophylla(Chla) concentration, a 200 mL water sample was gently passed through a 0.45 μm cellulose filter and extracted with acetone (90%v/v) for 24 h at 4℃ in darkness. The Chlaconcentration(unit: mg m-3) was then determined with a Turner design 10 AU fluorometer before and after acidification (Parsons,1984).
2.3 Laboratory Processing
Dry weight was measured with an electronic balance after oven-drying (24 h, 60℃) to calculate biomass. The difference in mass of the GF/F film before and after weighing was used to obtain the dry weight of zooplankton. The filtered water volume was determined by the rope length multiplied by the mouth size (unit: m3). All specimens were identified to species level wherever possible under a stereomicroscope (Leica Ml65C) based on currently available taxonomic information (Chen and Zhang, 1965; Chenet al., 1974; Zhenget al., 1984). The counts were converted to abundances expressed as the number of individuals per cubic meter (ind m-3).
2.4 Data Analyses
A species was defined as dominant whenY, the dominance indicator, was ≥0.02 (Xu and Chen, 1989).Ywas calculated as follows:Y=(ni/N)fi, whereiis the sample number,niis theith species abundance,fiis the frequency of occurrence of speciesi, andNis the total abundance of all zooplankton species. The Shannon-Wiener diversity index (H') was used to calculate the species diversity(Shannon and Weaver, 1963), and the Pielou evenness index (J) was employed to measure the relative abundances of species in the community (Omori and Ikeda,1984).
The hierarchical cluster and multidimensional scaling(MDS) analyses of similarity among the sampling stations or months were computed on the basis of the Bray-Curtis similarity index and log10(x+1)-transformed data from species followed by their abundance ranking from high to low, and their contribution to total abundance above 90%,using Primer software (v.6.0, Primer-E, Plymouth, UK)(Clarke and Gorley, 2006). A similarity matrix was constructed using the Bray-Curtis index. Non-metric multidimensional scaling (nMDS) was also applied to the similarity matrices to determine the similarity of sampling sites or seasons with respect to zooplankton composition.A one-way ANOVA (least significant difference or LSD)was used to analyze the differences in physical and biological parameters among groups. Pearson’s correlation analysis was used to examine possible relationships between sea temperature, salinity and Chlawith zooplankton abundance. The tests were deemed significant whenP< 0.05.
2.5 Historical Data
A one-year comprehensive survey was carried out from December 1986 to December 1987 by the Third Institute of Oceanography State Oceanic Administration (TIOSOA)before the DNPP and LNPP were in operation and was used as the background information on Daya Bay, including marine hydrology, chemistry and biology (TIOSOA,1990). The methods used to measure environmental factors (temperature, salinity and Chla) and zooplankton sampling and treatment were identical between 1986-987 and 2013-2014. Zooplankton was sampled by vertical hauls of a plankton net (mesh size: 160 μm). Historical data in comparison with the current survey were from the‘collections of papers on marine ecology in the Daya Bay’ published in 1990 (TIOSOA, 1990).
3 Results
3.1 Environmental Factors
Monthly variations in temperature, salinity and Chlaconcentration were found in Daya Bay (Fig.2). The surface temperature increased from 15.71℃ in February to 33.65℃ in September, while salinity showed the lowest values in September and the highest in February. Decreasing temperature and increasing salinity were observed from October to March, and the opposite pattern was found from April to September. The annual average Chlaconcentration was (2.05 ± 2.00) mg m-3with a wide range from 0.73 mg m-3in December to 4.33 mg m-3in May. Peak concentrations of Chlawere found in April,May, June and September (Fig.2).
The spatial distribution of temperature, salinity and Chlavaried seasonally (Fig.3). Temperature was higher at the surface layer than at the bottom layer and higher in the inner region than in the outer region of Daya Bay during the observation period. In contrast, high salinity was found at the bottom layer and in the outer region of the bay. High temperature was recorded around the thermal effluent of the nuclear power plants especially in the spring, autumn and winter (Figs.3a-d). An obvious water intrusion with low temperature and high salinity in the bottom bay was found in summer and autumn (Figs.3b, c,f, g). The concentration of Chlawas high in the western sections of Depeng’ao Bay and Aotou Bay during spring and summer, while high concentrations were found in the eastern sections of the bay during autumn and winter(Figs.3i-l).

Fig.2 Monthly variations in water temperature, salinity and chlorophyll a concentration at the surface layer in Daya Bay from May 2013 to April 2014. Values represent the average of 25 sampling stations. Bars denote standard errors.
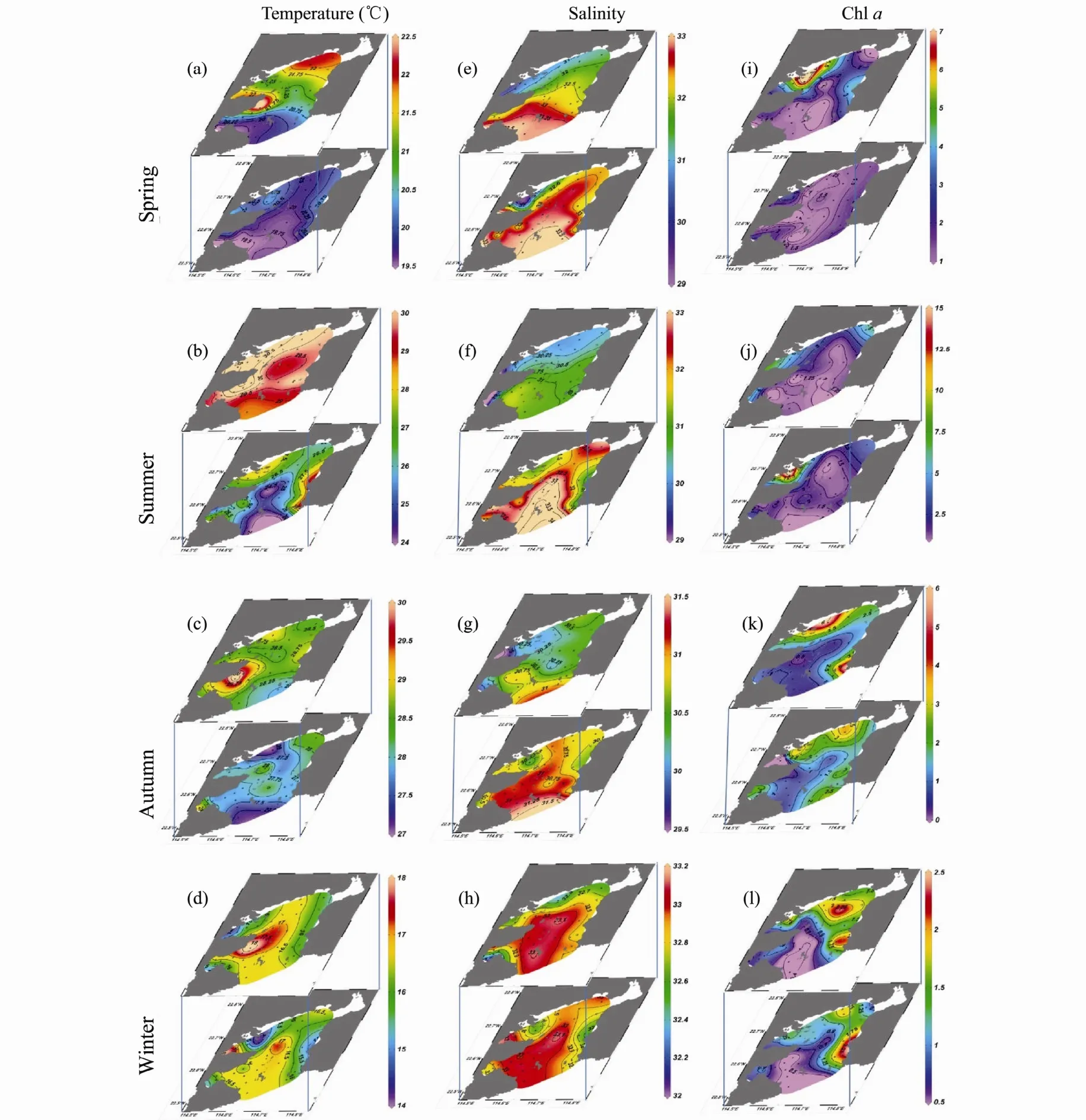
Fig.3 Spatial distribution of water temperature (℃), salinity and chlorophyll a concentration (mg m-3) at the surface and bottom layers in Daya Bay during spring (March, April, May), summer (June, July, August), autumn (September, October,November) and winter (December, January, February).
3.2 Biomass and Abundance
A wide monthly variation in zooplankton biomass (dry weight) and abundance was observed (Fig.4). The annual average biomass of zooplankton was 41.1 ± 30.9 mg m-3,with a maximum of 86.3 mg m-3in April and the minimum value of 8.2 mg m-3in March. High biomass of >60 mg m-3was recorded in August, January and February.Zooplankton biomass was below 40 mg m-3in other months. Zooplankton abundance fluctuated between 628.4 ind m-3in March and 34911.7 ind m-3in August. The abundance of zooplankton decreased from August to March in the following year. The abundance of zooplankton was less than 10000 ind m-3from November to March. Zooplankton biomass was not correlated with abundance during the study period (P> 0.05), because the dry weight biomass of zooplankton depends on the composition of species and their water content to some extent.

Fig.4 Monthly average of biomass (dry weight, mg m-3) and abundance (ind m-3) of mesozooplankton in Daya Bay from May 2013 to April 2014.
3.3 Community Structure
3.3.1 Species diversity
A total of 134 taxa of zooplankton (including 14 groups of planktonic larvae) were identified during the survey period, which mainly included copepods, Hydromedusae, appendicularians and siphonophores accounted for 47.10%, 12.69%, 5.9% and 5.22%, respectively, of total species richness. In other groups, their species richness was less than four (Table 1). The number of zooplankton species varied monthly, with a maximum in August and a minimum in April (Fig.5a).
Copepods were the most dominant group in terms of both species richness and numerical abundance contributing to 67.21% of annual zooplankton abundance, followed by cladocerans (Table 1). Although only three species of cladocerans (Penilia avirostris,Pseudevadne tergestinaandPodon polyphemoides) were observed,their average abundance reached 3918.8 ± 5449.7 ind m-3accounting for 25.03% of annual zooplankton abundance during 2013-2014. Copepods and cladocerans together contributed 80% to 98% to mesozooplankton abundance(Fig.5b). The percentage of cladoceran abundance reached 86.66% of the total abundance, far exceeding the proportion of copepods at 11.71% in April 2014. Cladocerans were also abundant from May to September. Appendicularians, planktonic larvae and chaetognaths were also important contributors to mesozooplankton abundance.

Table 1 Species richness, abundance (ind m-3) and percentage (%) in different groups of mesozooplankton

Fig.5 Monthly variation in the species number of zooplankton taxonomic groups (a) and their percentage of abundance (b)in Daya Bay from May 2013 to April 2014.
Results of the hierarchical cluster analyses revealed a seasonal pattern in the presence of the two groups among the sampling months at the similarity level of 60% (Fig.6). The zooplankton community sampled during the cold period (December to April of the following year) differed significantly from that during the warm period (2D stress= 0.09). The environmental factors during the warm period were characterized by higher temperature and Chla,and lower salinity in comparison with the cold period(Table 2). Zooplankton was more diverse during the warm period than in the cold period. Although high abundance of zooplankton was observed in the warm period, its biomass was lower than that in the cold period(Table 2).
Multivariate analysis of the data revealed spatial patterns across stations within the data where three station groups were significant with a Bray-Curtis similarity of 80%-85% (Fig.7a). Two-dimensional ordination of the MDS space confirmed the appropriateness of these groupings (Fig.7b), which was 0.14 in 2 dimensions. Spatially, the following major clusters were present: 1) group ONPP was around the outflow of the nuclear power plants, 2) group MCCA was located mainly in the marine cage-culture areas and coastal waters in the northern bay,3) group UPW was situated in the unpolluted waters of the southeast section of Daya Bay (Fig.7c).
Environmental factors and zooplankton abundance differed among stations at groups ONPP, MCCA and UWP:high Chlaconcentration and zooplankton abundance prevailed in MCCA, while high temperatures and low abundance occurred at ONPP (Table 2). The average zooplankton biomass was higher in group UPW than in groups ONPP and MCCA.

Fig.6 Results of the cluster and multidimensional scaling analyses of the zooplankton community for the monthly surveyed period in Daya Bay from May 2013 to April 2014. Black lines connect months that are statistically distinct groups (P < 0.05). Red lines connect samples that are not statistically unique (P > 0.05). Contours represent the 60% similarity level among stations.

Table 2 Comparison of environmental factors and zooplankton among the cluster groups based on season or region analyses
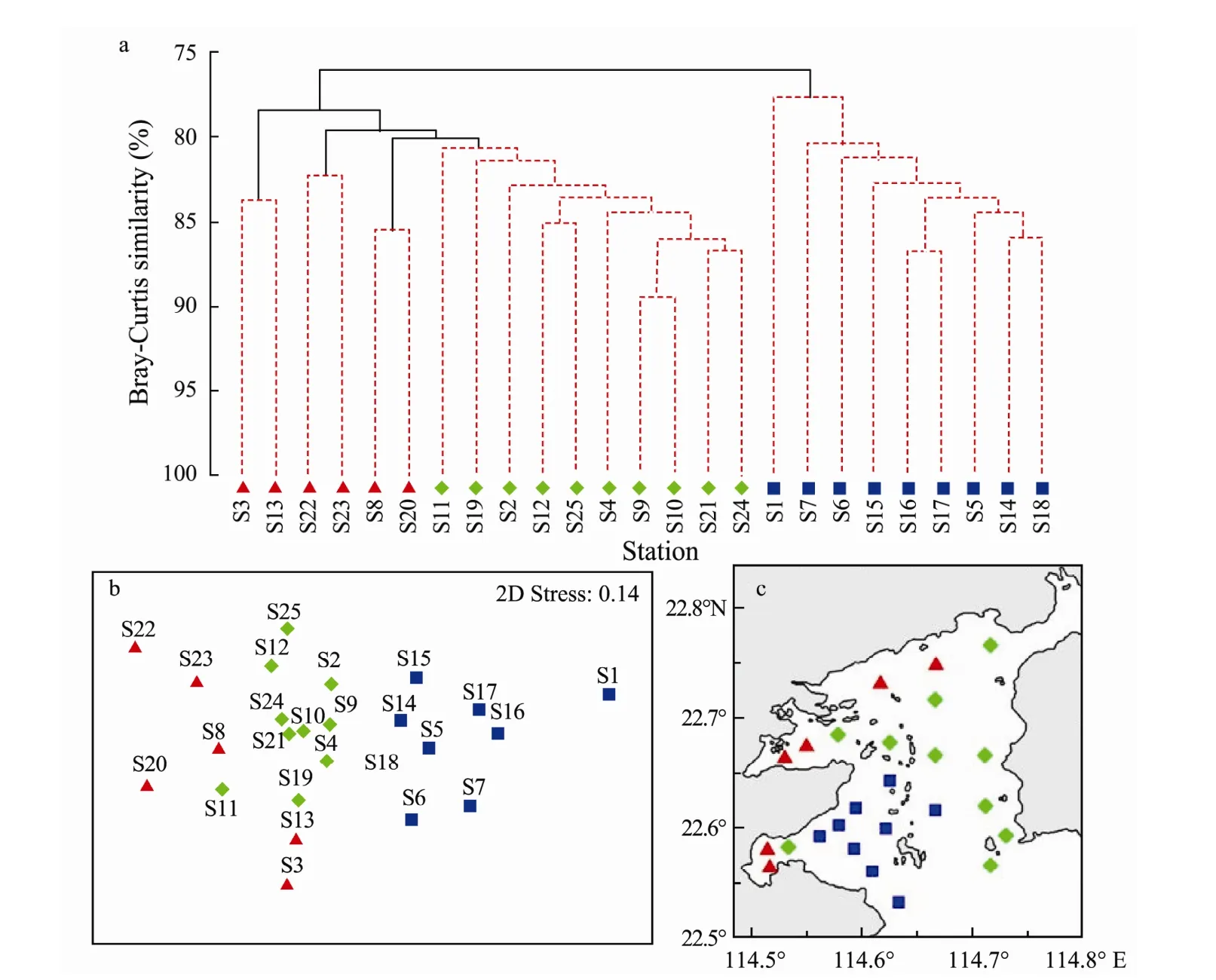
Fig.7 (a) Station similarity as determined by hierarchical clustering of zooplankton abundance in Daya Bay from May 2013 to April 2014. Red lines connect samples that are not statistically unique (P > 0.05). (b) Multidimensional scaling of zooplankton community abundances. (c) Spatial distribution of zooplankton clusters in Daya Bay. ■ represents the ONPP group; ▲ represents the MCCA group; ◆ represents the UPW group.
3.3.3 Dominant species
The abundance of zooplankton was attributed mainly to cladocerans, copepods, chaetognaths, appendicularians,salps and planktonic larvae (Table 1). The dominant species consisted mainly of 12 species includingPenilia avirostris,Pseudevadne tergestina,Acartia erythraea,Parvocalanus crassirostris,Paracalanus parvusandOikopleura rufescens(Figs.8a-i). The abundance of these 12 dominant species contributed 92% of the total zooplankton abundance.
The distribution patterns of dominant species varied seasonally and regionally. The seasonal pattern ofPenilia avirostris,Acartia erythraea,Parvocalanus crassirostris,Sagitta delicata,Oikopleura rufescensandDololium denticulatumwas more abundant in the warm period than in the cold period (Figs.8a, c, f, h, j, k). However, the abundance ofCalanus sinicusandParacalanus parvuswas higher in the cold period than in the warm period (Figs.8d,e).Sagitta enflatawas distributed widely during the surveyed period, and its abundance was mainly near the thermal drainage of nuclear power plants and the less polluted waters of the south-west section of Daya Bay(Fig.8i). The regional distribution of another speciesTemora turbinatawas the same as that ofSagitta enflatawith high abundance in the groups ONPP and UPW (Fig.8g). However, high abundance of Cirripedia larvae was observed in the region of marine aquaculture (Fig.8l). The abundance ofPseudevadne tergestinapeaked in April with a different distribution pattern to those of other species (Fig.8b).

Fig.8 Seasonal and regional variations in abundance of dominant species in Daya Bay from May 2013 to April 2014. Note the difference in abundance scales between panels (a)-(i). Dotted line represents the dividing line between the warm and cold periods based on the results from Fig.6.

Table 3 Correlation coefficient (R) between the abundance of 12 dominant species and the environmental factors assessed
3.4 Relationship Between the Zooplankton Community and Environmental Parameters
The characteristics of the zooplankton community were determined mainly by dominant species in terms of spatial-temporal variation. Changes in dominant species were closely related to temperature, salinity and Chlaconcentration (Table 3). There was a significant positive correlation between temperature, Chlaconcentration and the abundance of those species which occurred in the warm periods such asPenilia avirostris,Acartia erythraea,Parvocalanus crassirostrisandSagitta delicata. A negative correlation between their abundance and salinity was found. However, the abundance ofCalanus sinicusandParacalanus parvuswas negatively related to temperature and positively related to salinity (Table 3), which were abundant during the cold period (Figs.8d, e).
3.5 Comparison of Environmental Factors and Zooplankton Between 2013-2014 and 1986-1987
During these two periods, the Chlaconcentration increased. The concentration in 2013-2014 was 1.2 times that in 1986-1987 (Table 4). No significant variations in temperature and salinity were observed. The number of species and the dry weight of mesozooplankton were reduced to be almost 50% in 2013-2014, but the zooplankton abundance increased 2.5-fold after 30 years. The abundance of copepods and cladocerans increased accordingly.
And the Porcelain Maiden agreed to follow him, and after having given up her clothes, the young man bought a small horse for her, which went like the wind
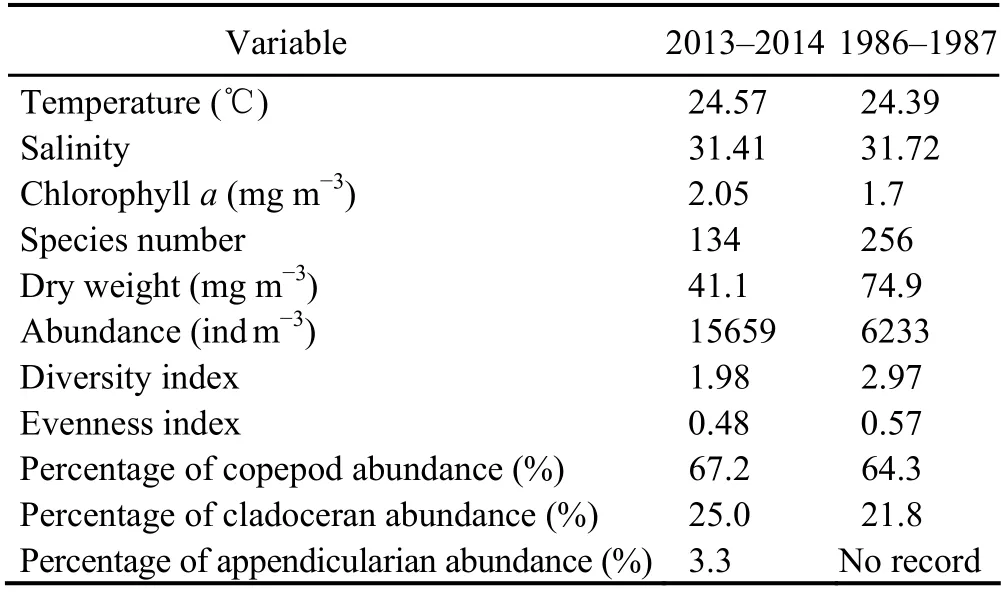
Table 4 Comparison of environmental parameters and species diversity of mesozooplankton between 2013-2014 and 1986-1987
4 Discussion
4.1 Spatio-Temporal Variation in the Dominant Species of Zooplankton
A plankton trawl is the main sampling method for marine zooplankton, and the application of different sized nets may affect the composition and abundance of the zooplankton species. For example, large-sized nets usually ignore the importance of small- and medium-sized zooplankton in the ecosystem (Panseraet al., 2014). It is known that investigations on zooplankton in Daya Bay started in the 1980s (Xu, 1989), however, many sampling events of zooplankton were conducted using 500 μm nets.As a result, the dominant species were large- and medium-sized zooplankton, includingSubeucalanus subcrassus,Canthocalanus pauper,Acartia erythraea,Calanus sinicus,Diphyes chamissonis,Sagitta enflata, andDoliolum denticulatum(Lianet al., 2011; Duet al., 2013).In this study, a planktonic net with 160 μm mesh size was used for zooplankton sampling, and our results highlighted the dominant roles of small- and medium-sized zooplankton in Daya Bay, such asParvocalanus crassirostris,Paracalanus parvus,Temora turbinata,Penilia avirostris,Pseudevadne tergestinaandOikopleura rufescens(Fig.8). In addition, an obvious seasonal variation was detected for the dominant species of zooplankton sampled using either 500 μm or 160 μm nets, consistent with previous studies (Lianet al., 2011; Duet al., 2013).
Zooplankton in Daya Bay displayed distinct temporal and spatial variations in community structure, which were mainly determined by the temporal and spatial variation of the dominant species. These dominant species contributed greatly to the total abundance of zooplankton, and their temporal and spatial variation may be driven by local environmental factors or their own ecological traits.The speciesParvocalanus crassirostrisis highly abundant in tropical and subtropical coastal waters and is smaller in size than other calanoid copepods. The mean annual abundance ofP. crassirostriswas (5702 ± 6352)ind m-3, accounting for over 36% of total zooplankton abundance. However,P. crassirostriswas largely understudied in previous studies on zooplankton in Daya Bay,and this species was only reported as one of the dominant species of zooplankton in an early study by Lianet al.(1990).P. crassirostrisusually occurred in the warm period, and its abundance in the warm period was ten times higher than that in the cold period. Regionally, high abundance ofP. crassirostrismainly occurred in the MCCA,followed by the UPW which is less affected by human activities and the DNPP which is affected by the thermal water discharge from nuclear power plants (Fig.8f). The optimal growth temperature forP. crassirostrisis 26℃,with a wide range of salinity (Alajmiet al., 2015). This species prefers to feed on microscopic algae (Alajmi and Zeng, 2015). The rapid expansion of mariculture was thought to be the most important source of increasing nutrients in Dapeng’ao Bay and Aotou Bay (Huanget al.,2005). The results indicate that the proportion of small-size phytoplankton (< 20 μm) is increasing, induced mainly by nutrient enrichment in Daya Bay based on in situ data and laboratory experiments (Xieet al., 2019).Hence, in Daya Bay, the amount ofP. crassirostrisin the warm period was much higher than that in the cold period,probably due to the higher mean water temperature (i.e.,28℃) which was beneficial for the species growth and reproduction (Table 2). Regionally, the higher abundance ofP. crassirostrisin the cage-culture area than in the other areas may be due to the fact that the cage-culture area contains abundant food resources whichP. crassirostrisprefers to feed on.P. crassirostrishas been highly recommended for use as live food in aquaculture due to its small size, fast generation time and is an ideal food for fish larva (Alajmi and Zeng, 2015). During the southwest monsoon, the favorable temperature, the abundant food sources in the cage-culture area and the short generation span of small copepods have jointly promotedP. crassirostristo become the dominant zooplankton species in Daya Bay.
Oikopleura rufescensis an overlooked species in studies of zooplankton in Daya Bay, and there are no reports or data on this species to date (Xu, 1989; Lianet al., 1990;Lianet al., 2011; Duet al., 2013; Liet al., 2014). Appendicularians are among the most numerous pan-global ‘gelatinous’ zooplankton and produce filter-feeding houses.They play an important trophic role as they are able to feed on particles as small as 0.2 mm (Flood and Deibel,1998), generating a shortcut in the marine food web by directly transferring energy from nanoparticles to larger predators (Flood and Deibel, 1998; Purcellet al., 2005).Previous studies found that appendicularian abundance was positively correlated with temperature and nutrient levels (Troedssonet al., 2013). The annual variation inO.rufescensin Daya Bay showed that this species was 20 times more abundant in the warm period than in the cold period. The average abundance ofO. rufescensin the cage-culture area was 8625 ind m-3, much higher than that in the UPW and DNPP areas (Fig.8j). The abundance ofO. rufescenswas significantly and positively correlated with temperature and Chlaconcentration (Table 3). These results suggest that the typical subtropical climate characteristics of Daya Bay and the rich food sources in the MCCA may jointly promote the growth ofO. rufescens,resulting in it being one of the dominant species. Current studies on the ecology ofOikopleuraare mainly focused onOikopleura dioicawhich is widely distributed and is easily cultured in an indoor environment. By contrast,small and gelatinousO. rufescensis mainly distributed in tropical and subtropical coastal waters, and the population dynamics of this species and its role in the marine ecosystem require more attention and further studies.
The spatial and temporal abundance variations in dominant speciesParvocalanus crassirostrisandOikopleura rufescenswere mainly affected by temperature and food supply. In this bay, temperature was mainly regulated by the East Asian monsoon, and phytoplankton, especially small-sized phytoplankton, were capable of mass propagation in culture zones as a result of eutrophication in the MCCA. In addition, given the specific feeding pattern and food choice of these two species, the abundance of these two species during the southwest monsoon was higher than during the northeast monsoon, and the abundance of both species in the cage-culture area was also higher than in the other two areas. The amount of variation in the other two dominant speciesCalanus sinicusandDoliolum denticulatumwas influenced mainly by the ocean current in different monsoons.Calanus sinicusmainly occurred during the period between January and April, and its abundance was negatively correlated with temperature, and positively correlated with salinity(Fig.8d, Table 3).Calanus sinicusis a warm temperate species, which is mainly distributed and reproduces in the coastal areas of the Yellow Sea and the East China Sea.The population recruitment ofCalanus sinicusis mainly due to the ocean current along the coast of Fujian and Zhejiang Provinces during the northeast monsoon.C.sinicusis capable of growth and reproduction at low temperature from January to April in Daya Bay (Liet al.,2016). When the temperature increases in May, this species cannot survive at temperatures higher than > 27℃(Uye, 1988). The seasonal variation ofC. sinicusin Daya Bay was mainly due to the temperature change regulated by the monsoon. From January to April,C. sinicusin Daya Bay also showed significantly spatial variations,which was likely caused by the exchange cycle of ocean current and bay water (Liet al., 2016). Linet al.(1990)showed thatD. denticulatumwas the dominant species ofThaliaceain Daya Bay, which mainly occurred at higher temperature in summer and autumn. The distribution and aggregation ofD. denticulatumin Daya Bay was mostly correlated with the warm current of the South China Sea caused by the southwest monsoon. Consistent with this finding, our study suggested thatD. denticulatumoccurred mainly during the period between May and August,and it was rarely detected in the MCCA.
4.2 Spatio-Temporal Variation in the Main Groups of Zooplankton
Zooplankton was rich in species in Daya Bay, with copepods and cladocerans being the most dominant groups in number. These two groups accounted for more than 92% of the total zooplankton abundance (Table 1). In addition, copepod was the most abundant groups in terms of either diversity or abundance (Fig.5). Among the zooplankton species, the predominant role of copepods in Daya Bay has not changed since the 1980s (Xu, 1989;Lianet al., 1990; Lianet al., 2011; Duet al., 2013). However, the percentage abundance of copepods accounted for the increase in zooplankton species in Daya Bay compared to the data collected 30 years previously (Table 4). In eutrophic bays, zooplanktons tend to miniaturize(Uye, 1994; Wanget al., 2008). The community composition and structure of zooplankton are also significantly different, with an increased proportion of small-sized zooplankton in bays heavily impacted by eutrophication(Uye, 1994). Small- and medium-sized species including
Paracalanus parvus,Parvocalanus crassirostris, andAcartia erythraeashowed an advantage in terms of abundance among copepods and even zooplankton, indicating that the abundance of small-sized zooplankton in Daya Bay is indeed increasing. In addition, the increasing Chlaconcentration caused by natural conditions and human activities in the sea area provides sufficient food sources for these species.
Although only three cladoceran species were found in Daya Bay,Penilia avirostrisandPseudevadne tergestinacontributed up to 28% of the total zooplankton abundance.Penilia avirostrisoccurred mainly in the warm period,while the abundance ofPseudevadne tergestinaincreased suddenly in April (Figs.8a-b). In addition, the abundance ofPenilia avirostrisin the DNPP (an area near thermal drainage) was significantly higher than that in the other two areas. In Daya Bay, the average abundance ofPenilia avirostrisin the warm and cold periods was 3437 ind m-3and 0.59 ind m-3, respectively. The seasonal variation in cladocerans was significantly and positively correlated with water temperature (Table 3). Water temperature change can alter the reproductive pattern of cladocerans(Johnset al., 2005). At high temperatures, cladoceran species reproduce by means of parthenogenesis, with a large amount of spawning, a high reproduction rate, and a sharp increase in abundance. At low temperatures, the species reproduceviasexual reproduction, species abundance decreases, and the population may even disappear(Zhenget al., 1984). The average temperature during the warm period in Daya Bay was higher than that in the cold period (Table 2). High temperature promotes the mass propagation of cladoceransviaparthenogenesis. Warm conditions have contributed to the success ofPeniliaavirostrisin the coastal waters by favoring their resting eggs and aiding colonization (Johnset al., 2005; Miyashitaet al.,2010). High abundance of cladocerans was achieved rapidly due to the influence of favorable temperatures and parthenogenetic reproduction in Daya Bay.
Although cladoceran species composition remained unchanged, the abundance and proportion of cladocerans in this study were both higher than the data collected 30 years previously (Cai, 1990). According to the survey conducted during 1986-1987, the peak abundance of cladocerans occurred in August. By contrast, based on our study, the peak abundance of these species was found to be earlier, during the period between April and June(Fig.8a-b). The results of the short-term study on cladocerans in Daya Bay also showed that in the sea area near the nuclear power plant, the abundance of cladocerans ranged widely from 16 to 7267 ind m-3from April to June and peaked at the ONPP outflow (Liet al., 2014). At the monthly and annual levels, cladocerans proliferated in the sea area near the nuclear power plant. At the long-term level, the seasonal time-series of peak abundance of cladocerans was advanced (Cai, 1990; Liet al., 2014). A 1-3℃ temperature increase was associated with the discharge of cooling water from the ONPP in Dapeng Cove(Tanget al., 2003). Rising temperatures from the power plant’s thermal discharge have strongly influenced the phytoplankton community, favoring dinoflagellates over diatoms (Liet al., 2011). The plankton in Daya Bay showed an ecological response to the thermal drainage of the nuclear power plant. For cladoceran species, thermal drainage can not only increase species abundance, but can also promote earlier peak abundance.

Fig.9 The percentage abundance of gelatinous zooplankton and non-gelatinous zooplankton during two different time periods. Notes: zooplankton data were collected based on a 500 μm net. Data collected during 1994-2004 were sourced from the ecological network survey of Daya Bay experimental station. Data collected during 2004-2014 were sourced from the ecological monitoring area of Daya Bay of the State Oceanic Administration.
Over the past 20 years, non-gelatinous zooplanktons have remained dominant species of zooplankton in Daya Bay, with an average abundance of 83.24%. The average abundance of gelatinous zooplankton was 16.74%, highlighting the dominant roles of non-gelatinous zooplankton in Daya Bay. However, the percentage abundance of gelatinous zooplankton among the zooplankton species is increasing. From 2000 to 2010, the abundance of non-gelatinous zooplankton decreased from 87.19% to 75.86%,while the abundance of gelatinous zooplankton increased from 12.81% to 24.14% (Fig.9). There are many explanations for the increase in abundance of gelatinous zooplankton, including climate change, eutrophication, overfishing, aquaculture, construction of water conservancy projects, and biological invasion (Purcell, 2005; Purcellet al., 2007; Richardsonet al., 2009). Although the abundance of gelatinous zooplankton increased in Daya Bay,the increase in diversity and amount of gelatinous zooplankton species was not as significant as reported from the Jiaozhou Bay in the Bohai Sea (Purcell, 2005; Sunet al., 2011).
4.3 Spatio-Temporal Variation in the Zooplankton Community
Under the control of the East Asian monsoon, the seawater temperature in Daya Bay showed a significant seasonal variation. However, in recent years, the ecological environment has changed due to the influence of human activities, such as cage culture and nuclear power plant operation in Dapeng’ao Bay and Aotou Bay (Wanget al.,2008; Wuet al., 2010, 2017). The community structure of zooplankton in Daya Bay also showed significant temporal and regional variations (Fig.10). For example, the diversity and abundance of zooplankton species were higher during the southwest monsoon than during the northeast monsoon. Regionally, the zooplankton communities were clearly divided into three groups, the MCCA,ONPP, and UPW. Group MCCA was mainly influenced by cage culture. The abundance of small-sized phytoplankton led to the aggregation ofParvocalanus crassirostris,Temora turbinata,Acartia erythraea,Oikopleura rufescensand other species during the southwest monsoon. High-abundance planktonic larvae were also detected in the cage-culture area. Group ONPP was affected by thermal drainage of the nuclear power plant, with a generally higher temperature, and cladoceran species such asPenilia avirostriswere found to be abundant (Liet al.,2014). In the group UPW which was less affected by human activities, zooplankton community composition was complex, and mainly dominated by some cosmopolitan species and exotic species such asParacalanus parvus,Sagitta enflataandDoliolum denticulatum. Overall, the species composition and variation of zooplankton in Daya Bay were affected not only by natural factors such as monsoon and ocean currents, but also by ecological environment change caused by human activities.
Compared to the data collected 30 years ago, the overall temperature in Daya Bay was slightly higher, and the temperature of the seawater near the thermal discharge of the nuclear power plant had increased significantly (Tanget al., 2003). In addition, Chlaconcentration was also higher than that reported 30 years ago (Wuet al., 2017).In the past 30 years, the most significant changes in zooplankton in Daya Bay were the decrease in species diversity and the increase in zooplankton abundance (Table 4).Although previous studies have suggested that zooplankton biomass (wet weight) in Daya Bay was increased (Duet al., 2013), the dry weight of zooplankton biomass was decreased, suggesting that water-rich zooplankton may have increased or the individual size of zooplankton may have become smaller (i.e., miniaturized). The composition of zooplankton in Daya Bay was significantly lower than that in the survey conducted during 1986-1987. The number of copepods and jellyfish has significantly decreased, but the abundance of both has significantly increased, mainly due to a single dominant species (Lianet al., 1990), such as the ball-shapedPleurobrachia globosain the cage-culture area, which has increased substantially (Lianet al., 2011).Parvocalanus crassirostrisis a typical small copepod compared toCalanus sinicus(Alajmiet al., 2015).Oikopleura rufescensis small in size, but it contains a high content of water (Flood and Deibel, 1998). Therefore, the increase in the number of small-sized copepods or gelatinous species may increase the abundance of zooplankton and decrease its dry biomass before and after 30 years in Daya bay (Fig.10). In our study, appendicularians accounted for more than 3%of the total zooplankton abundance (Table 4). Thermal discharge from nuclear power plants increased the temperature in the surrounding sea area, changed the reproduction pattern of cladocerans, and increased the speed of the growth cycle of small-sized species. In addition, eutrophication in the cage-culture area improved the Chlaconcentration, and provided enough food sources for zooplankton growth. Overall, these environmental factors caused by East Asia monsoon, ocean current, marine aquaculture and thermal water discharge contributed to the increase in the amount of small-sized zooplankton and gelatinous zooplankton in Daya Bay.

Fig.10 Conceptual pattern of the zooplankton community affected by monsoon, ocean current and anthropogenic activities in Daya Bay.
5 Conclusions
Our study shows significant temporal and spatial variations in the community structure of zooplankton in Daya Bay. The temporal variation is mainly driven by environmental factors such as monsoon and ocean current,while the spatial variation is mainly detected in the sea area which is affected by human activities. More specifically, the spatial variation at the species level is a result of multiple factors. In addition, zooplankton shows a miniaturization tendency and an increased percentage abundance of gelatinous zooplankton before and after 30 years.These factors may further affect the structure and function of the entire ecosystem. The overall miniaturization and gelatinization of plankton may limit the stability and regulatory function of the marine ecosystem, and may affect the energy flow and material circulation of the food web as well as the biodiversity, thereby affecting the yield of the marine fishery in Daya Bay. Therefore, under the dual impact of climate change and human activities, it is necessary to strengthen ecological monitoring and research data accumulation of the bay.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 31971432, 41976112),the Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) (Nos. GML2019ZD0401, GML 2019ZD0405), the Science and Technology Basic Resources Investigation Program of China (No. 2017FY20 1404) and the Science and Technology Planning Project of Guangdong Province, China (No. 2020B1212060058).
杂志排行
Journal of Ocean University of China的其它文章
- Paleosalinity and Its Association with Organic Matter:A Case Study from the Eocene Shahejie Formation,Laizhou Bay Sag, Bohai Bay Basin (China)
- Studies on the Inversion Phenomenon of Physical Properties Observed in the Huagang Formation Reservoir in the Xihu Sag Based on the Water-Rock Reaction Experiments
- Effect of Sand Body Enrichment Under the Restriction of a Tectonic Transfer Zone: A Case Study on the Pinghu Formation in the Kongqueting Region on the Pinghu Slope
- Relationship Between Paleogene Reservoir Densification and Hydrocarbon Accumulation in the Xihu Depression
- Adjoint Method-Based Algorithm for Calculating the Relative Dispersion Ratio in a Hydrodynamic System
- Analysis of the Leading Modes of Autumn Precipitation over the Yangtze River Basin
