Comparison of the Digestion and Absorption Characteristics of Docosahexaenoic Acid-Acylated Astaxanthin Monoester and Diester in Mice
2021-09-01LIYeYUJieXUJieCHEHongxiaWANGYumingXUEChanghuHUANGQingrongandZHANGTiantian
LI Ye, YU Jie, XU Jie, CHE Hongxia,2),*, WANG Yuming,3), XUE Changhu,3),HUANG Qingrong, and ZHANG Tiantian,*
1) College of Food Science and Engineering, Ocean University of China, Qingdao 266003, China
2) College of Marine Science and Biological Engineering, Qingdao University of Science and Technology, Qingdao 266042,China
3) Laboratory for Marine Drugs and Bioproducts, Pilot National Laboratory for Marine Science and Technology, Qingdao 266237, China
4) Rutgers State University, Department of Food Science, New Jersey 08901, USA
Abstract Docosahexaenoic acid-acylated astaxanthin (DHA-AST) esters exhibit distinct bioactivities in improving brain function.However, the digestion and absorption characteristics of DHA-AST esters in vivo are unclear, thereby restricting the molecular mechanism analysis of their superior activities. This study compared the digestion and absorption characteristics of DHA-AST monoester and diester by determining the levels of AST and DHA in the serum, liver, small intestinal content and wall, and feces at different time points after a single-dose oral administration of the esters. After oral gavage with 2 mg AST equivalent of the DHA-AST monoester and diester for 18 h, the excretion rates were approximately 51% and 84%, respectively. This result indicates that DHAAST monoester was better than diester for the absorption of AST. The results in serum, liver, and small intestinal content and wall also agreed with this finding. Moreover, the excretion rates of DHA in the feces at 24 h in the DHA-AST monoester and diester groups were approximately 40% and 36% after gavage with 5 mg DHA equivalent, respectively. This result indicates that DHA-AST diester exhibited a better tendency than monoester for the absorption of DHA. Interestingly, the results in the liver and small intestinal wall showed an apparent difference, indicating that DHA-AST diester was better than monoester for the absorption of DHA.These findings provide a scientific basis for the molecular mechanism analysis and utilization of DHA-AST monoester and diester as functional ingredients.
Key words DHA-acylated astaxanthin monoester; DHA-acylated astaxanthin diester; digestion; absorption
1 Introduction
Astaxanthin (AST) is an important xanthophyll carotenoid that is widely distributed in marine animals and microalgae (Husseinet al., 2006). AST possesses various physiological activities, such as antioxidant and anti-inflammatory properties; in addition, it can effectively improve cardiovascular health and immune system, and prevent diabetes and neurodegenerative disorders (Yasuiet al., 2011;Yanget al., 2013; Grimmiget al., 2017; Visioliet al., 2017;Guanet al., 2019). AST naturally exists in the form of unesterified AST or fatty acid-acylated AST ester (monoester or diester) (Johnsonet al., 1991). Nevertheless, unesterified AST is unstable owing to its potential hydroxylation,which restricts its utilization in functional nutraceuticals(Perez-Galvezet al., 2005). Interestingly, esterification can enhance the stability of AST (Zhouet al., 2019). AST esters are chemically composed of AST and various types of fatty acids, such as palmitic acid, stearic acid, oleic acid,and linoleic acid. The AST in the pelagic red crab langostilla (Pleuroncodes planipes) is composed of approximately 70% diester, 12% monoester, and 10% unesterified AST (Coral-Hinostrozaet al., 2002). Recently, AST esters have attracted increasing attentions because of their stability and biological activities.
Docosahexaenoic acid (DHA), an n-3 long-chain polyunsaturated fatty acid (PUFA) rich in marine creatures, displays various health benefits by preventing chronic degenerative diseases, such as neurodegenerative diseases, cardiovascular disease, cancer, and type II diabetes, and by improving the intelligence and vision of fetus (Jainet al.,2015; Wanget al.,2016; Wenet al., 2016; Newellet al.,2019; Zhanget al., 2019). However, DHA is chemically unstable and susceptible to oxidation (Yildizet al.,2018).Combining DHA with AST may be a good strategy to increase their stability and synergistic bioactivities. DHAacy lated AST esters, including monoesters and diesters,are widely present in marine organisms (Gómez-Estacaet al., 2017). Docosahexaenoic acid-acylated astaxanthin(DHA-AST) diester can attenuate cognitive disorders better than unesterified AST by reducing pathological features in model mice with Alzheimer’s disease (Cheet al.,2018). However, the digestion and absorption properties of DHA-AST esters are unclear, thereby hindering the molecular mechanism analysis of their unique bioactivities.
The bioavailability of short-chain fatty acid-acylated AST ester is greater than that of unesterified AST (Qiaoet al.,2018). Nevertheless, the digestion and absorption of longchain fatty acid-acylated AST esters, especially DHA-AST esters, have not been characterized. Moreover, a comparison of the digestion and absorption features of long-chain fatty acid-acylated AST monoester and diester is lacking.The objective of this study was to compare the digestion and absorption characteristics of DHA-AST monoester and diester by determining the AST levels in the serum,liver, small intestinal content and wall, and feces after a single-dose oral administration of DHA-AST esters in healthy mice. Unesterified AST served as a reference. The absorption kinetics of DHA in DHA-AST esters was also studied by measuring the DHA level in different tissues of mice deficient of n-3 PUFAs and compared with that of traditional fish oil DHA-ethyl ester (DHA-EE).
2 Materials and Methods
2.1 Materials
DHA-AST monoester and diester (purity ≥ 90%) were donated by the Laboratory of Food Science and Human Health from Ocean University of China (Yanget al., 2017). The chemical structures of DHA-AST monoester and diester are shown in Figs.1A and B. DHA-EE and AST were purchased from Huihaiyuan Biotech Co., Ltd. (Yibin, Sichuan,China) and Xinweipu Addictive Co., Ltd. (Shaoxing, Zhejiang, China), respectively. The standard sample of AST(purity ≥ 97%) was obtained from Solarbio Biotech Co., Ltd.(Beijing, China). Fatty acid methyl ester (FAME) standard was obtained from Sigma-Aldrich (St. Louis, MO, USA).Methyl tert-butyl ether (MTBE) and methanol were of chromatographic grade from Merck Co. (Hohenbrunn, Germany). All other chemical reagents were of analytical grade.
2.2 Animals and Diets
Animal experiments were carried out in accordance with the guidelines of the Ethical Committee of Experimental Animal Care at Ocean University of China (Certificate no.:SYXK2015012). The mice were kept in a controlled environment with a 12 h/12 h light-dark cycle at 24 ± 1℃ with 60% ± 10% relative humidity. The mice were provided with standard diet and waterad libitum.
2.2.1 Absorption kinetics of AST
Six-week-old male Balb/c mice were obtained from Vitar River Laboratory Animal Technology Co., Ltd. (Beijing, China). The mice were provided with standard dietad libitum, and the ingredients of the diet are listed in Table 1. Animals were grouped randomly as unesterified AST (AST), DHA-AST monoester (monoester), and DHAAST diester (diester) after 1 week for adapting to laboratory environment. The dose of oral gavage was 100 mg kg-1body weight according to the AST equivalent in the corn oil vehicle. The mice were fasted for 10 h with free access to water before gavage. Subsequently, the mice (n= 8 per group at every time point) were sacrificed by cervical dislocation after inhalation anesthesia with diethyl ether at different time points (0, 3, 6, 8, 10, 12, and 18 h)after gavage.

Table 1 Ingredients in the standard diet and n-3 PUFA-deficient experimental diet
2.2.2 Absorption kinetics of DHA
Female and male ICR strain mice aged 10 weeks were purchased from Vitar River Laboratory Animal Technology Co., Ltd. (Beijing, China). All animals were allowed to adapt to the environment for a week before the experiments. The mice were mated on 1:1 to produce offspring.An n-3 PUFA-deficient diet was provided during gestation and lactation. It was modified on the basis of AIN-93G, and the ingredients are displayed in Table 1. The pups were still fed with the n-3 PUFA-deficient diet after weaning until 8-week old. Afterward, the male offspring were randomly divided into three groups: DHA-EE, DHA-AST monoester (monoester), and DHA-AST diester (diester).The dose of oral gavage was 250 mg kg-1body weight based on the DHA equivalent in the corn oil vehicle. The mice were fasted for 10 h with free access to water before gavage. Subsequently, the mice (n= 8 per group at every time point) were sacrificed by cervical dislocation after inhalation anesthesia with diethyl ether at different time points (0, 2, 3, 5, 8, and 24 h) after gavage, respectively.
Whole blood was collected, and the serum was acquired by centrifugation at 7500 ×gfor 15 min at 4℃. Serum samples were aliquoted and stored at -80℃ until further analysis. The small intestinal content was collected by flushing the small intestinal tract sufficiently with 4 mL of physiological saline solution per mouse using an injector. The feces, liver, and small intestinal wall (from duodenum to ileum) were weighed and collected quickly. Fecal samples were excreted normally and collected completely before the mice were sacrificed at every time point. All fresh samples were snap frozen with liquid nitrogen before storage at -80℃.
2.3 Extraction and Detection of AST from Tissue Samples
AST was extracted from the serum and small intestinal content in accordance with a previously described method with minor modifications (Qiaoet al., 2018). In brief, methanol was added to the samples to precipitate the protein.The mixture was combined with chloroform to dissolve AST as much as possible to the chloroform phase. After centrifugation at 6900 ×gfor 5 min at 4℃, the chloroform layer was collected. The above extraction process was repeated two times. AST samples in the liver, small intestinal wall, and feces were obtained using the classic method (Folchet al., 1957). In brief, samples were homogenized with chloroform and methanol (2:1, v/v) in a glass homogenizer. Homogenization was performed in a constant-temperature water bath at 37℃ for 30 min. Purified water about one-fifth of the total volume was then added,and the chloroform layer was collected after standing for one night.
After the chloroform layer was dried with nitrogen, 1 mL of methanol/MTBE (1:1, v/v) was added to dissolve AST.The samples were then filtered through a 0.22 μm filter membrane. AST content was detected through high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) equipped with a diode array detector. Light was avoided during the experiment because of the unstable property of astaxanthin.
2.4 Lipid Extraction and Gas Chromatography
The total lipids in the serum and small intestinal content were extracted following a previously described method with modifications (Khachiket al., 1992). In brief,the samples were extracted with chloroform/methanol (1:1,v/v) at 40℃ for 30 min and then centrifuged. The chloroform phase was obtained twice with the same volume of chloroform. The total lipids from the liver, small intestinal wall, and feces were extracted with chloroform/methanol (2:1, v/v) following a previously described method(Folchet al., 1957). The extraction was mixed with onefifth volume of purified water and placed into a separatory funnel for 24 h after water bath at 37℃ for 30 min.Subsequently, the chloroform layer was collected. The obtained chloroform phase was dried with nitrogen and then dissolved in petroleum ether. The lipid samples were stored at -80℃ until analysis.
The obtained lipids were transmethylated to form FAMEs with hydrochloric acid/methanol (1:5, v/v) by heating at 90℃ for 3 h. The derivatives were extracted using n-hexane and then analyzed using an Agilent 7820A gas chromatograph equipped with a flame-ionization detector and a HP-INNOWAX capillary column (30 m × 0.32 mm, 0.25 μm). All injections were carried out in split mode with a ratio of 10:1. Meanwhile, the injector and detector temperatures were set at 240℃ and 250℃, respectively. The column temperature was 170℃ initially, then was increased to 210℃ at a speed of 3℃ min-1, and finally was held at 210℃ for 30 min. The results of DHA referred to total DHA and could not distinguish the specific forms (unesterified or esterified).
2.5 Statistical Analysis
Data were expressed as means ± SEM (standard error of the mean). A comparison of means between different groups was performed using one-way ANOVA followed by Tukey’s test. Differences withP< 0.05 were considered significant. All statistical analyses were carried out using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). The area under the curve (AUC) was calculated by the Trapezoidal integration of GraphPad prism 5 (Graph-Pad Software, Inc., San Diego, CA, USA).
3 Results
3.1 Level of AST in the Feces After Administration with DHA-AST Monoester and Diester
Whole feces were collected at 6, 8, 10, 12, and 18 h after a single-dose oral gavage of AST or DHA-AST esters with 100 mg kg-1body weight (AST equivalent, about 2 mg AST per mouse) in healthy mice to evaluate the bioavailability of DHA-AST esters. As shown in Fig.1C, the highest amount of total AST in the feces was found in the DHA-AST diester group after administration with samples for 18 h, and this amount was 1.65-fold and 1.15-fold higher than those in the unesterified AST and DHA-AST monoester groups. The excretion rates calculated as AST equivalent at 18 h following gavage of DHA-AST monoester and diester were approximately 51% and 84%, respectively. This result indicates that DHA-AST monoester possessed better bioavailability than diester.
The contents of the three forms of AST (non-esterified AST, monoester, and diester, expressed in AST equivalents) in the feces were detected to investigate the specific change in AST form following a single-dose oral administration of unesterified AST and AST esters. As expected,only non-esterified AST was found in the feces after a single-dose oral administration of AST, and the level gradually increased with the extension of time (Fig.1D). Interestingly, no AST diester was found in the fecal samples after gavage with DHA-AST monoester. In addition, the content of monoester was significantly higher in DHAAST monoester than in unesterified AST (Fig.1E). Notably, the AST in the feces of mice in the DHA-AST diester group was mainly in the form of diester, and the levels of monoester and non-esterified AST showed no significant differences (Fig.1F). These findings suggest that DHAAST monoester and diester were differentially excreted after gavage.

Fig.1 Chemical structures of DHA-AST esters and time course curves of AST equivalents and different forms of AST in the feces at 6, 8, 10, 12, and 18 h after oral gavage of AST and AST esters using healthy mice. A, Structure of DHA-AST monoester; B, Structure of DHA-AST diester; C, Amount of AST equivalents in the feces at different specific time points; D, Time course curves of different forms of AST in the AST group; E, Time course curves of different forms of AST in the DHA-AST monoester group; F, Time course curves of different forms of AST in the DHA-AST diester group. Different letters indicate significant differences at P < 0.05 among various forms of AST. Data are presented as means ± SEM(n = 8 per group at every time point).
3.2 Levels of AST in the Serum and Liver After Administration with DHA-AST Monoester and Diester
The contents of AST in the serum and liver at 3, 6, 8, 10,12, and 18 h were detected by HPLC-MS/MS after oral administration with unesterified AST and DHA-AST monoester and diester to illustrate the absorption kinetics of ASTin vivo. However, unlike in the feces, only unesterified AST was detected in the serum and liver. As described in Fig.2A, the span of total AST (unesterified AST)in the serum was about 12 h after gavage of AST and AST esters. Notably, the DHA-AST monoester group achieved the maximum absorption of 0.52 ± 0.031 μg mL-1in the serum at 6 h, which was approximately twofold than that of unesterified AST (0.27 ± 0.013 μg mL-1at 6 h). The total AST level in the serum of the DHA-AST diester group reached the peak of 0.12 ± 0.011 μg mL-1at 10 h (Fig.2A).The areas under the curve from 0 h to 18 h (AUC0-18) of total AST (unesterified AST) in the serum were 2.07 ± 0.042,2.74 ± 0.063, and 0.77 ± 0.025 μg h mL-1in the non-esterified AST, DHA-AST monoester, and DHA-AST diester groups, respectively. This result indicates that DHA-AST monoester had higher bioavailability than unesterified AST and DHA-AST diester in Balb/c mice (Fig.2B). Moreover,the maximum concentrations of AST in the liver after a single-dose oral administration of unesterified AST, DHAAST monoester, and DHA-AST diester were 1.28 ± 0.12,1.55 ± 0.017, and 1.00 ± 0.019 μg g-1tissue, respectively(Fig.2C). Meanwhile, the hepatic maximum of AST in the monoester group was attained at 6 h, which was faster than that in the diester group. This result indicates that DHA-AST monoester exhibited good absorption property and slow transfer efficiency to other tissues (Fig.2C). The AUC0-18values of total AST (unesterified AST) in the liver were 15.96 ± 1.42, 12.36 ± 1.13, and 8.35 ± 0.86 μg h g-1tissue in the non-esterified AST, DHA-AST monoester,and DHA-AST diester groups, respectively (Fig.2D).

Fig.2 Unesterified AST (equivalents) level in the serum and liver at 0, 3, 6, 8, 10, 12, and 18 h after oral gavage of AST and AST esters in healthy mice. A, Time course curves of unesterified AST in the serum; B, Area under the curve of unesterified AST in the serum; C, Time course curves of unesterified AST in the liver; D, Area under the curve of unesterified AST in the liver. Different letters indicate significant difference at P < 0.05 among three groups at the same time points. Data are presented as means ± SEM (n = 8 per group at every time point).
3.3 Levels of AST in the Small Intestinal Content and Wall After Administration with DHAAST Monoester and Diester
The small intestine is an important organ for the absorption of ingredients into the blood. Hence, the levels of AST in the small intestinal content and wall after administration with DHA-AST monoester and diester were determined. Results showed that the content of total AST in the small intestinal content at 3 h were approximately 0.47± 0.10, 0.21 ± 0.029, and 0.64 ± 0.10 mg, respectively, following administration with unesterified AST and DHAAST monoester and diester (Fig.3A). Notably, DHA-AST diester exhibited a fast decreasing rate from 3 h to 8 h compared with unesterified AST and DHA-AST monoester.Hence, we speculated that DHA-AST diester possesses a low absorption efficiency and can be excreted into the colon rapidly. Meanwhile, the AUC0-18of AST equivalents in the small intestinal content in the DHA-AST diester group was significantly higher than that in the DHA-AST monoester group (Fig.3B).
In addition, the concrete changes in the three forms of AST (unesterified AST, monoester, and diester, shown as AST equivalents) in the small intestinal content after gavage with AST and AST esters were determined to investigate the hydrolytic ability of esterase on DHA-AST monoester and diester. Only unesterified AST existed in the small intestinal content in the AST group, and the maximum was 0.47 ± 0.10 mg (Fig.3C). After administration with DHA-AST monoester, the content of unesterified AST attained a maximum value (0.18 ± 0.026 mg) at 3 h, which was about 4.5-fold higher than that of monoester (0.039 ±0.0055 mg). This result indicates that DHA-AST monoester was hydrolyzed quickly under the action of enzymes in the gastrointestinal tract (Fig.3D). Similarly, the main form of AST was unesterified AST (0.33 ± 0.060 mg) at 3 h after oral administration with DHA-AST diester (Fig.3E). Notably, the total level of monoester and diester was only about half of the total AST in the small intestinal content at 3 h, indicating that the DHA-AST diester was hydrolyzed slower than monoester in general (Figs.3A and E). The contents of monoester and diester were 0.14 ± 0.023 and 0.19± 0.054 mg at 3 h, respectively, and no apparent difference was observed.
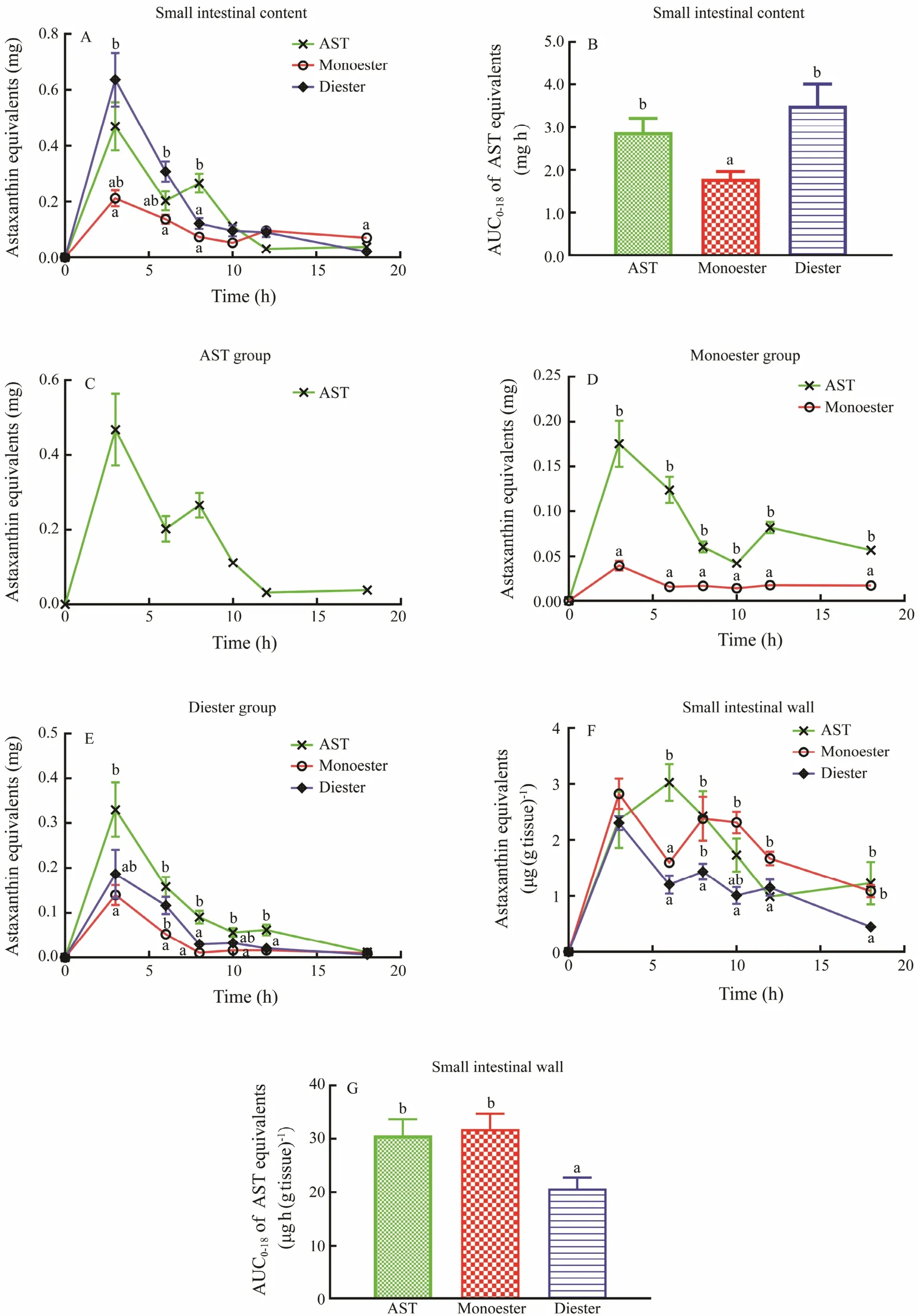
Fig.3 Time course curves of AST equivalent level and area under the curve from 0 h to 18 h (AUC0-18) in the small intestinal content and wall, and different forms of AST in the small intestinal content at 0, 3, 6, 8, 10, 12, and 18 h after oral gavage of AST and AST esters with a dose of 100 mg kg-1 body weight (equivalents of AST) in healthy mice. A, time course curves of AST equivalents in the small intestinal content; B, area under the curve of AST equivalents in the small intestinal content; C, time course curves about different forms of AST in the AST group; D, time course curves about different forms of AST in the DHA-AST monoester group; E, time course curves about different forms of AST in the DHAAST diester group; F, time course curves of AST equivalents in the small intestinal wall; G, area under the curve of AST equivalents in the small intestinal wall. Different letters indicate significant difference at P < 0.05 among three groups at the same time points. Data are presented as means ± SEM (n = 8 per group at every time point).
Unesterified AST was absorbed into the blood through the small intestinal wall. As shown in Fig.3F, the AST content in the small intestinal wall showed a trend of fluctuating decline from 3 h after oral administration of unesterified AST and AST esters. DHA-AST monoester possessed a relatively higher level of total AST at 3 h than DHA-AST diester, although the difference was not significant. In addition, the changing trends of AST equivalents in the AST monoester and diester groups were similar, whereas the unesterified AST group showed a peak at 6 h. The AUC0-18of total AST in the small intestinal wall after gavage with DHA-AST monoester was significantly higher than that in the DHA-AST diester group, suggesting that DHA-AST monoester had higher bioavailability for AST in comparison with DHA-AST diester in Balb/c mice (Fig.3G). These findings suggest that DHA-AST monoester and diester exhibited different digestion and absorption processes and efficiency levels.
3.4 Level of DHA in the Feces After Administration with DHA-AST Monoester and Diester
Dietary α-linolenic acid can be converted into DHAin vivo. A model of n-3 PUFA-deficient mice was established successfully by feeding with n-3 PUFA-deficient diet for two generations, which can alleviate or avoid the influence of DHA in tissues, to investigate the absorption kinetics of DHA in DHA-AST esters (Pawloskyet al.,2001). Whole feces were collected at 5, 8, and 24 h after a single-dose oral gavage with DHA-EE and DHA-AST esters at 250 mg kg-1body weight (approximately 5 mg DHA per mouse, shown as DHA equivalent). As presented in Fig.4A, the excretion amount of DHA in the DHA-EE,DHA-AST monoester, and DHA-AST diester groups showed remarkable differences in the first 8 h with values of 0.68 ± 0.079, 1.84 ± 0.25, and 1.16 ± 0.21 mg, respectively,and followed the order of DHA-AST monoester > DHAAST diester > DHA-EE. The results suggest that DHA was excreted faster in the DHA-AST monoester group than in the DHA-AST diester group. The excretion rates of DHA in the feces until 18 h in the DHA-AST monoester and diester groups were approximately 40% and 36% after gavage with 5 mg DHA equivalent, respectively, indicating that DHA-AST diester exhibited a better tendency than monoester in the absorption of DHA (Fig.4A).
3.5 Levels of DHA in the Serum and Liver After Administration with DHA-AST Monoester and Diester
The serum and hepatic DHA concentrations were determined at 0, 2, 3, 5, 8, 10, and 24 h after a single-dose oral gavage with DHA-EE and DHA-AST esters to understand the absorption kinetics of DHA from DHA-AST esters.Fig.4B shows that all of the three groups emerged with two peaks. The peak times of the DHA-EE group were 2 and 8 h, whereas those of the monoester and diester groups were 3 and 8 h after gavage with the same equivalent of DHA in different forms. The maximum values of the DHAEE group were significantly higher than those of the DHAAST monoester and diester groups, whereas no apparent differences were observed between the DHA-AST monoester and diester groups (Fig.4B). The areas under the curve from 0 h to 24 h (AUC0-24) of the DHA-EE, monoester,and diester groups in the serum were 3.79 ± 0.15, 2.15 ±0.069, and 2.21 ± 0.082 μg h μL-1, respectively (Fig.4C). This result indicates that the bioavailability of the DHA-EE group was significantly higher than that of the DHA-AST ester group. Meanwhile, no remarkable difference in AUC0-24was found between the monoester and diester groups (Fig.4C). Interestingly, the results in the liver revealed that DHA-AST diester was better absorbed than monoester in the absorption of DHA (Fig.4D). Notably, the AUC0-24values of DHA in the liver after gavage with DHAAST monoester and diester were 25.11 ± 2.49 and 36.58 ±3.63 μg h g-1, respectively, indicating that DHA-AST diester had a higher bioavailability for DHA than DHAAST monoesterinvivo(Fig.4E).
3.6 Levels of DHA in Small Intestinal Content and Wall After Administration with DHA-AST Monoester and Diester
Considering that the small intestine plays a critical role in the digestion and absorption of substances, we evaluated the levels of DHA in the small intestinal content and wall over time after a single-dose oral administration of DHA-AST monoester and diester. As depicted in Fig.4F,the DHA level in the small intestinal content similarly decreased quickly in the DHA-AST diester and DHA-EE groups after 2 h with the extension of time after gavage.In particular, the value of DHA in DHA-AST diester decreased from 1.77 ± 0.22 mg to 0.37 ± 0.067 mg during 2 h to 3 h (Fig.4F). Relatively, the decline rate of DHA in the DHA-AST monoester group slowly reached the bottom level until 5 h, indicating that the absorption of DHA in the DHA-AST monoester group was inferior to that in the DHA-AST diester group (Fig.4F). The AUC0-24values of DHA in the small intestinal content after gavage with DHAEE, DHA-AST monoester, and DHA-AST diester were 2.42 ± 0.18, 9.24 ± 0.82, and 8.20 ± 0.85 mg h, respectively(Fig.4G). Significant differences in DHA absorption were found between DHA-EE and DHA-AST esters (Fig.4G).Meanwhile, no significant difference in the AUC0-18of the small intestinal content was found between DHA-AST monoester and diester (Fig.4G). In addition, the results of the small intestinal wall indicated that the DHA content in the DHA-AST diester group (0.82 ± 0.12 mg) was slightly higher than that in the DHA-AST monoester group (0.55 ±0.078 mg) at 8 h. This result suggests that DHA-AST diester was better absorbed than monoester in the absorption of DHA, but the difference was not significant (Fig.4H).Interestingly, no conspicuous change in DHA content was found between the DHA-AST monoester and diester groups from 8 h to 24 h (Fig.4H). The AUC0-24trend about DHA in the small intestinal wall after gavage with DHA-EE,DHA-AST monoester, and DHA-AST diester was consistent with that in the liver (Fig.4I). In conclusion, DHAAST diester exhibited a better efficiency to absorb DHA than DHA-AST monoester.

Fig.4 Time course curves of DHA level in different tissues after oral gavage of DHA-EE and AST esters with a dose of 250 mg kg-1 body weight (DHA equivalents) using n-3 PUFA-deficient mice. A, time course curves of DHA level in feces at 5, 8, and 24 h; B, time course curves of DHA level in the serum at 0, 2, 3, 5, 8, and 24 h after oral gavage of DHA-EE and AST esters; C, area under the curve of DHA level in the serum; D, time course curves of DHA level in the liver; E,area under the curve of DHA level in the liver; F, time course curves of DHA level in the small intestinal content; G, area under the curve of DHA level in the small intestinal content; H, time course curves of DHA level in the small intestinal wall; I, area under the curve of DHA level in the small intestinal wall. Different letters indicate significant difference at P< 0.05 among three groups at the same time points. Data are presented as means ± SEM (n = 8 per group at every time point).
4 Discussion
Previous studies investigated the oral absorption capacity of AST esters, but the experimental materials used were mostly mixed AST esters extracted from natural materials or synthetic short-chain and medium-chain fatty acidacylated AST esters (Fukamiet al., 2006; Qiaoet al., 2018;Zhouet al.,2019). Our previous study reported that DHAAST diester exhibits superior physiological activity in improving brain function (Cheet al., 2018). However, no studies focused on the oral absorption characteristics of DHA-AST esters. DHA-AST esters can be found in marine creatures, which are important ingredients in food(Gómez-Estacaet al., 2017). In the present study, the absorption kinetics of AST and DHA were illustrated to explore the digestion and absorption of DHA-AST monoester and diesterin vivo. Our data showed that the absorption capacity of AST was higher in the DHA-AST monoester group than in the unesterified AST and DHA-AST diester groups, whereas the absorption of DHA was better in the DHA-AST diester group than in the DHA-AST monoester group (Fig.5).
It is well-known that carotenoids are difficult to be digested and absorbed because of their hydrophobic structure, while lipid ingredients can promote their absorption(Erdman Jr.et al., 1993; Brownet al., 2004). The similar result was also observed in DHA-AST monoester in this study. Our results showed that DHA-AST monoester utilized AST better than unesterified AST, which might be attributed to the DHA that was released from AST esterviathe zymolytic effect of esterase in the digestive tract.Fatty acids could promote the formation of micelles containing fat-soluble nutrients in the hydrophobic zone (Mcclementset al.,2014; Mcclements, 2015). A previous study found that n-octanoic acid-acylated AST monoester significantly increased the concentration of unesterified AST in the plasma and liver compared with diester (Fukamiet al.,2006). Similarly, in the present study, the excretion rates calculated as AST equivalent of unesterified AST, DHAAST monoester, and DHA-AST diester at 18 h following gavage were approximately 73%, 51%, and 84%, respectively. This result indicates that DHA-AST monoester was better than unesterified AST and diester in the absorption of AST. In the DHA-AST diester group, the excretion rates calculated as AST equivalent of unesterified AST and DHA-AST monoester and diester at 18 h following gavage were approximately 10%, 16%, and 60%, respectively, suggesting that most DHA-AST diester was excreted from the feces in the prototype. Numerous studies have proven that AST in esters could be absorbed in the form of unesterified AST under the action of esterase in the gastrointestinal tract (Coral-Hinostrozaet al., 2002; Showalteret al., 2004; Aoiet al., 2018). In the present study,only unesterified AST was detected in the serum and liver after gavage with DHA-AST esters and unesterified AST.This finding is consistent with the previous result that only monomer lutein exists in tissues after gavage with lutein ester (a carotenoid ester) (Zhanget al., 2007). Meanwhile,a low level of AST in the serum at first several time points might mean low absorption efficiency from the gut or rapid transfer from the serum to other tissues.

Fig.5 Digestion and absorption characteristics of AST and DHA after a single-dose oral gavage of DHA-AST monoester and diester.
The peak time of AST in the serum after gavage with noctanoic acid-acylated AST monoester and diester in the previous study of Fukamiet al.(2006) was earlier than that in our study. The reason for this result might be that the catalytic efficiency of the enzyme was related to the steric hindrance of the substrate, and the esterase in the gastrointestinal tract was more prone to react on the ester bonds formed by fatty acids with less steric hindrance and AST (Estellet al., 1986). In the present study, the peak time of AST in the serum of the DHA-AST diester group was 4 h later than that in the DHA-AST monoester and unesterified AST groups, which might be due to the presence of two ester bonds in the diester that need longer time to degrade into unesterified AST. The levels of different forms of AST in the small intestinal content could reflect the specific process of digestion and absorption after administration with DHA-AST esters. Our data showed that DHA-AST monoester was almost completely hydrolyzed to unesterified AST within 3 h after gavage, whereas only about half of DHA-AST diester was hydrolyzed into free form during the same time span. Taken together,these results suggest that the priority of AST absorption was in the following order of DHA-AST monoester > DHAAST diester. Interestingly, the high bioavailability might contribute to improved health outcomes. Raoet al. (2013)found that AST monoesters and diesters isolated fromHaematococcus pluvialisexhibit better anticancer potency than unesterified AST because of increased bioavailability. Further studies need to be conducted to compare the health effects of DHA-AST monoester and diester, which provide a scientific basis for utilization as potential functional ingredients.
Exogenous lipids are hydrolyzed under the action of enzymes, such as gastric lipase and pancreatic lipase, in the gastrointestinal tract. Hydrolyzates, especially free fatty acids with more than 12 carbon atoms, are emulsified by bile salts from the cholecyst and then mixed with other ingredients to form micelles before entering the intestinal mucosa layer (Thomsonet al., 1989; Phanet al., 2001).Absorbed lipids are re-esterified to assemble triacylglycerol (TG) and phospholipids (PL) in the smooth endoplasmic reticulum. These substances are then packed with cholesterol and apolipoproteins to synthesize chylomicrons,which are excreted into cell spaces by exocytosis and then enter the blood stream through the lymphatic circulation(Ramirezet al.,2001; Schuchardtet al., 2013). The absorption efficiency and bioactivity of fatty acids can be influenced by many factors, such as chemical structures,formulation form, and other ingredients in food (Michalskiet al., 2013). Different forms of DHA might have different characteristics of digestion and absorptionin vivo(Bannoet al., 2002). In the present study, DHA-EE was absorbed faster than DHA-AST monoester and diester,which might be attributed to enzymatic efficiency. Our data showed that all three groups exhibited two peaks of DHA in the serum, and the increase in DHA concentration in the serum corresponded to the decrease in DHA concentration in the liver. The reason for this result might be that absorbed DHA in the liver is excreted into the blood in the form of very low-density lipoprotein-TG (Schaapet al., 2004; Ghasemifardet al., 2014). The small intestine is the most important organ for digestion and absorption (Iqbalet al., 2009). Interestingly, the results in the present study indicated that the DHA concentration in the small intestinal wall of the DHA-AST diester group was generally higher than that in the DHA-AST monoester group. These data revealed that DHA-AST diester was slightly better than DHA-AST monoester in terms of DHA absorption.
5 Conclusions
A comparative analysis was carried out to illustrate the digestion and absorption characteristics between DHA-AST monoester and diester by determining the levels of AST and DHA in the serum, liver, small intestinal content and wall, and feces. Results showed that the absorption capacity of AST was higher in the DHA-AST monoester group than in the DHA-AST diester group, whereas the absorption capacity of DHA in the DHA-AST diester group was higher than that in the monoester group. The obtained findings might provide insights into the molecular mechanisms related to distinct bioactivities of DHA-AST esters as well as development and utilization as functional ingredients in the future.
Acknowledgements
The work was supported by the National Key R&D Program of China (No. 2018YFD0901103), the National Natural Science Foundation of China (Nos. 31901688 and 31571864), the Natural Science Youth Foundation of Shandong Province (Nos. ZR2019QC004 and ZR2020QC236),the Laboratory for Marine Drugs and Bioproducts of Pilot National Laboratory for Marine Science and Technology(Qingdao, No. LMDBKF201807).
杂志排行
Journal of Ocean University of China的其它文章
- Paleosalinity and Its Association with Organic Matter:A Case Study from the Eocene Shahejie Formation,Laizhou Bay Sag, Bohai Bay Basin (China)
- Studies on the Inversion Phenomenon of Physical Properties Observed in the Huagang Formation Reservoir in the Xihu Sag Based on the Water-Rock Reaction Experiments
- Effect of Sand Body Enrichment Under the Restriction of a Tectonic Transfer Zone: A Case Study on the Pinghu Formation in the Kongqueting Region on the Pinghu Slope
- Relationship Between Paleogene Reservoir Densification and Hydrocarbon Accumulation in the Xihu Depression
- Adjoint Method-Based Algorithm for Calculating the Relative Dispersion Ratio in a Hydrodynamic System
- Analysis of the Leading Modes of Autumn Precipitation over the Yangtze River Basin
