Morphology and Molecular Phylogeny of Two Marine Folliculinid Ciliates Found in China (Ciliophora, Heterotrichea)
2021-09-01YETingtingZHAOXuetongCHIYongZHENGBohanZHANGHuiHUANGJieWARRENAlanandCHENXiangrui
YE Tingting, ZHAO Xuetong, CHI Yong, ZHENG Bohan,ZHANG Hui, HUANG Jie, WARREN Alan, and CHEN Xiangrui,*
1) School of Marine Sciences, Ningbo University, Ningbo 315800, China
2) Institute of Evolution and Marine Biodiversity, Ocean University of China, Qingdao 266003, China
3) Key Laboratory of Aquatic Biodiversity and Conservation of Chinese Academy of Sciences, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China
4) Department of Life Sciences, Natural History Museum, London SW7 5BD, UK
Abstract Ciliates of the heterotrich family Folliculinidae are characterized by their transparent lorica, conspicuous peristomial lobes pair and dimorphic life cycle, a sessile trophont and a motile swarmer. However, understanding their biodiversity and systematics is relatively poor. In the present study, two species representing two genera of folliculinids, which were collected from marine and brackish habitats in China, were investigated with morphological and molecular methods. The genus Diafolliculina n. gen. is established for D. longilobata n. sp. Detailed morphological redescriptions for another species Eufolliculina is provided. Moreover, phylogenetic analysis based on small subunit ribosomal DNA (SSU rDNA) sequences showed that 1) all two genera locate in the Folliculinidae clade; 2) Metafolliculina clusters with Eufolliculina; and 3) two genera (Diafolliculina and Ampullofolliculina) with a lorica closure device have close affinities with Folliculina. The lorica of Diafolliculina and Ampullofolliculina lacks a closure device, thereby the phylogeney contradicts the morphology-based classification.
Key words Diafolliculina longilobata n. gen., n. sp.; Folliculinidae; morphology; SSU rDNA phylogeny
1 Introduction
Ciliated protozoa (ciliates) are an extremely diverse and ubiquitous group with more than 10000 nominal species from a wide range of habitats and constitute an important component of the microbial food web (Lynn, 2008; Huanget al., 2021; Liuet al., 2021). In addition, many species are widely used in scientific research fields such as cell biology, ecology, systematics, genomics and epigenetics(Chenget al., 2019; Jianget al., 2019; Zhaoet al., 2020).Members of the class Heterotrichea Stein, 1859, which are characterized by their large body size, undifferentiated somatic ciliary pattern, and well-developed oral apparatus,have been widely used in studies of basic and applied sciences, and as a representative group for ciliate research(Lobbanet al., 2007; Fernandeset al., 2016; Perrottaet al.,2016; Wancuraet al., 2017; Campello-Nuneset al., 2018;Chenet al., 2019; Shazibet al., 2019; Chiet al., 2020a, b,2021).
Folliculinidae Dons, 1914 is the largest family of Heterotrichea. Its members are mainly found in brackish and marine habitats including deep-sea hydrothermal vents although there are four freshwater species. Folliculinids are characterized by their lorica, which comprises a flask and a neck, a conspicuous pair of peristomial lobes, and a dimorphic life cycle with alternating mobile and sessile phases(Mulischet al., 1998; Lynn, 2008; Luoet al., 2019). They feed on a wide range of organisms from bacteria to microalgae and constitute a food source for metazoans, thus play important roles in energy transfer between different trophic levels in aquatic ecosystems (Corliss 1979; Kouriset al., 2007, 2010; Lynn, 2008). In addition, some folliculinids have been reported to be harmful, for exampleHalofolliculinasp., which pose a threat to some coral species (Cróqueret al., 2006). A total of 33 genera and about 80 nominal species have been documented since the description ofFolliculina ampulla(Müller, 1786) Lamarck,1816, the type species of the genus. Folliculinids are usually attached to the surfaces of immersed substrates and host organisms, thus not easy to collect. Furthermore, many species are known solely from a few fixed individuals and empty loricae. Currently, only seven species have been studied using silver-staining and electron microscopy, and three of them have their small subunit ribosomal DNA(SSU rDNA) sequences available in public databases such as GenBank (Kahl, 1932; Hadži, 1938, 1951; Mulischet al.,1987, 1993; Song and Packroff, 1997; Luoet al., 2019).Therefore, the morphological and phylogenetic data are either lacking or insufficient for most folliculinids (Mulisch and Markmann-Mulisch, 1999; Gaoet al., 2016; Chiet al., 2021).
In the present study, two folliculinids, namelyDiafolliculina longilobatan. gen., n. sp. andEufolliculina moebiusi(Kahl, 1932) Hadži, 1951, collected from a subtropical brackish wetland and a temperate intertidal zone in China, respectively, are described based on the observations of living cells and protargol-stained specimens. In addition, phylogenetic analysis was performed based on their SSU rDNA sequences to reveal the evolutionary relationships of folliculinids.
2 Material and Methods
2.1 Sample Collection, Observation, and Identification
Two species were collected from two different locations in China (shown in Fig.1).Diafolliculina longilobatan.gen., n. sp. was collected on 27 November, 2018 from a small wetland with an abundance of the common reed(Phragmites australis) in Meishan Island (29°46΄33΄΄N,121°57΄47΄΄E), which is in subtropical coastal waters of the East China Sea off Ningbo where the water temperature is 16℃ and salinity is 5 (Fig.1C1). The new species was mainly attached to the surface of the stems and leaves of plants immersed in water. Because of frequent rainfall,the salinity of water at the sampling site decreased from 5 in 2018 to 2 in 2020. It was collected in the winter of 2018 when the salinity was about 5 and the water temperature was about 16℃. When the salinity of the wetland fell below 5, we failed to isolate the new species despite repeated attempts.
Eufolliculina moebiusiwas collected on 23 Junuary, 2019 from Taiping Cape Park (36°3΄N, 120°21΄36΄΄E), on the coast of Yellow Sea, Qingdao, where the water temperature was 22℃ and salinity was 30 (Fig.1B1). Specimens were collected using glass microscope slides as artificial substrates. The slides were fixed in reef crevices at low tide to keep them constantly immersed in seawater and left for about one month to allow colonization by folliculinids.The slides were then transported to the laboratory in habitat water. The numerous individuals ofE. moebiusion the freshly collected glass slides were visible to the naked eye and could be maintained for about 3 - 5 days at room temperature.

Fig.1 Maps showing the locations of Qingdao and Ningbo, and photographs of the sampling sites. A, Map of China; B,Map of portion of China, showing the location of Qingdao; B1, Sampling site for Eufolliculina moebiusi; C, Map of portion of China, showing the location of Ningbo; C1, Sampling site for Diafolliculina longilobata n. gen., n. sp.
Raw cultures of two species were maintained for 5 - 7 days with habitat water at room temperature. Pieces of aquatic plants (stems and leaves) from the sampling site and rice grains were added to promote the growth of bacteria as food for the ciliates.
Living cells attached to their substrate were examined at (40 - 400)× magnification using an inverted microscope(Nexcope NIB400) to describe theirin vivomorphological characteristics,e.g., peristomial lobes, holdfast organelle, lorica closure device amomg others. Trophonts were squeezed out of their lorica using needles and transferred to a clean glass slide using a micropipette. Cells were observed under a bright field and differential interference contrast (DIC) microscopy (Leica DM2500) at (400 - 1000)×magnification. The ciliature was revealed with protargol staining method (Wilbert, 1975). Counts, measurements and drawings of stained specimens were performed at 1000×magnification.
2.2 DNA Extraction, PCR Amplification and Sequencing
For each species, genomic DNA was extracted according to Wuet al. (2019). In brief, total genomic DNA was extracted from a single cell of trophont using the DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany). The SSU rDNA was amplified using the primers 18SF (5’-AAC CTG GTT GAT CCT GCC AGT-3’) and 18SR (5’-TGA TCC TTC TGC AGG TTC ACC TAC-3’) (Medlinet al., 1988).The cycling conditions were as follows: an initial denaturation step of 98℃ for 30 s followed by 35 cycles of 10 s at 98℃, 20 s at 56℃ and 100 s at 72℃; and a final extension step at 72℃ for 5 min. To minimize the possibility of amplification errors, Q5 Hot Start High-Fidelity DNA Polymerase (New England BioLabs, United States) was used.Sequencing was performed bidirectionally by the Tsingke Biological Technology Company (Beijing, China).
2.3 Phylogenetic Analysis
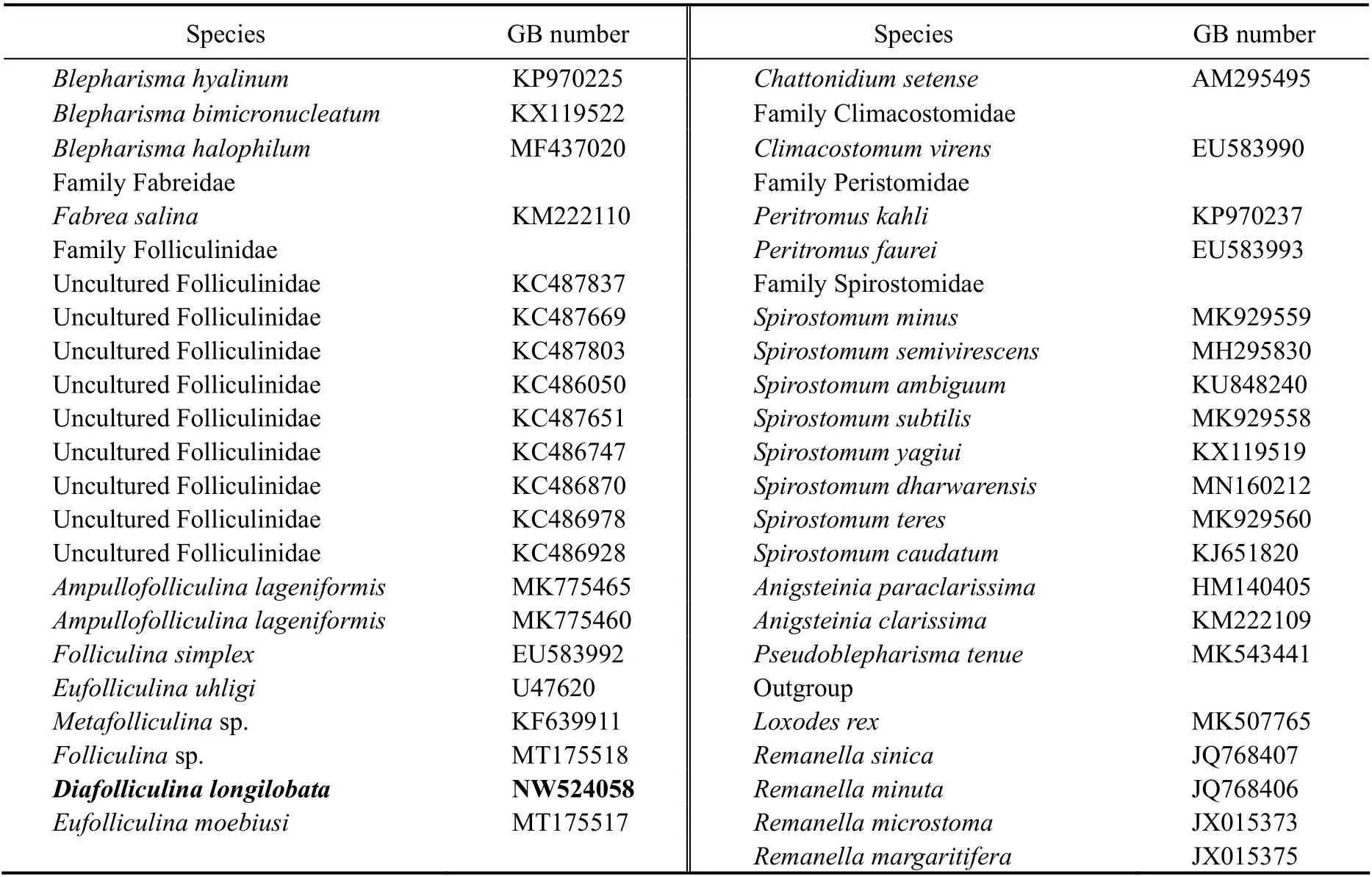
The new SSU rDNA sequence from present work and 73 sequences downloaded from the GenBank were used in the phylogenetic analysis. The downloaded sequences represent the species in Stentoridae (9 sp.), Blepharismidae (11 sp.), Fabreidae (1 sp.), Folliculinidae (7 sp. and 9 environmental sequences), Maristentoridae (1 sp.), Gruberiidae (2 spp.), Condylostomatidae (14 spp.), Climacostomidae (1 sp.), Peritromidae (2 sp.), Spirostomidae (11 sp.)and Karyorelictea (5 sp.; as outgroup) (Table 1). The 74 sequences were aligned using MUSCLE implemented in GUIDANCE with default parameters (Pennet al., 2010).The ends of the resulting alignments were trimmed manually using the program BioEdit v7.0.5.3 (Hall, 1999).Maximum likelihood (ML) analysis was conducted on CIPRES Science Gateway with RAxML-HPC2 on XSEDE v8.2.4 (Stamatakiset al., 2008) using the GTR + I + G model as selected according to the Akaike Information Criterion (AIC) by Modeltest v3.4 (Posada and Crandall, 1998).Support for the best ML tree was from 1000 bootstrap replicates. Bayesian inference (BI) analysis was performed on CIPRES Science Gateway with MrBayes on XSEDE v3.2.6 (Ronquist and Huelsenbeck, 2003) using the GTR+ I + G model selected by MrModeltest v2.2 (Nylander,2004). The chain length for the analysis was 106generations with trees sampled every 100th generation. The first 2500 trees were discarded as burn-in. The tree topologies were visualizedviaMEGA v6.0 (Tamuraet al., 2011) and TreeView v.1.6.6 (Page, 1996). Systematic classification mainly followed Hadži (1951), Lynn (2008) and Shazibet al.(2014).

Table 1 Genbank accession numbers of all sequences included in phylogenetic analyses
(to be continued)
(continued)
3 Results
3.1 Order Heterotrichida Stein, 1859 Family Folliculinidae Dons, 1914
Diafolliculinan. gen.
Synonym:DiafolliculinaHadži, 1951
3.1.1 ZooBank registration
Number: urn:lsid:zoobank.org:act:5F0C06C5-6A02-45DFB721-965AC4E674D1.
Hadži (1951) erected this genus for three folliculinids with two closure devices and single macronucleus and supplied a brief diagnosis. However, he did not assign the type species, so it was a nomen nudum. Based on our detailed taxonomic data, we reactivate this genus, give an improved diagnosis, and assign the type species here.
3.1.2 Improved diagnosis
Lorica surface smooth, flask rounded and recumbent,neck short and unsculptured; closure device in the form of two delicate flaps, one ventral and one dorsal; peristomial lobes similar or dissimilar in size and shape; holdfast organelle spatula-shaped or stout stem-shaped; single macronucleus.
3.1.3 Etymology
The nameDiafolliculinais a composite of the Greek prefixdia+ (through, across, between) and the name of the folliculinid genusFolliculina, and refers to the similar morphology ofDiafolliculinaandFolliculina. Feminine gender according to Article 30.1.1 of the International Code of Zoological Nomenclature (ICZN, 1999).
3.1.4 Type species
Diafolliculina longilobataYeet al., 2021
3.1.5 Species assigned
D. longilobatan. gen., n. sp.;D. rotunda(Hadži, 1951)n. comb.;D. similis(Hadži, 1951) n. comb.;D. thomseni(Hadži, 1951) n. comb. (syn.Folliculina boltoniKent, 1881 sensu Thomsen, 1921).
3.2 Diafolliculina longilobata n. gen., n. sp.
3.2.1 ZooBank registration
Number: urn:lsid:zoobank.org:act:593CF447-F0D4-4DBC-8A59-09AE12405075.
3.2.2 Diagnosis
Lorica about (120 - 155) × (75 - 100) μm in size; neck obliquely raised above substratum, about (45 - 65) × (40 - 45)μm in size; ventral flap of closure device larger than dorsal one; fully extended trophont about (270 - 375) × (20 - 30)μm; peristomial lobes relatively inflexible, long and slender, equal in shape and size, each possessing a pointed tip;posterior region of trophont gradually narrowed; holdfast organelle small, spatula-shaped; cortical granules ellipsoidal, greenish in color, forming 1-2 irregular rows between adjacent kineties; pigment granules globular, light-green in color; single macronucleus ellipsoidal, usually closely associated with two globular micronuclei; oral apparatus with 283 - 477 adoral membranelles and single-rowed paroral membrane; 35 - 49 somatic kineties; brackish water habitat (Table 2).
3.2.3 Etymology
The species-group namelongilobatais a composite of the Latinlongi- (adjective: long, slender), Latinlobat- (noun:lobe, lobate), and Latin -a[feminine suffix, agreement in gender with genus, according to Article 31.2 of the International Code of Zoological Nomenclature (ICZN, 1999)],and refers to this species having long, slender peristomial lobes.

Table 2 Morphometric characteristics of Diafolliculina longilobata n. gen., n. sp. (D. long)and Eufolliculina moebiusi (E. moeb)
3.2.4 Type material
Ten slides with protargol-stained specimens have been deposited in the Laboratory of Protozoology, Ocean University of China (OUC), including one slide (registration number: LJ-20181127-01) with the holotype specimen circled in black ink and nine slides (registration number: LJ-20181127-02, 03···10) with paratype specimens.
3.2.5 Type locality
Subtropical brackish wetland in Meishan Island of Ningbo (29°46΄33΄΄N, 121°57΄47΄΄E), near East China Sea, the water temperature was 16℃ and salinity was 5.
3.2.6 General morphology and ciliary pattern
Lorica transparent and colorless, consisting of swollen recumbent flask and unsculptured neck (Figs.2B - G, 3A,D, E). Flask pyriform when viewed from top, about (120 -155) × (75 - 100) μm in size, ratio of width to length about 3:5 (Figs.2A, 3G). Neck short and wide, about (45 - 65) ×(40 - 45) μm, obliquely raised at an angle of 60°-90° relative to basal plate (Figs.2B - G, 3A, D, E, H), occasionally with two parallel ring strips (Fig.2E, arrowhead). Neck opening with narrow brim slightly curved outwards (Figs.2A -E, 4A, B). Closure device comprising two transparent flaps inserted asymmetrically at posterior end of neck, one dorsal and one ventral; flaps unequal in size and shape, ventral flap (Figs.2A, B, green arrows) longer, slightly bent forwards and overlaps dorsal flap (Figs.2A, B, red arrows).Lorica and basal plate (Figs.3E, G, purple arrowheads) usually covered by a thin layer of biofilm in mature specimens.
Trophont (270 - 375) μm × (20 - 30) μmin vivowhen fully extended; if distributed or otherwise stimulated, trophont retracts rapidly into lorica and the two flaps closely sealing the cell inside the flask; contracted trophont about (100- 145) μm × (30 - 85) μm (Figs.2C, G, 3A, D, G). Peristomial lobes relatively inflexible, long and slender, about equal in shape and size ((95 - 120) μm × (15 - 20) μm), each with a pointed tip at its anterior end (Figs.2G, 3A - C, F).Posterior part of trophont gradually narrowed. Holdfast organelle small spatula-shaped (Figs.2G, 4C, yellow arrow). Cortical granules greenish, oval to elliptical in outline (about 0.7 × 0.9 μm), forming one or two irregular lines between adjacent ciliary rows (Figs.2K, 4E). Pigment granules light-green, globular, about 0.5 μm in diameter, forming several irregular rows between adjacent kineties (Figs.2K, 4F, orange arrows). Cytoplasm colorless, usually containing several food vacuoles (Figs.2C, G, 3D, G). One macronucleus, ellipsoidal, about (19 - 36) μm × (14 - 31) μm in size (Figs.4H, I); two globular micronuclei closely associated with macronucleus (Figs.2I, 4D, double arrowheads). Contractile vacuole absent.
Oral apparatus complex (Figs.2H - J, 4G - I). Adoral zone of membranelles (AZM) conspicuous, composed of about 283 - 477 membranelles, cilia about 17 - 25 μm longin vivo.AZM commences on ventral side at base of right lobe(Figs.2I, 4G), extends along margin of each peristomial lobe and spirals into buccal cavity along left lobe margin,making about one-and-a-half further turns within buccal cavity (Figs.2I, J, 4G, I). Bases of membranelles of distal portion of AZM longer than those of proximal and main portions (Figs.2J, 4G). Paroral membrane single-rowed,arranged along margin of peristomial lobes parallel to AZM, but not entering buccal cavity (Fig.2J). About 35 -49 somatic kineties composed of dikinetids, cilia about 8 -10 μm longin vivo(Figs.2H, I, 4H, I).
3.3 Eufolliculina moebiusi (Kahl, 1932) Hadži, 1951
Eufolliculina moebiusiwas originally reported asFolliculina ampullaby Möbius (1887). Since then, many populations and synonyms of this species have been reported worldwide without detailed morphological data (Kahl,1932; Fauré-Fremiet, 1936; Hadži, 1938; Mulisch and Patterson, 1983). An improved diagnosis is provided here based on previous and current data.
3.3.1 Improved diagnosis
Lorica wall thick, colorless, light bluish or yellowish;flask recumbent, elliptical in outline when viewed from top,(115 - 265) × (90 - 100) μm in size, ratio of length to width about 5:2; neck short, about (80 - 150) × (40 - 65) μm, raised at angle of 30°- 45° relative to basal plate, with spiral ridge that makes 4 - 6 indistinct turns; trophont (475 - 560) × (35- 50) μm when fully extended; peristomial lobes flexible and slender, equal in shape and size; posterior portion of body gradually narrowed; holdfast organelle slightly swollen; cortical granules elliptical in outline, dark greenish in color,forming 2 - 3 irregular rows between adjacent somatic kineties; pigment granules globular, bluish; macronucleus moniliform, comprising 4 - 14 nodules; AZM with 353 -524 membranelles; paroral membrane composed of dikinetids; 42 - 58 somatic kineties (Table 2).
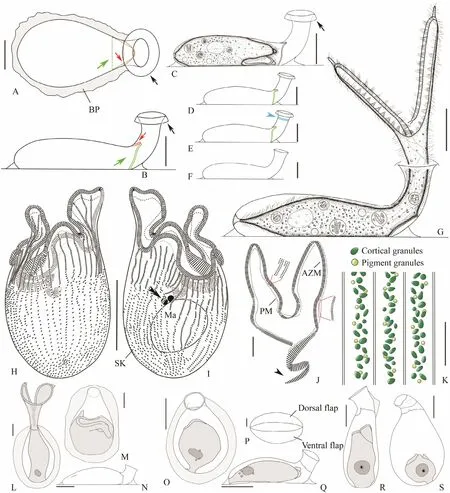
Fig.2 Living morphology and infraciliature of Diafolliculina longilobata n. gen., n. sp. (A - K) and three congeners (L - S).A, B, Top (A) and lateral (B) views of empty lorica, red arrows to show the dorsal flap of closure device, green arrows to show the ventral flap of closure device, black arrows denote the conspicuous brim of neck opening; C, A contracted individual, arrow marks the brim of neck opening; D - F, Lateral views of different loricae, blue arrow in (E) marks transverse striations near the neck opening which are only present in some individuals, (F) to show a newly formed lorica without closure device or brim; G, Lateral view of a representative individual; H, I, Infraciliature of the ventral (H) and dorsal (I)sides of the holotype specimen, double arrowheads show the micronucleus; J, Detailed illustration of buccal apparatus,including the adoral zone of membranelles and paroral membrane, arrow shows the distal end of the AZM; K, Showing the distribution of cortical granules and pigment granules of the trophont; L - N, Diafolliculina thomseni (redrawn after Thomsen, 1921); O - Q, D. rotunda (redrawn after Hadži, 1951); R, S, D. similis (redrawn after Hadži, 1951). AZM, adoral zone of membranelles; BP, basal plate; Ma, macronucleus; PM, paroral membrane; SK, somatic kineties. Scale bars = 50 μm (A - J, L -O, Q - S), 5 μm (K, P).

Fig.3 Photomicrographs of Diafolliculina longilobata n. gen., n. sp. in vivo. A, Lateral view of a representative extended individual, red and green arrows show the closure device; B, Peristomial lobes; C, Showing the distal end of the adoral membranelles spiraling into the buccal cavity, arrow denotes brim of neck opening; D, Lateral view showing a contracted individual inside the lorica, red and green arrows show the dorsal and ventral flaps, respectively, of closure device; E, An ageing individual with atrophied body and almost empty lorica to show the flask and neck, black arrow shows the brim of neck opening, purple arrowheads show the basal plate; F, Detail of peristomial lobes, red rectangle to show the pointed tip at the top of the lobe; G, Top view of a contracted individual inside the lorica, arrowhead shows the basal plate; H, Lateral view of anterior portion of lorica, showing the flaps of the closure device (red and green arrows show dorsal and ventral flaps, respectively) and brim of neck opening (black arrow); I, Detail of closure device (red and green arrows) in an extended individual. Scale bars = 50 μm.

Fig.4 Photomicrographs of Diafolliculina longilobata n. gen., n. sp. in vivo (A-F) and after protargol staining (G - I). A,Lateral view of the neck opening to show the conspicuous brim of the neck opening (arrow); B, Top view of brim (arrow);C, Posterior end of cell to show the holdfast organelle (arrow); D, Nuclear apparatus comprising a globular macronucleus and two micronuclei (double arrowheads); E, Grey-greenish elliptical cortical granules; F, Light-green spherical pigment granules (arrows); G, Detailed illustration of buccal apparatus; H, I, Infraciliature of the ventral (H) and dorsal (I) sides of the holotype specimen. Ma, macronucleus. Scale bars = 50 μm (C, H, I), 10 μm (A, B, D - G).
3.3.2 Voucher slides
Six voucher sliders (registration numbers: CY-2019022 301) with protargol-stained trophonts are deposited in the Laboratory of Protozoology, Ocean University of China(OUC).
3.3.3 Morphological description of Qingdao population
Lorica transparent, wall thick, light bluish or yellowish in color. Flask recumbent, pyriform when viewed from above,about (115 - 255) μm × (90 - 100) μm in size, ratio of length to width about 5:2 (Figs.5A - D, F, 6A, E, G, H). Neck short,about (80 - 110) μm × (55 - 65) μm in size, obliquely raised at 30° - 45° angle relative to basal plate, weakly sculptured by spiral ridge that makes 4 - 6 turns (Figs.5A - D, F, 6A, E),neck opening with brim slightly curved outwards (Figs.5A -D, F, 6F). Junction of flask and neck near the substrate with bracket-like fold when viewed from top (Figs.5A, C,6G, arrows). Basal plate transparent, thick, and conspicuous (Figs.5A - D, F, 6A, E).
Trophont (475 - 560) μm × (35 - 50) μmin vivowhen fully extended (Figs.5D, F, 6A, E). Peristomial lobes flexible and slim, equal in shape and size, about 140-185 μm long,connected by pellicular flap that extends from right lobe(Figs.5A, D, 6A - D). Right lobe of some individuals with pointed tip (Fig.6D). Posterior part of cell gradually narrowed. Holdfast organelle slightly swollen (Figs.5D, 6L).Cortical granules dark greenish, oval to elliptical in outline, about 0.8 × 0.9 μm, forming 2-3 irregular rows between somatic kineties (Figs.5E, 6M). Pigment granules bluish, spherical, about 0.8 μm in diameter, forming several irregular rows intermingled with rows of cortical granules between adjacent somatic kineties (Figs.5E, 6M).Cytoplasm colorless, usually containing numerous food vacuoles (Fig.6H). Macronucleus moniliform, comprising 4-14 nodules (Figs.5I, 6P). Micronucleus not observed. Contractile vacuole absent.
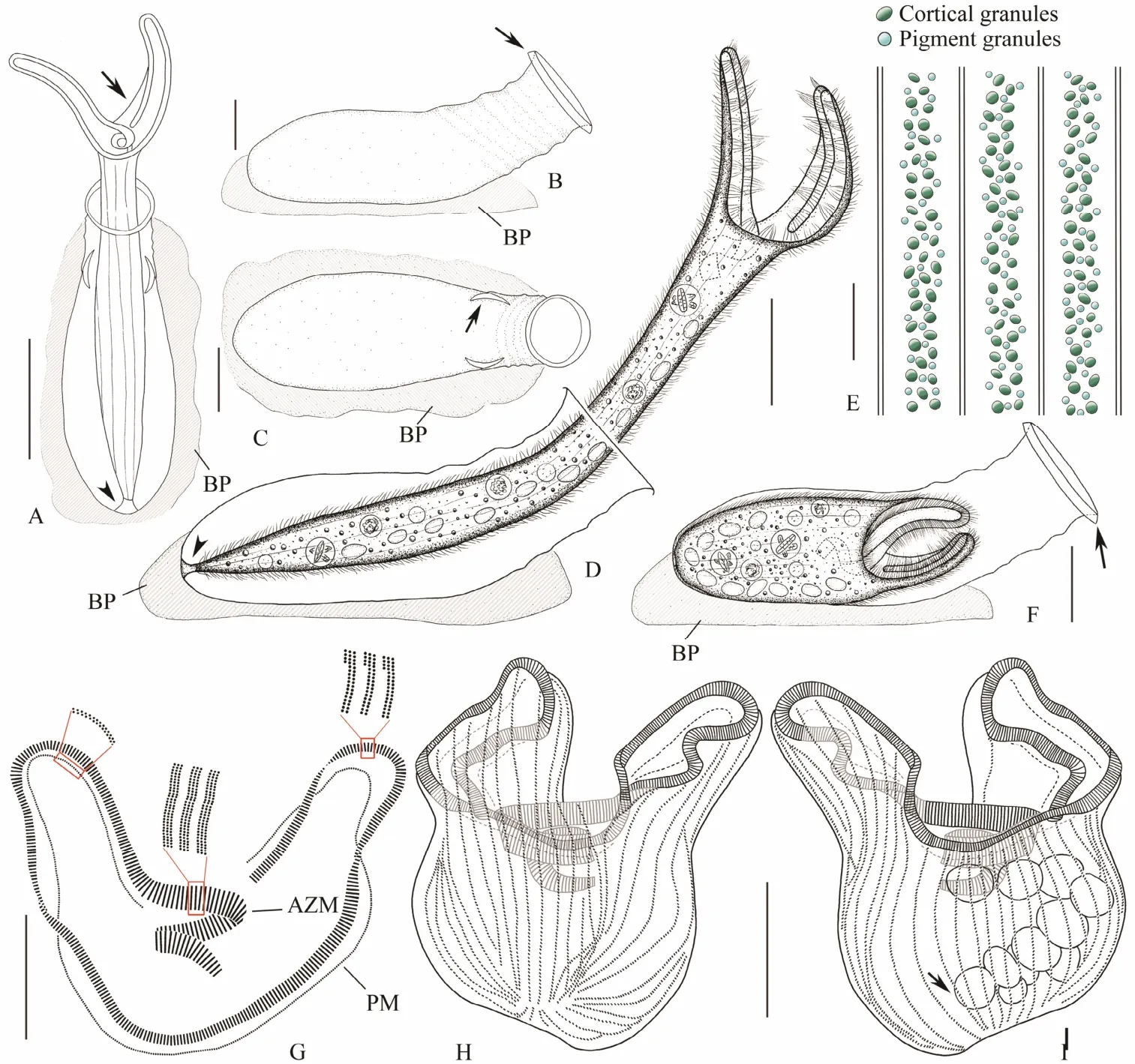
Fig.5 Living morphology and infraciliature of Eufolliculina moebiusi. A, Top view of an extended individual, arrow to show the pellicle of right peristomial lobe, arrowhead to show holdfast organelle; B, C, Lateral (B) and top (C) views of an empty lorica, arrow in (B) to show the brim of neck opening, arrow in (C) to show the bracket-like fold at the junction of lorica neck and substrate; D, Lateral view of a representative extended individual, arrowhead to show holdfast organelle; E, Cortical granules and pigment granules of trophont; F, Lateral view of a contracted individual, arrow to show brim of neck opening; G, Detailed illustration of buccal apparatus, including the adoral zone of membranelles and paroral membrane; H, I, Infraciliature of the ventral (H) and dorsal (I) sides of the same specimen, arrow to show the macronucleus. AZM, adoral zone of membranelles; BP, basal plate; PM, paroral membrane. Scale bars = 100 μm (A, D), 50 μm (B,C, F - I), 5 μm (E).
Oral apparatus complex (Figs.5G, I, 6N, P). AZM conspicuous and composed of 353 - 524 membranelles, cilia about 10 - 17 μm in lengthin vivo(Figs.5D, G - I, 6B, P).AZM commences on ventral side at base of right lobe, runs along margin of peristomial lobes and spirals into buccal cavity along edge of left lobe, making about two-and-ahalf turns in total (Figs.5G, I, 6N, P). Bases of membranelles three-rowed, those along margin of peristomial lobes composed of two long and one short row, bases of membranelles that spiral into buccal cavity composed of three equal-length rows (Figs.5G, 6N). Paroral membrane parallel to AZM and composed of dikinetids, two kinetosomes almost parallel (Fig.5G). About 42 - 58 somatic kineties,including several short rows on both ventral and dorsal sides, cilia about 8 μm longin vivo(Figs.5H, I, 6O, P).
Swarmer about (200 - 225) μm × (40 - 60) μm in size,emerald greenish (Fig.6J, K). Color of swarmer always darker than that of trophont (Fig.6I).

Fig.6 Photomicrographs of Eufolliculina moebiusi in vivo (A-M) and after protargol staining (N-P). A, Lateral view of a representative extended individual; B-D, Peristomial lobes, arrow in (C) to show the pellicle of right peristomial lobe,arrow in (D) to show the pointed tip of right peristomial lobe; E, Lateral view of a contracted individual; F, Top view of neck opening, arrow to show brim; G, Top view of a contracted individual, arrows to show the bracket-like fold at the junction of lorica neck and substrate; H, Posterior part of an extended individual, showing yellowish thick lorica covered by diatom debris; I, Swarmer and trophont in the same lorica; J, K, Different shapes of swarmer; L, Posterior end of an extended individual, arrow to show the holdfast organelle; M, Cortical granules and pigment granules of trophont; N, Detail of oral region showing that adoral zone of membranelles makes one-and-a-half turns as it spirals into the buccal cavity,note that each membranelle is composed of three equal rows (arrows); O, P, Infraciliature of the ventral (O) and dorsal (P)sides of the same specimen. Scale bars = 100 μm (A-E, G-K), 50 μm (F, L, O, P), 10 μm (M, N).
3.4 Phylogenetic Analysis
The newly obtained SSU rDNA sequence was deposited in the GenBank database with lengths, G + C contents and accession numbers as follows:Diafolliculina longilobatan. gen., n. sp. (1650 bp, 47.70%, NW524058). The SSU rDNA sequence deposited in the GenBank database by Chiet al. (2021) with accession number MT175517 was misidentified as aFolliculinopsisand is, in fact,Eufolliculina moebiusi.
The topologies of the ML and BI trees were almost same,therefore only ML is shown here with support values from both analyses (Fig.7). According to this tree, Blepharismidae and Folliculinidae are paraphyletic asBlepharisma halophilumand nine sequences deposited under the name‘uncultured Folliculinidae’ group with the single sequence of the family Fabreidae. Each of the other eight families of Heterotrichea is monophyletic. All species of Folliculinidae for which detailed morphological information are documented form a well-supported clade (90% ML, 1.00 BI), including two strongly supported subclades.Maristentor dinoferus, the only member of the family Maristentoridae, is sister to the family Folliculinidae.
There are two genera in Subclade I of Folliculinidae,namelyMetafolliculinaandEufolliculina. The Qingdao population ofEufolliculina moebiusiclusters withEufolliculina uhligi(U47620) with medium to high support values (ML 98%, BI 0.87). Subclade II includes three genera,Folliculina,Ampullofolliculina, andDiafolliculinan.gen., with the former branching off first, then the two sequences ofAmpullofolliculina lageniformisgroup withD.longilobatan. gen., n. sp. (ML 61%, BI 0.81).
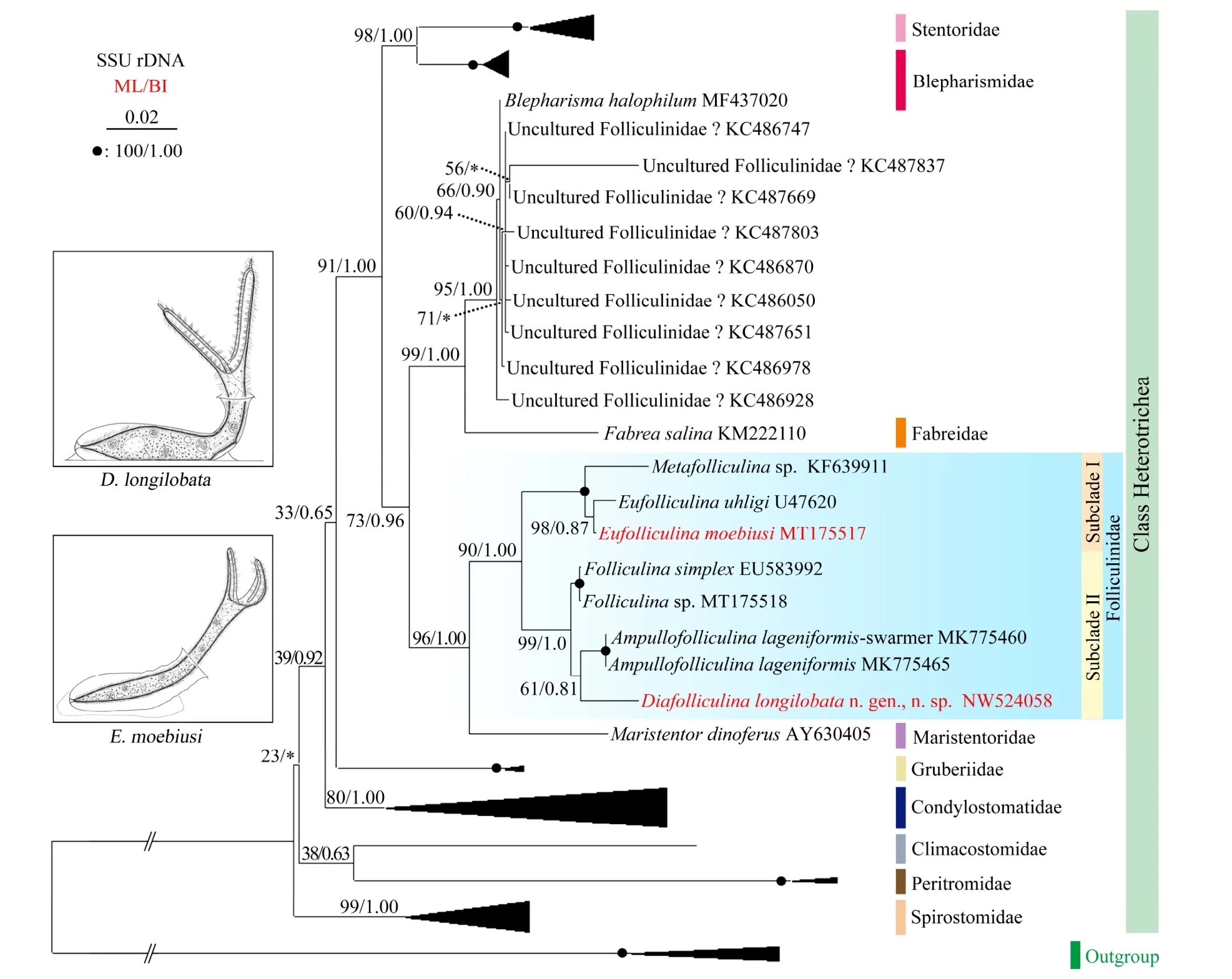
Fig.7 Maximum likelihood tree inferred from SSU rDNA sequences showing the positions of Diafolliculina longilobata n.gen., n. sp. and Eufolliculina moebiusi (bold red font). Numbers near the nodes represent the ML bootstrap values and BI posterior probability values. Asterisks indicate the disagreement between the ML and BI trees, question marks denote sequences the identities of which are controversial. Fully supported (100/1.00) branches are marked with solid circles. The bar corresponds to 2 substitutions per 100 nucleotide positions.
4 Discussion
4.1 Comments on Diafolliculina n. gen.
Thomsen (1921) collected and described a species from South America,Folliculina boltoniKent, 1881. In Thomsen’s description and illustration, this organism has an obvious closure device and spatula-shaped holdfast organelle.This combination of features was not found in any known genus; therefore a new genus was established by Hadži(1951) who renamed Thomsen’s isolate asD. thomseni.Two other species,D. rotundaandD. similis, were collected from the Adriatic Sea by Hadži and briefly described(Hadži, 1951). However, as noted by Aescht (2001) and Lynn (2008),Diafolliculinais a nomen nudum for the lack of type species fixation. We here reactivate the genusDiafolliculinaand designatethe well studied new speciesD.longilobataas the type species.
Diafolliculina thomseniwas regarded as a freshwater species by Hadži (1951) because the type locality was a freshwater pond near Montevideo, Uruguay. However, the salinity of the samples was not supplied by Thomsen (1921)and the original description was given simply as ‘Ein aufgestauter Teiche in der Nähe Montevideos auf Süsswasserpflanzen und abgefallenen Blättern von Uferbäumen’(English: an uprooted pond near Montervideo on freshwater plants and fallen leaves of shore trees). A diagnosis ofD. thomseniinferred from Thomsen (1921) and Hadži(1951) descriptions as following: lorica flat and transparent, light-greenish in color; flask recumbent, pyriform when viewed from above, about 212 × 153 μm in size; neck short with enlarged opening, raised at angle of about 60° relative to basal plate; closure device in form of two flaps, ventral flap larger than dorsal one; trophont about 420 μm long when fully extended, with short peristomial lobes that differ in shape and size, left lobe wide, spoon-shaped,and longer than slim, pointed right one; holdfast organelle spatula-shaped, about as wide as body; one ellipsoidal macronucleus.
Diafolliculina rotundawas collected from coastal waters of the Adriatic Sea at a shallow port near Split, in July,1937 (Hadži, 1951). Although most specimens were contracted trophonts, Hadži (1951) provided many important morphological features and gave the following diagnosis:lorica transparent and light-greenish in color; flask recumbent, ellipsoidal when viewed from above, about (100- 135) μm × (68 - 100) μm in size; neck short and unsculptured, (25 - 40) μm × (27 - 35) μm in size; closure device in form of transversely stretched membrane with a central gap(that is, ventral and dorsal flaps equal in shape and size);peristomial lobes unequal in shape and size; holdfast organelle spatula-shaped; single macronucleus; 2 - 3 micronuclei.
Diafolliculina similiswas collected from coastal waters of the Adriatic Sea at Trieste. LikeD. rotunda, most morphological features are based on observations of contracted trophonts (Hadži, 1951). It was diagnosed by Hadži(1951) as follows: lorica transparent and colorless; flask flat, recumbent, about (110 - 170) μm × (70 - 130) μm in size;neck short, (25 - 60) μm × (32 - 45) μm in size, with wide opening; closure device in the form of dorsal and ventral flaps, ventral one larger than dorsal one; peristomial lobes unequal; holdfast organelle columnar; single macronucleus with one or two heterogenous inner cores.
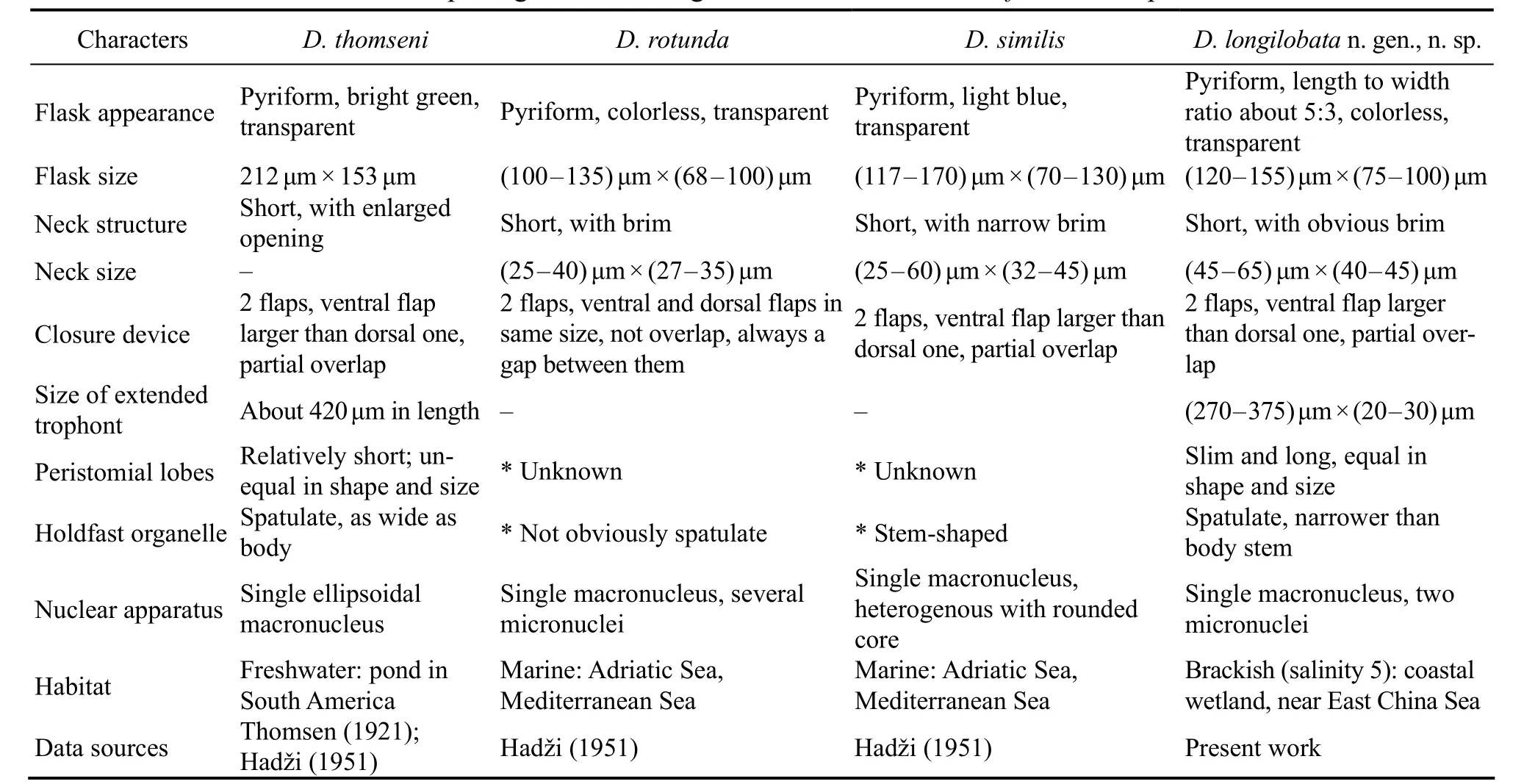
Table 3 Morphological and ecological characters of four Diafolliculina species
4.2 Comments on Eufolliculina moebiusi (Kahl,1932) Hadži, 1951
Hadži (1951) established the genusEufolliculinapartly based on its short, unsculptured neck, thus differentiating it fromMetafolliculinaDons, 1924.Eufolliculina moebiusi(Kahl, 1932) Hadži, 1951 was originally described asFolliculina ampullaby Möbius (1887). Kahl (1932) considered that organism to be a separate species and renamed it asFolliculina moebiusi. Hadži (1951) placed this species in the newly erected genusEufolliculinaand fixed it as the type species. Five other species have previously been assigned to the genusEufolliculina, namelyE. ampullaceaHadži, 1951,E. latemarginata(Hadži, 1938)Hadži, 1951,E. lignicola(Fauré-Fremiet, 1936) Hadži,1951,E. uhligiMulisch & Patterson, 1983, andE. bruneaJankowski, 2009. Mulisch and Patterson (1983) provided a detailed review ofEufolliculinaand regardedE. ampullacea,E. latemarginata, andE. lignicolaas synonyms ofE. moebiusi. We accept this conclusion. As discussed above,several populations or synonyms ofE. moebiusihave been reported, but none were adequately described and are therefore ambiguous. Thus, an improved diagnosis and revised circumscription of this species are provided here based on the previous and current data.
In terms of the living characteristics and lorica structure,E. moebiusiandE. uhligiare very similar. However, the former can be distinguished from the latter by having a thick (vs. thin) lorica wall and 42 - 58 (vs. 60 - 65) somatic kineties. Moreover, the SSU rDNA sequences of these two species differ from each other by 14 nucleotides which further supports their distinction as separate species.
Eufolliculina bruneawas collected from the Barents Sea,Russia (Jankowski, 2009). All morphological data were obtained from fixed samples. For instance, the lorica was brown in color and (202 - 234) μm × (54 - 71) μm in size,flask margin was straight or slightly wavy, neck was unsculptured, trophont had about 50 somatic kineties, and moniliform macronucleus including 12 - 18 nodules. It is therefore possible thatE. bruneais a population ofE. moebiusias there are no significant differences between these two organisms based on the above-mentioned morphological features. The validity ofE. bruneatherefore awaits details of its morphologyin vivoand gene sequence data.
4.3 Phylogenetic Analyss
As shown in previous molecular phylogenetic studies(Shazibet al., 2014; Fernandeset al., 2016; Yanet al., 2016;Chenet al., 2019; Luoet al., 2019; Campello-Nuneset al.,2020), the class Heterotrichea is a well-supported clade in the SSU rDNA tree (Fig.7). The phylogenetic relationships of five heterotrich families,i.e., Stentoridae, Blepahrismidae, Fabreidae, Folliculinidae and Maristentoridae,are very stable in all known studies, while the other five families (Spirostommidae, Peritromidae, Climacostomidae,Condylostomatidae, and Gruberiidae) are not well resolved as indicated by the low support values for these taxa both in previous and present analyses. Recently, the phylogeny of Heterotrichea was studied based on a combination of morphological features and multi-gene information (Chiet al., 2021). Here we focus on evolutionary relationships within the family Folliculinidae.
Nine sequences deposited under the name ‘uncultured Folliculinidae’, collected from the hypersaline Lake Tyrrell, Australia, clustered with the hypersaline heterotrichean speciesBlepharismahalophilumandFabreasalina(Song and Packroff, 1997; Chenet al., 2011; Heidelberget al., 2013; Pan and Stoeck, 2017). This hypersaline cluster suggests that habitat may play an important role in the evolution of heterotrichean ciliates (Chiet al., 2021).
Except for nine uncultured sequences, the rest of folliculinid sequences are clustered into two subclades: Subclade I (MetafolliculinaandEufolliculina) and Subclade II (Folliculina,AmpullofolliculinaandDiafolliculina). The close relationship betweenMetafolliculinaandEufolliculinais supported by their morphological similarities that both genera have a moniliform macronucleus and very flexible peristomial lobes (Hadži, 1951; Andrews, 1952).By contrast, the only unique character that the three genera in Subclade II (Folliculina,AmpullofolliculinaandDiafolliculina) have in common is that they all have relatively inflexible peristomial lobes. The present grouping pattern (very flexiblevs. relatively inflexible peristomial lobes)supports the assertion that the structure of the peristome is a more phylogenetically informative character than the morphology of the macronucleus or lorica, as proposed by Mulischet al. (1993). Other morphological characters used for species circumscription and identification of folliculinids, such as the macronucleus shape, presence or absence of a closure device, flask shape, and holdfast organelle shape, are not concordant with the topology of the SSU rDNA tree. For example, species with different macronuclear shapes,e.g.,Ampullofolliculina(moniliform macronucleus) andFolliculinaandDiafolliculina(single globular or flattened macronucleus) cluster together. However, in the family Spirostomidae, species with the same macronucleus type cluster together,i.e., all species with a moniliform macronucleusvs. those with a single globular or band-like macronucleus (Boscaroet al., 2014; Chiet al.,2021). Species with loricae that lack a closure device do not, however, cluster separately from those that possess such a device. For example,Folliculina(closure device absent) is the closest relative ofAmpullofolliculinaandDiafolliculina(closure device present). It is noteworthy that SSU rDNA gene sequences are only available for five out of 33 folliculinid genera and morphological data revealed by silver staining or transmission electron microscopy are available for less than 10% of folliculinid species. Therefore, such data are needed for a greater number of taxa in order to determine more scientific and reasonable evolutionary relationships within the family Folliculinidae.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31970398), the National Key Research and Development Program of China (No.2018YFD0900701), and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (No. 201 9333).
杂志排行
Journal of Ocean University of China的其它文章
- Paleosalinity and Its Association with Organic Matter:A Case Study from the Eocene Shahejie Formation,Laizhou Bay Sag, Bohai Bay Basin (China)
- Studies on the Inversion Phenomenon of Physical Properties Observed in the Huagang Formation Reservoir in the Xihu Sag Based on the Water-Rock Reaction Experiments
- Effect of Sand Body Enrichment Under the Restriction of a Tectonic Transfer Zone: A Case Study on the Pinghu Formation in the Kongqueting Region on the Pinghu Slope
- Relationship Between Paleogene Reservoir Densification and Hydrocarbon Accumulation in the Xihu Depression
- Adjoint Method-Based Algorithm for Calculating the Relative Dispersion Ratio in a Hydrodynamic System
- Analysis of the Leading Modes of Autumn Precipitation over the Yangtze River Basin
