Fucoidans from Thelenota ananas with 182.4 kDa Exhibited Optimal Anti-Adipogenic Activities by Modulating the Wnt/β-Catenin Pathway
2021-09-01LIUYuanyuanWANGNaTIANYingyingCHANGYaoguangandWANGJingfeng
LIU Yuanyuan, WANG Na, TIAN Yingying, CHANG Yaoguang,*,and WANG Jingfeng,*
1) College of Food Science and Engineering, Ocean University of China, Qingdao 266003, China
2) Marine Biomedical Research Institute of Qingdao, Qingdao 266061, China
Abstract In this study, fucoidans were extracted from the sea cucumber Thelenota ananas (Ta-FUCs) by enzymatic degradation.Four products with molecular weights of 1380.3, 524.0, 182.4, and 110.3 kDa were obtained, and the Ta-FUC showing optimal antiadipogenic activities was determined. Results of MTT and Oil red O staining analyses showed that the Ta-FUCs inhibited the proliferation and differentiation of 3T3-L1 adipocytes. Futhermore, Ta-FUCs significantly downregulated the key transcriptional factors,such as SREBP-1c, PPARγ, and C/EBPα of adipocytes. The Ta-FUCs also activated Wnt/β-catenin pathway-related genes, such as β-catenin, LRP5, and FrZ. The Ta-FUCs suppressed lipid accumulation in 3T3-L1 adipocytes possibly by decreasing the expression of genes ACC, FAS, ME, GPAT, DGAT, and PILN, which are important in the synthesis of fatty acids and triglycerides; and by increasing the expression of genes PPARα, CPT-1α, and ACOX, which are crucial in fatty acid β-oxidation. The anti-adipogenic activities initially increased and then declined with decreasing molecular weight. Among the Ta-FUCs, the 182.4 kDa Ta-FUC exhibited optimal bioactivities. This study reports for the first time that Ta-FUCs can prevent obesity by regulating the differentiation and lipid accumulation of adipocytes.
Key words fucoidan from Thelenota ananas; molecular weight; anti-adipogenic activities; adipocyte differentiation; lipid accumulation; Wnt/β-catenin pathway
1 Introduction
Adipocytes secrete various adipocytokines that can regulate many metabolic activities, such as endocrine regulation (Salmeronet al., 2013). Obesity is associated with hypertension, type 2 diabetes, coronary heart disease, and arthritis (Rahmouniet al., 2005; Teichtahlet al., 2008; Logueet al., 2011; Kauret al., 2017). It has become a chronic epidemic disease affecting human health. Increases in the number and volume of adipocytes can directly cause obesity. Therefore, controlling adipocyte differentiation and lipid accumulation may be a strategy for the clinical prevention and treatment of obesity.
Fucoidans belong to marine acid mucopolysaccharide families and are major functional components in the body wall of sea cucumbers (Vo and Kim, 2013). Fucoidans from sea cucumber (SC-FUCs) exhibit multiple bioactivities, such as modulation of metabolic syndromes (Liet al., 2019), prevention of tumor (Kwak, 2014), improvement of liver injury (Limet al., 2015), prevention of ethanol gastric ulcer(Wanget al., 2014), and other physiological functions. However, primary polysaccharides are characterized by a heavy molecular weight over 1000 kDa, high viscosity, and low solubility, which limit their bioactivities. Several studies have suggested that the molecular weight of polysaccharides greatly influences their bioactivities. Zuoet al. (2015)degraded SC-FUCs into fragments with different molecular weights and compared their protective effects on the intestinal mucosa. Results showed that 10 kDa fragments exhibit no apparent protection of intestinal mucosal activity relative to 50 -100 kDa fragments. Xuet al. (2018) found that fucoidans with different molecular weights and chain conformations could prevent ethanol-induced gastric ulcers and that their protective activity declines with decreasing molecular weight. Understanding the relationship between the molecular weights and bioactivities of polysaccharides is important for the design and bioactivity optimization of polysaccharides.
The present study aimed to clarify the molecular mechanism by whichTa-FUCs with different molecular weights inhibit the differentiation and adipogenesis of 3T3-L1 adipocytes and identify theTa-FUC exerting optimal bioactivities. This research will provide a basis for the exploitation and high-valued utilization of SC-FUCs in food science and marine medicine.
2 Materials and Methods
2.1 Materials
Wild sea cucumberThelenota ananaswas harvested from the South China Sea (Hainan, China). Mouse 3T3-L1 fibroblast cells were purchased from the American Type Culture Collection (CL-173, Manassas, VA, USA). High-glucose Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Biological Industries (Kibbutz Beit-Haemeck, Israel). 3-(4, 5 Dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT),insulin, dexamethasone, 3-isobutyl-1-methylxanthine (IBXM), and Oil red O colorant were provided by Sigma (St.Louis, MO, USA). Bovine serum albumin (BSA), triglyceride (TG) assay kit and bicinchoninic acid (BCA) kit were purchased from Solarbio (Beijing, China). Trizol reagent was a product of Life Technologies (Gaithersburg,MD, USA). M-MLV reverse transcriptase was obtained from Takara Bio Inc (Otsu, Shiga, Japan). SYBR Green mix was obtained from Roche (Basel, Switzerland). The primers of genes were synthesized by GENEWIZ (Suzhou,China).
2.2 Preparation of Ta-FUCs with Different-Molecular-Weights
Ta-FUCs were prepared as described below (Yuet al.,2014). Their primary structure has been determined as[→3-α-L-Fucp-1→3-α-L-Fucp-1→3-αL-Fucp2, 4 (OSO3-)-1→3-α-L-Fucp2 (OSO3-)-1→]n, and the main chemical structure is shown in Fig.1. The intracellular enzyme of marine bacterialFlavobacteriaceaeCZ1127 with different enzyme activities (0.25, 1.00, and 3.00 mU mL-1) was added to 50 mL of 0.4%Ta-FUC solution (pH 7.2) containing 0.3 mol L-1NaCl and 20 mmol L-1Tris-HCl (Chenet al., 2014). The enzymatic hydrolysis reaction was incubated at 35℃ for 12 h to obtain three low-molecular-weight fucoidans. Finally, the solution was retained at 100℃ for 10 min to terminate the enzymatic reaction. Products were subsequently purified with a HiPrep 26/60 Sephacryl S-300/400/500 HR column (GE Healthcare, Sweden, USA). Fractions around the major elution peak were pooled, dialyzed,and then lyophilized. Finally, three low-molecular-weight fucoidans were obtained. UntreatedTa-FUC was desinated asTa1, and the three low-molecular-weight fucoidans were designated asTa2,Ta3, andTa4. The sulfate contents of the products were analyzed with the method previously described to determine their structural integrity (Xuet al.,2018). The sulfate contents ofTa1,Ta2,Ta3, andTa4 were 28.2% ± 3.5%, 29.5% ± 2.5%, 28.6% ± 1.8%, and 29.0% ±2.2%, respectively. No obvious difference in sulfate content was found among the four groups, suggesting that the sulfate groups of theTa-FUCs were not hydrolyzed and theTa-FUCs possessed a uniform functional structure. The molecular weights (Mw) and polydispersity index (PDI) of theTa-FUCs were determined by using the HPSEC-MALLSVisc-RI system. The PDI of polysaccharides indicates their purity. The PDI values ofTa1,Ta2,Ta3, andTa4 were 1.21,1.18, 1.28, and 1.53, re- spectively. The PDI values of the fourTa-FUCs are close to 1, indicating the uniform molecular weight distribution and high purity of theTa-FUCs.The Mw values ofTa1,Ta2,Ta3, andTa4 were 1380.3 ±8.0, 524 ± 17.1, 182.4 ± 3.6, and 110.3 ± 5.2 kDa, respectively.The spatial images of the different-molecular-weightTa-FUCs were measured with the method previously described (Xuet al., 2018). In brief, theTa-FUCs were dissolved in deionized water with a concentration of 10 μg mL-1. A 10 μL sample solution was added onto muscovite mica substrate and then dried for more than 1.5 h. Images of the samples were recorded under tapping mode in air (25℃,ambient pressure, and humidity). The undecomposed monolayers of theTa-FUCs present semi-rigid chains, with less branches and crimp chain.Ta2 andTa3 are displayed as random coil chains, andTa4 shows a uniform spherical chain.
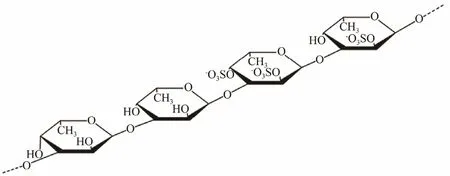
Fig.1 Primary structure of Ta-FUCs.
2.3 Cell Culture and Adipocyte Differentiation
3T3-L1 fibroblast cells were cultured in DMEM containing 10% FBS (v/v), 0.1 mg mL-1streptomycin, and 100 U mL-1penicillin at 37℃ under a humidified atmosphere with 5% CO2. 3T3-L1 cells were induced to mature adipocytes by using a standard cocktail method previously described (Hsuet al., 2010). In brief, 3T3-L1 cells were seeded into 24-well plates (2 × 104cells per well) and then incubated for 48 h until confluence (marked as day 0). Cells were treated with 0.01 mg mL-1insulin, 0.5 mmol L-1IBXM, and 10-3mmol L-1dexamethasone in DMEM with 10%FBS (v/v) for 48 h. Then the cell culture medium was replaced with DMEM containing 0.01 mg mL-1insulin and 10% FBS for another 48 h. The culture medium was changed with DMEM containing 10% FBS (v/v) every 2 days until day 8.
2.4 Cells Viability and Cytotoxicity
Cell viability was determined by using the MTT assay.3T3-L1 adipocytes in the logarithmic growth phase were seeded into 96-well plates (2 × 103cells per well). After being cultured for 24 h, cells were treated with various concentrations (0, 20, 40, and 80 μg mL-1) of different-molecular-weightTa-FUCs for 24 and 48 h. Then MTT solution (0.5 mg mL-1in DMEM medium) was added into the culture media and the cells were incubated for 4 h. The absorbance was obtained at 570 nm. Cell culture supernatant was collected at 48 h. Intracellular cytotoxicity was assessed through the activity of lactate dehydrogenase(LDH) by using a LDH kit.
2.5 Oil Red O Staining
3T3-L1 adipocytes were seeded into 24-well plates (2 ×104cells per well) and then divided into five groups: control,Ta1-treated,Ta2-treated,Ta3-treated, andTa4-treated groups. 3T3-L1 adipocytes were induced to mature adipocytes in accordance with the method described above.Ta-FUCs were not added to the control group, whereas the experimental groups were treated with different-molecular-weightTa-FUCs (60 μg mL-1) at days 0, 2, 4, and day 6. At day 8, the cell culture supernatant was discarded, and the cells were washed twice with phosphate buffer saline(PBS). The cells were fixed through incubation with 2.5%glutaraldehyde in PBS for 0.5 h, washed three times with PBS, and then stained with filtered 0.5% Oil red O solution for 0.5 h. The stained cells were washed with 60% isopropanol and photographed with an inverted microscope(BX41, Olympus, Tokyo, Japan). Images were collected,and the absorbance value was obtained at 570 nm. ImageJ software was used to analyze the red values of Oil red O staining.
2.6 Intracellular TG Measurement
3T3-L1 adipocytes were seeded into 24-well plates (3 ×104cells per well) and induced to mature adipocytes in accordance with the method described above. Cells were lysed with the lysis solution for 0.5 h at 4℃. The cell lysate was thawed and frozen three times and then centrifuged to obtain the supernatant. TG level was determined using a commercial kit, and the protein content was measured using a BCA kit. TG content was expressed as mg TG per mg protein.
2.7 Real-Time Polymerase Chain Reaction(qRT-PCR) Analysis
The expression levels of key genes that regulate adipocyte differentiation, such asWnt10b,FrZ,LRP6,GSK3β,β-catenin,c-myc,C/EBPα, andPPARγwere measured using qRT-PCR. 3T3-L1 cells were seeded into six-well plates(4.5 × 104cells per well) and then induced to mature adipocytes with the method described above. 3T3-L1 adipocytes were treated with different-molecular-weightTa-FUCs (60 μg mL-1) from day 0, and culture medium was changed every 2 days until day 8. Total ribonucleic acid(RNA) was extracted by using Trizol reagent. RNA (1 μg)was transcribed to cDNA with M-MLV. The cDNA was amplified in a 25 μL solution containing SYBR Green mix by using a quantitative real-time PCR thermocycler (iQ5,Bio-Rad, Hercules, CA, USA). The amplification conditions were as follows: One cycle of predenaturation at 95℃ for 10 min; 45 cycles including denaturation at 95℃for 15 s, annealing at 60℃ for 10 s, and extension at 72℃for 45 s. The relative mRNA expression levels of the tar-get genes were analyzed using the 2-ΔΔCtmethod, and βactin served as the internal control for quantification. Primer sequences of the genes examined are listed in Table 1.

Table 1 Primer sequences of different genes examined
2.8 Western Blot
The differentiation of 3T3-L1 cells was adopted as shown in Section 2.7. Total proteins were obtained by using radioimmunoprecipitation assay buffer. Adipocytes were digested with trypsin and then nuclear proteins were obtained using a nuclear extract kit. Proteins were separated by SDSPAGE and then transferred onto polyvinylidene fluoride membranes. The membranes were blocked with 5% (w/v)BSA for 2 h and then incubated with antibodies against βcatenin, TATA binding protein (TBP), and β-actin (Cell Signaling Technology, Danvers, MA, USA) overnight at 4℃.Subsequently, the membranes were washed with Tris-HCl +Tween and then incubated with horseradish peroxidaseconjugated secondary antibody for 2 h. Protein bands were visualized by using automatic chemiluminescence apparatus (Tanon 5200, Shanghai, China).
2.9 Statistical Analysis
Results were presented as means ± standard deviation(S.D.) for three independent experiments. Data were analyzed using SPSS software (Statistics 21.0, SPSS Inc., Chicago, USA). One-way Analysis of Variance (ANOVA) followed by the Turkey’s test was used to assess the differences between individual groups. Differences were considered significant atP< 0.05.
3 Results
3.1 Effects of Different-Molecular-Weight Ta-FUCs on the Proliferation of 3T3-L1 Adipocytes
The inhibitory effects of different-molecular-weightTa-FUCs on the proliferation of 3T3-L1 adipocytes were measured by the MTT assay. As shown in Fig.2, cell viability decreased after treatment with theTa-FUCs in a dose-dependent manner. After treatment with theTa-FUCs (80 μg mL-1) for 48 h,Ta1,Ta2,Ta3, andTa4 reduced cell viability by 28.83%, 29.78%, 31.77%, and 34.15%, respectively. The results indicate that low-molecular-weightTa-FUCs exert a better inhibition rate. The release of LDH is due to membranolysis resulting from cytotoxicity. Thus LDH activity is used to evaluate the cytotoxicity of cells treated withTa-FUCs. Results showed that all four testedTa-FUCs are nontoxic to 3T3-L1 adipocytes (Fig.2C). Considering the low dose can bring effective action, the dose of 60 μg mL-1was used in subsequent experiments.

Fig.2 Effect of Ta-FUCs on the proliferation and cytotoxicity of adipocytes. A, Relative activity of 3T3-L1 adipocytes treated with different-molecular-weight Ta-FUCs for 24 h; B, Relative activity of 3T3-L1 adipocytes treated with differentmolecular-weight Ta-FUCs for 48 h; C, LDH activity. 3T3-L1 adipocytes were treated with different-molecular-weight Ta-FUCs for 48 h, and the supernatant was collected for the detection of LDH activity. Data are presented as means ± S.D. for three independent experiments. Multiple comparisons were performed using one-way ANOVA. *P < 0.05 versus control;**P < 0.01 versus control.
3.2 Effects of Different-Molecular-Weight Ta-FUCs on the Differentiation of 3T3-L1 Adipocytes
Oil red O dye can specifically stain TG to reflect the accumulation of lipid droplets in cells. As shown in Fig.3,the number of lipid droplets in theTa-FUC-treated cells was significantly lower than that in the control group. Semiquantitative results showed thatTa3 decreased Oil red O absorbance by about 11.27% (P< 0.01). The measurements of TG content further showed thatTa2 andTa3 significantly reduced the lipid accumulation in 3T3-L1 cells by 11.23% and 11.67%, respectively. These results indicate thatTa3, with a Mw of 182.4 kDa, exhibited the optimal antiadipogenic effect.

Fig.3 Ta-FUCs suppressed the differentiation of 3T3-L1 adipocytes. 3T3-L1 adipocytes were induced to mature adipocytes and stained with Oil red O at day 8. A, Lipid droplets were evaluated by Oil red O staining, and images were photographed under an inverted microscope at 10× objective; B, Global diagrams about adipocyte differentiation; C, Semiquantitative analysis with Oil red O; D, Quantification analyses of Oil red O by Image J; E, TG contents were measured by TG assay kit. Data are presented as means ± S.D. for three independent experiments. Multiple comparisons were performed using one-way ANOVA. *P < 0.05 versus control; **P < 0.01 versus control.
3.3 Effects of Different-Molecular-Weight Ta-FUCs on the Expression of Adipocyte Differentiation Markers
Adipocyte differentiation is controlled by complex transcriptional cascades.SREBP-1c,PPARγ, andC/EBPαare the central engine of adipocyte differentiation and lipid accumulation. As shown in Fig.4, the mRNA expression levels ofSREBP-1c,PPARγ, andC/EBPαwere significantly downregulated after treatment with theTa-FUCs. Compared with the control group,Ta1,Ta2,Ta3, andTa4 decreased the expression ofSREBP-1cby 15.35%, 20.34%, 20.97%, and 16.55%, respectively, and downregulated the expression ofPPARγby 17.14%, 26.29%, 31.86%, and 20.22%, respectively.Ta1,Ta2,Ta3, andTa4 also decreased the expression ofC/EBPαby 10.99%, 24.21%, 30.50%, and 13.99%, respectively.Ta3 exhibited the most notable effect, which is consistent with the result of Oil red O staining.

Fig.4 Ta-FUCs suppressed the mRNA expression of adipocyte markers. mRNA expression was measured by qRT-PCR.β-actin was used as an internal control. A, SREBP-1c mRNA expression; B, PPARγ mRNA expression; C, CEBPα mRNA expression. Data are presented as mean ± S.D. for three independent experiments. Multiple comparisons were performed using one-way ANOVA. *P < 0.05 versus control; **P < 0.01 versus control.
3.4 Wnt/β-Catenin Pathway Key Genes Were Enhanced by Different-Molecular-Weight Ta-FUCs During Adipogenesis
The Wnt/β-catenin pathway negatively regulates adipogenesis by promoting β-catenin translocation into the nucleus. As shown in Fig.5, theTa-FUCs significantly upregulated the mRNA expression levels of their receptorFrZand co-receptorsLRP5andLRP6. However, theTa-FUCs exerted no effect on the mRNA expression of Wnt10b.This result is similar to our previous finding that IL-6 could activate the Wnt/β-catenin pathway without affecting the expression of Wnt10b. Meanwhile,Ta3 markedly enhanced the mRNA expression levels ofβ-cateninand its transcriptional productsCCND1andc-mycby 46.94%, 38.28%,and 37.58%, respectively. In addition, theTa-FUCs significantly upregulated the β-catenin protein levels in the total cell lysate and in the nuclear extract, indicating the elevated nuclear translocation of β-catenin. The above results indicate that theTa-FUCs can inhibit adipocyte differentiation by activating the Wnt/β-catenin signaling pathway.

Fig.5 Effects of Ta-FUCs on the Wnt/β-catenin pathway in 3T3-L1 adipocytes. mRNA expression was measured by qRTPCR. β-Actin was used as an internal control. A, Wnt10b mRNA expression; B, FrZ mRNA expression; C, LRP5 mRNA expression; D, LRP6 mRNA expression; E, GSK-3β mRNA expression; F, β-catenin mRNA expression; G, c-myc mRNA expression; H, CCND1 mRNA expression; I, Expression of β-catenin protein. Data are presented as mean ± S.D. for three independent experiments. Multiple comparisons were performed using one-way ANOVA. *P < 0.05 versus control; **P < 0.01 versus control.
3.5 Effects of Different-Molecular-Weight Ta-FUCs on the Expressions of Key Genes Involved in the Aynthesis of Fatty Acids and Triglycerides in 3T3-L1 Adipocytes
The formation of lipid droplets includes the synthesis of fatty acids and triglycerides. ACC, FAS, and ME are the rate-limiting enzymes of fatty acid synthesis.Ta2 andTa3 significantly improved the mRNA expression ofACCandFAS(P< 0.05,P< 0.01). However,Ta1 andTa4 exhibited minimal effects (Fig.6). DGAT and GPAT are key enzymes in the regulation of triglyceride synthesis. Figs.6D and 6E show thatTa2 andTa3 significantly reduced the mRNA expression levels ofGPATandDGAT.PLINcoats triglycerides to form lipid droplets.Ta1,Ta2,Ta3, andTa4 decreased the mRNA expression ofPLINby 20.23%, 27.89%,30.78%, and 23.89%, respectively, compared with the control group. The results suggest thatTa-FUCs with intermediate molecular weights, such as 524 and 182.4 kDa,can decrease lipid accumulation significantly.
3.6 Effects of Different-Molecular-Weight Ta-FUCs on the Expression of Key Genes Involved in Fatty Acid β-Oxidation in 3T3-L1 Adipocytes
The key genes related to lipidolysis were measured to further explore howTa-FUCs regulate lipid metabolism.PPARαis a key transcriptional regulator of fatty acid βoxidation and regulates the expression of target genes, includingCPT-1α,CPT-2, andACOX. As shown in Fig.7,Ta1,Ta2,Ta3, andTa4 markedly increasedPPARαmRNA expression by 31.30%, 36.65%, 43.45%, and 36.46%, respectively, compared with the control. The expression levels ofCPT-1α,CPT-2, andACOXwere clearly upregulated byTa-FUCs. In specific,Ta3 raisedCPT-1α,CPT-2, andACOXexpressions by 40.35%, 31.38%, and 20.13%, respectively. These results suggest that theTa-FUCs can reduce lipid accumulation by promoting fatty acid β-oxidation.

Fig.6 Ta-FUCs inhibited the synthesis of fatty acids and triglycerides in 3T3-L1 adipocytes. mRNA expression was measured by qRT-PCR. β-Actin was used as an internal control. A, ACC mRNA; B, FAS mRNA; C, ME mRNA; D, GPAT mRNA; E, DGAT mRNA; F, PLIN mRNA. Data are presented as mean ± S.D. for three independent experiments. Multiple comparisons were performed using one-way ANOVA. *P < 0.05 versus control; **P < 0.01 versus control.
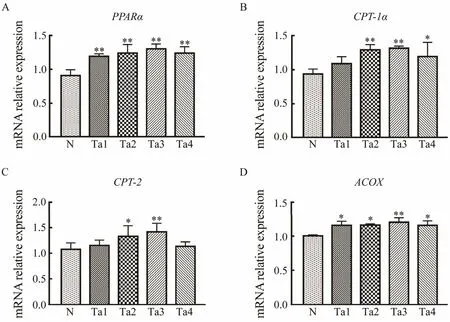
Fig.7 Ta-FUCs promoted fatty acid β-oxidation in 3T3-L1 adipocytes. mRNA expression was measured by qRT-PCR. βactin was used as an internal control. A, PPARα mRNA expression; B, CPT-1α mRNA expression; C, CPT-2 mRNA expression; D, ACOX mRNA expression. Data are presented as mean ± S.D. for three independent experiments. *P < 0.05 versus control; **P < 0.01 versus control.
4 Discussion
Obesity is characterized by increases in the number and volume of adipocytes. Suppressing the proliferation and differentiation of adipocytes is an effective strategy to control obesity. In the present study, we extracted fucoidans from the sea cucumberT. ananasand degraded the primaryTa-FUCs to three low-molecular-weightTa-FUCs by using the enzyme CZ1127 isolated from marine bacteriaFlavobacteriaceae. The three low-molecular-weightTa-FUCs suppressed the proliferation and lipid accumulation of 3T3-L1 cells. The mechanisms involved in the anti-adipogenic effects were activation of the Wnt/β-catenin pathway and upregulation of the expression of genes related to fatty acid β-oxidation. Our findings indicate thatTa-FUCs have a therapeutic potential to obesity.
Adipocyte differentiation is controlled by complex transcriptional cascades, in which transcription factors such as SREBP-1c, PPARγ, and C/EBPα play critical roles (Rosenet al., 1999, 2002; Tanget al., 2004; Wanget al., 2016).DecreasingSREBP-1cexpression can significantly suppress lipogenesis (Seoet al., 2013).PPARγknockout causes a loss in the formation of adipose tissue, whereas PPARγ agonists strongly promote adipocyte differentiation (Barbieriet al., 2012). Overexpression ofC/EBPαin pre-adipocytes can result in the automatic differentiation of adipocytes. TheC/EBPαknockout mice died within a week of birth due to having no adipose tissue and exhibiting a severe metabolic disorder (Rosenet al., 2002). In the present study, theTa-FUCs inhibited adipocyte differentiation in 3T3-L1 cells, and this result was associated with decreased levels ofSREBP-1c,C/EBPα, andPPARγ.
The classic Wnt/β-catenin signaling pathway plays an important role in regulating adipocyte differentiation (Prestwichet al., 2007; Liet al., 2008; Chenet al., 2014; Xuet al.,2014). The transcription products ofβ-catenin,CCND1,andc-myccan negatively regulate adipocyte differentiation by inhibiting the expression ofPPARγandC/EBPα(Kimet al., 2014), and these products also regulate the proliferation of 3T3-L1 cells. Our results indicate that, at least in part,Ta-FUCs suppress adipocyte differentiation by activating Wnt/β-catenin signaling.
SREBP-1ccan regulate fatty acid synthesis, triglyceride synthesis, cholesterol conversion, and glucose metabolism(Miserezet al., 2002; Priceet al., 2016; DeBose-Boyd and Ye, 2018). In the present study, theTa-FUCs reduced the mRNA expression ofSREBP-1cand further downregulated the expression of key genes in the synthesis of fatty acids and triglycerides, which decreased lipid accumulation in adipocytes.PPARαand its target genesCPT-1α,CPT-2, andACOXaccelerate fatty acid catabolism (Kimet al., 2004; De Filippiset al., 2011). The present results showed increased levels of the transcription factor PPARα and its target genes by treatment withTa-FUCs, suggesting that stimulating β-oxidation and reducing fatty acid levels are important ways forTa-FUCs to relieve lipid accumulation in adipocytes.
Ta-FUCs inhibited the adipogenesis, but differences existed in the anti-adipogenic effect of the four differentmolecular-weight fucoidans. When the molecular weights of fucoidans changed from 1380.3 kDa to 182.4 kDa, the inhibitory effects on lipogenesis increased. However, the anti-adipogenic activities decreased when the molecular weight of fucoidans was 110.3 kDa. Overall, the 182.4 kDaTa-FUC exhibited the optimal capacity of lipid inhibitory activities. Several studies have shown that a change in molecular weight of polysaccharides can lead to the changes in biological activities (Imet al., 2005; Yanget al., 2008; Choet al., 2010; Anastyuket al., 2012; Sunet al., 2012; Jianget al., 2016; Xuet al., 2016). Sunet al. (2012) suggested that polysaccharides fromPorphyridium cruentumhave six molecular weights and that the minimum fragments exhibit the strongest immunomodulation activities. However,other studies suggested that the optimal biological activity for polysaccharides should reside within a suitable range,where molecular weights that are excessively high or low would limit their bioactivities. Imet al. (2005) confirmed that polysaccharides with molecular weights in the range of 5 - 400 kDa demonstrate the best immunomodulating and antitumor activities. However, polysaccharides with molecular weights larger than 400 kDa or less than 5 kDa exhibit no bioactivities. Jianget al. (2016) also indicated thatAstragaluspolysaccharides within a certain molecular weight range exert significant immune activity. The results of these studies were similar to our findings.
Previous studies have indicated that the chain conformation, determined by the primary structure and molecular weight, influences bioactivity (Saitôet al., 1977; Falchet al., 2000). Chenet al. (2014) found that the hyper branched (1→4)-α-d-glucan with spherical conformation has weak biological activity. However, sulfation and carboxymethylation modifications changed the chain conformation into irregular curly chains and enhanced the antitumor activity.This result is consistent with our finding that the random coil chain ofTa-FUCs exhibited better biological activity than the spherical chain. The activity of fucoidans with higher molecular weights diminished, which may be attributed to their poor absorption. The absorption of minimum-molecular-weight fucoidans should be optimal, but their bioactivity was not greater than that of medium-molecularweight fucoidans. This result may be attributed to the degradation of the active domain of the minimum-molecular-weight polysaccharides, resulting in reduced bioactivity. Further in-depth investigation is required and in progress to understand the mechanism by whichTa-FUCs suppress adipocyte differentiation and lipid accumulation.
5 Conclusions
This study provided evidence thatTa-FUCs exhibit antiadipogenic activities by activating the Wnt/β-catenin pathway. The molecular weight ofTa-FUCs can influence their anti-adipogenic effects, while the 182.4 kDaTa-FUC exhibits the optimal activity.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (No. 2018YFC0311 203), and the Key Research and Development Plan in Shandong Province (No. 2016YYSP017). Moreover, we acknowledge all the staff for their valuable assistance in conducting this study.
Abbreviations
ACC, acetyl-CoA carboxylase;ACOX, acyl-CoA oxidase;CCND1, cyclin D1;C/EBPα, CCAAT/enhancer binding protein α;CPT-1α, carnitine palmitoyl transferase-1α;CPT-2,carnitine palmitoyl transferase-2; DGAT, diacylglycerol acyltransferase;FAS, free fatty acid;FrZ, frizzled;GPAT,glycerol-3-phosphate acyltransferase;GSK-3β, glycogen synthase kinase 3 β;LRP, lipoprotein receptor-related protein;ME, malic enzyme;PLIN, perilipin;PPARγ, peroxisome proliferator-activated receptor γ;PPARα, peroxisome proliferator-activated receptor α;SREBP-1c, sterol regulatory element-binding protein-1c;Wnt 10b, Wnt family member 10b.
杂志排行
Journal of Ocean University of China的其它文章
- Paleosalinity and Its Association with Organic Matter:A Case Study from the Eocene Shahejie Formation,Laizhou Bay Sag, Bohai Bay Basin (China)
- Studies on the Inversion Phenomenon of Physical Properties Observed in the Huagang Formation Reservoir in the Xihu Sag Based on the Water-Rock Reaction Experiments
- Effect of Sand Body Enrichment Under the Restriction of a Tectonic Transfer Zone: A Case Study on the Pinghu Formation in the Kongqueting Region on the Pinghu Slope
- Relationship Between Paleogene Reservoir Densification and Hydrocarbon Accumulation in the Xihu Depression
- Adjoint Method-Based Algorithm for Calculating the Relative Dispersion Ratio in a Hydrodynamic System
- Analysis of the Leading Modes of Autumn Precipitation over the Yangtze River Basin
