In situ construction of oxygen-vacancy-rich Bi0@Bi2WO6-x microspheres with enhanced visible light photocatalytic for NO removal
2021-08-26XioqinXieQdeerUlHssnHunLuFeiRoJinzhiGoGngqingZhu
Xioqin Xie,Qdeer-Ul Hssn,Hun Lu*,Fei Ro,Jinzhi Go,*,Gngqing Zhu,*
a School of Physics and Information Technology,Shaanxi Normal University,Xi’an 710062,China
b School of Geography and Tourism,Shaanxi Normal University,Xi’an 710062,China
ABSTRACT Surface oxygen vacancy defects and metal deposition on semiconductor photocatalysts play a critical role in photocatalytic reactions.In this work,oxygen-deficient Bi2WO6 microspheres have been prepared by a facile ethylene glycol-assisted solvothermal method.Bi0 nanoparticles were reduced by in situ thermaltreatment on Bi2WO6 microspheres to obtain Bi0@Bi2WO6-x as well as maintaining the oxygen vacancies(OVs)under N2 atmosphere.Afterwards,photocatalytic NO oxidation removal activities of these photocatalysts were investigated under visible light irradiation and Bi0@Bi2WO6-x shows the best NO removal activity than other samples.The photogenerated charge separation and transfer are promoted by Bi0 nanoparticles deposited on the surface of semiconductor catalysts.OVs defects promote the activation of reactants(H2O and O2),thereby enhancing the formation of the active substance.Moreover,both OVs defects and Bi0 metal have the characteristics of extending light absorption and enhancing the efficient utilization of solar energy.Besides,the photocatalytic NO oxidation mechanism of Bi0@Bi2WO6-x was investigated by in situ FTIR spectroscopy for reaction intermediates and final products.This work furnishes insight into the synthesis strategy and the underlying photocatalytic mechanism of the surfacemodified Bi0@Bi2WO6-x composite for pollutants removal.
Keywords:Oxygen vacancies Bi metal Photocatalytic reaction In situ FTIR Bi2WO6 microspheres
Recent investigations have shown that the formation of oxygen vacancy defects is considered an effective method for adjusting the semiconductor band gap and its electronic structure[1-3],thereby enhancing visible light absorption[4-6].Research has confirmed that the construction of oxygen vacancies(OVs)defect promote the activation of reactants(H2O and O2),thereby enhancing the formation of the active substance[7,8].In addition,it is also a wellestablished phenomenon that the catalytic performance of the photocatalysts can improve by depositing metal nanoparticles due to its surface plasmon resonance(SPR)effect[9-13].Compared with precious metals,low-cost Bi0nanoparticles also can produce SPR effect[14,15],which can enhance the visible light adsorption.Furthermore,Bi0metal as a cocatalyst on the surface of the semiconductor can effectively accelerate the charge-carrier separation.Thus,it is of considerable significance to develop a simple and feasible method for constructing OVs and depositing Bi0nanoparticles on the surface of the photocatalyst to improve its photocatalytic performance.
As a typical visible-light-driven photocatalyst,Bi2WO6can be excited by the light with a wavelength less than 460 nm,which merely occupies a little fraction of the visible light[16-19].Moreover,the fast recombination of photogenerated electron-hole pairs also significantly decrease the photocatalytic efficiency of the pristine Bi2WO6[20].Therefore,expanding the wavelength range of light absorption and promoting the separation of photoinduced charges are two effective methods for enhancing the photocatalytic activity of Bi2WO6[21].
In this work,a convenient and effective in situ thermaltreatment method was used to simultaneously construct oxygen vacancies and load Bi0nanoparticles on the surface of Bi2WO6microspheres.In the thermal treatment process,Bi3+was reduced by the carbon species on the sample surface and Bi0nanoparticles was loaded on the surface of the Bi2WO6-xmicrospheres under N2atmosphere.The presence of oxygen vacancies and the metal SPR effect can increase the utilization of light and reduce the recombination of photogenerated carriers.Subsequently,photocatalytic NO oxidation removal was investigated under visible light irradiation to evaluate the photocatalytic performance of these obtained photocatalysts.
A solvothermal method with the assisted of ethylene glycol was conducted in order to prepare Bi2WO6microspheres.The synthesis process is in Text S1(Supporting information).Then,the obtained Bi2WO6was thermal-treated at different temperatures(260,300,350,400 and 450°C,respectively)in a nitrogen surrounding for 2 h.Moreover,the thermal-treated samples were named as BWO-260,BWO-300,BWO-350,BWO-400,and BWO-450,respectively,and pristine Bi2WO6was designated as BWO.In addition,the Bi2WO6powders synthesized with deionized water as solvents are named as BWO-DW.
As shown in Fig.1a,all diffraction peaks of the pristine BWO can be indexed to the orthorhombic russellite phase of Bi2WO6(PDF No.73-2020),and no peak of impurity was observed.With the increasing of the thermal treatment temperature to 450°C,the characteristic peaks of metal bismuth phase that can be accurately indexed to the hexagonal phase of Bi0(PDF No.44-1246).Moreover,with the thermal treatment temperature increased,the color of the synthesized samples gradually darkens from white to black.The above results indicate that Bi0metal phase was increased gradually with the increasing thermal treatment temperature.
FT-IR spectra were employed to further characterize these samples prepared by different solvents,as shown in Fig.S1a(Supporting information).The peaks in BWO spectrum over the range of 2800-3000 cm-1matched the antisymmetrical and symmetrical vibrations of-CH2[22].The peaks at 1385 and 1086 cm-1can be attributed to the C--OH and C--O bending vibrations[23,24],respectively.However,the vibration of the chemical bond or functional group related to the C element cannot be detected in the BWO-DW sample(Text S4 and Fig.S1 in Supporting information).As shown in Fig.S1b,X-ray diffraction(XRD)patterns also prove that without Bi0metal phase will appear after thermal treatment if there is no organic carbon adsorbed on the sample surface.In Fig.1b,the organic carbon adsorbed on the BWO catalyst surface has been significantly reduced after the thermal treatment process.Therefore,the organic solvent used in the synthesis of BWO is essential for the reduction of Bi0metal from BWO by in situ method.The characteristics absorption bands of pristine BWO sample can be observed in the range of 400-800 cm-1(Fig.1b),which are originated from the Bi-O stretching vibrations,W--O stretching vibrations and W--O--W bridge stretching modes[25,26].These peaks still exist after BWO thermal treatment at different temperatures,which also indicates that the structure of BWO has not changed during the process of generating Bi0phase.
Based on the Bi 4f spectrum of pristine BWO(Fig.1c),the peaks around 159.05 and 164.35 eV could be ascribed to Bi3+4f7/2and Bi3+4f5/2spin-orbit splitting photoelectrons states being inBi3+chemical state[14,27],respectively.However,two newly peaks located at 157.8and163.25 eVwereobservedinBWO-350,whichareaccording to the Bi04f7/2and Bi04f5/2,respectively.The two peaks are resulted by part of the Bi3+reduced to Bi0phase during thermal treatment process,which also confirm the presence of Bi0metal phase after thermal treatment[15].Furthermore,the Bi 4f peak of the BWO-350 sampleshows a slight shift towards higher bindingenergy dueto the altering of the electron distribution near the Bi atom[28].As shown in Fig.1d,the asymmetric O 1s X-ray photoelectron spectroscopy(XPS)spectrum of BWO can be fitted to three gaussian peaks at 529.75,530.45and531.3eV,which are ascribed to the latticeoxygen,oxygen vacancies and surface chemisorbed oxygen of BWO[29-31],respectively.The formation of Bi0nanoparticles also causes the increasing of oxygen vacancies over BWO-350 during thermal treatment,which is well consistent with the electron paramagnetic resonance(EPR)results in Fig.1f.
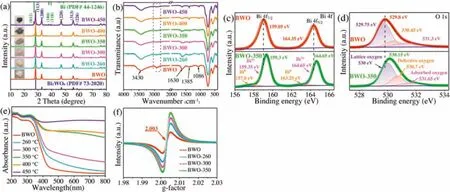
Fig.1.XRD patterns(a)and FT-IR spectra(b)of the samples synthesized at different thermal treatment temperatures.High-resolution XPS spectra of Bi 4f(c)and O 1s(d)of BWO and BWO-350 samples.(e)UV-vis spectra of the sample with different thermal treatment temperatures.(f)EPR spectra of BWO,BWO-260,BWO-300 and BWO-350 samples.
Pristine BWO exhibited an intrinsic visible light absorption edge of approximately 460 nm(Fig.1e).Interestingly,the samples after thermal-treated exhibited appearance redshift and evident amelioration in visible light absorption compared to pristine BWO.With the thermal treatment temperature increased,photocatalysts exhibited strong absorption in the visible-near infrared(vis-NIR)region due to the OVs and the SPR effect originated from the Bi0metal on the semiconductor surface[32].However,ultravioletvisible diffuse reflectance spectrum(UV-vis DRS)in Fig.S3c(Supporting information)shows that the BWO-DW sample without obvious redshift as well as optical absorption changes after thermal treatment at different temperatures(Text S6 and Fig.S3 in Supporting information).
EPR analysis was performed to determine the presence of oxygen vacancy visually.As illustrated in Fig.1f,BWO,BWO-260,BWO-300 and BWO-350 samples exhibit a symmetric EPR signal gradient at about g=2.003 attributing to the electrons trapped on the oxygen vacancies,where the EPR signal gradually increases with the increase of thermal treatment temperature[27,28].These results indicate that the samples after thermal-treated has more oxygen vacancies.In addition,the thermal-treatment has little effect on the specific surface area and pore size distribution of the samples(Text S6 and Fig.S3d).
It can be observed from the scanning electron microscopy(SEM)and transmission electron microscopy(TEM)images in Figs.2a-c and Fig.S4(Supporting information)that all the prepared samples exhibit a self-assembled microsphere morphology composed of nanosheets with a thickness about 6-7 nm.From the SEM image of BWO-450 in Fig.2b,it can be seen that some irregular nanoparticles loaded on the microspheres.In addition,the high resolution TEM(HRTEM)image of BWO-450 in Fig.2d displays that the lattice fringes is about 0.315 nm,which matching the(113)crystal plane of Bi2WO6crystal.The energy-dispersive spectroscopy(EDS)results in Fig.S5 and Text S7(Supporting information)also demonstrate that the Bi0particles were produced on the surface of BWO photocatalyst during the process of thermal-treatment.According the above discussion and analysis,the generation of Bi0particles and OVs in the BWO during the thermal treatment are illustrated Fig.2e.
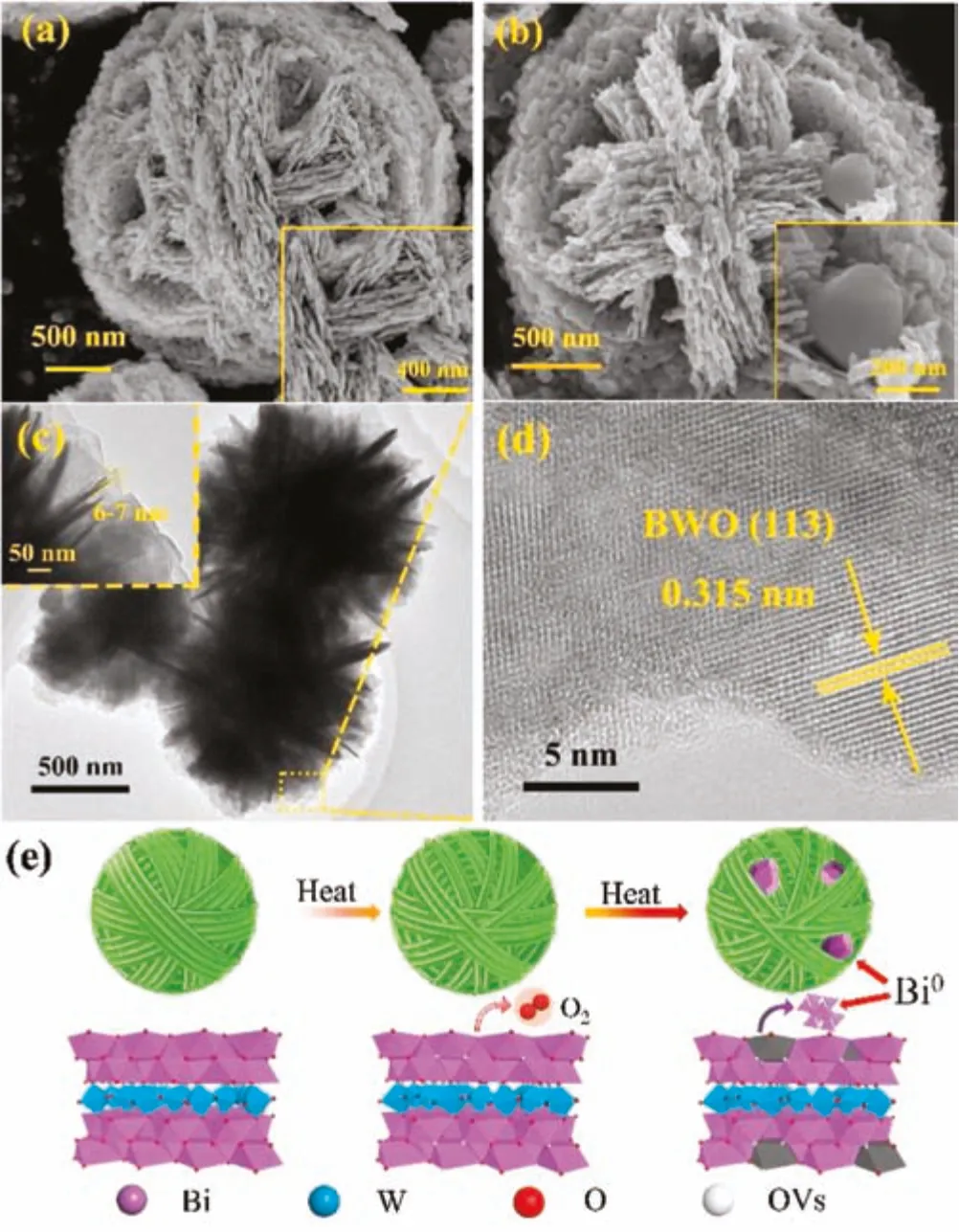
Fig.2.SEM images of BWO(a)and BWO-450(b)samples.TEM image(c)and HRTEM image(d)of BWO-450.(e)Schematic diagram of the generation of OVs and Bi0 nanoparticles during the thermal treatment process.
In this study,the NO photocatalytic removal performances of the photocatalysts were investigated under visible light irradiation(λ≥420 nm).Fig.3a displays the variation of NO concentration with the illumination time of all samples under visible light irradiation.As shown in Fig.S6b(Supporting information),the photocatalytic removal efficiencies of NO oxidation removal over these samples of BWO,BWO-260,BWO-300,BWO-350,BWO-400 and BWO-450 photocatalysts were about 46.5%,50.5%,51.3%,53.4%,45.1% and 33.1%,respectively.Among them,the BWO-350 sample exhibited the best photocatalytic performance,which may be attributed to the OVs and Bi0nanoparticles with enhanced light utilization and effective charge transfer.In order to further confirm whether OVs and Bi0nanoparticles both have enhanced light utilization and effective charge transfer,the results of controlling a single variable are provided(Text S9 and Figs.S7a-e in Supporting information).The results show that for the synthesized samples,the presence of OVs and Bi0can indeed enhance the effective absorption and utilization of light and the separation and transfer of photogenerated carriers.It also proved that the in-situ synthesized Bi0@Bi2WO6-xcontaining oxygen vacancies has better photocatalytic efficiency than the general chemical loading method.Moreover,the BWO-350 sample has good photocatalytic recyclability and structure stability(Text S10 and Figs.S8a and b in Supporting information).
In order to investigate the photocatalytic mechanism,active substance trapping experiments were performed.As shown in Fig.3b,the addition of K2Cr2O7into the reaction system obviously reduces the photocatalytic efficiency of BWO-350.In addition,the addition of p-benzoquinone(PBQ),KI and isopropanol(IPA)also have a certain influence on the photocatalytic efficiency of theBWO-350 photocatalyst.The trapping experiments results indicated that theelectrons,holes,O2•-and•OH,especialtheelectrons,playagreat role in th ephotocatalytic NO removal.Electron spinresonance(ESR)spectra were further to verify the presence of O2•-and•OH in the system under visible light illumination over BWO-350 photocatalyst.From Figs.3c and d,there is no signal under dark condition,indicating no active radicals.However,under visible light irradiation,the characteristic peaks of DMPO-•OH and DMPO-O2•-are clearly detected,which indicating that both active substances participated in the photocatalytic NO removal process.The ESR spectra are well consistent with the trapping experiments results.
The photoluminescence(PL)spectra,photocurrent,electrochemical impedance spectroscopy(EIS)measurements and surface photovoltage spectroscopy(SPS)were conducted to analyze the separation efficiency of photo-induced electron-hole pairs of the samples(Text S11 and Fig.S9 in Supporting information).Compared with other samples,the photo-induced carriers of BWO-350 sample have lower recombination rate,faster transmission rate,and higher separation efficiency.Therefore,the BWO-350 shows better photocatalytic activity than other samples.
In situ FTIR spectra were used to monitor the adsorption and reaction of NO and O2gases on the BWO-350 catalyst surface.Under the dark condition(Fig.3e),the absorption bands at 987,1005 and 1059 cm-1are matched the vibration of the bridging nitrate and the bidentate nitrate(NO2-)[33],while 1085,1131 and 1160 cm-1can be ascribed to the vibration of NO gas adsorbed on the surface of photocatalyst[3,33,34].The absorption peak at 1365 cm-1is ascribed to NO2due to the part of NO oxidized to NO2under dark condition(Eq.1)[3].Furthermore,the absorption bands at 1105,1394,1457 and 1641 cm-1can be properly allocated to trans-hyponitrites(N2O22-),monodentate nitrites,linear nitrites,and NO3-(Eqs.2 and 3)[27,35],respectively.The intensity of the peaks at 1394 and 1641 cm-1increased slightly over time.The possible reaction paths are shown in the following equations(Eqs.1-3):
Fig.3f shows the photocatalytic NO oxidation process of the BWO-350 sample under visible light irradiation.After light irradiation,a series of new absorption bands at 1008,1068,1116,1276,1342,1434 and 1482 cm-1can be detected.The absorption bands at 1116,1342,and 1482 cm-1could be originated from the bridge nitrites and bidentate nitrates[27,35,36].Also,the absorption bands at 1008,1068,1276 and 1434 cm-1can be assigned to cis-(N2O22-),NO-,monodentate nitrates and NO2-(chelated)[7,15,27,37],respectively.The absorption bands allocated to nitrate species increased significantly with irradiation time.After light on,e-and h+are generated by photoexcited and react with O2and OH-on the catalyst surface to form O2•-and•OH,respectively.Then,the reaction between the NO gas on the catalyst surface and various reactive species is switched in the direction of formation of more stable nitrates.The possible reaction paths of the whole process of photocatalysis are as following(Eqs.4-11):
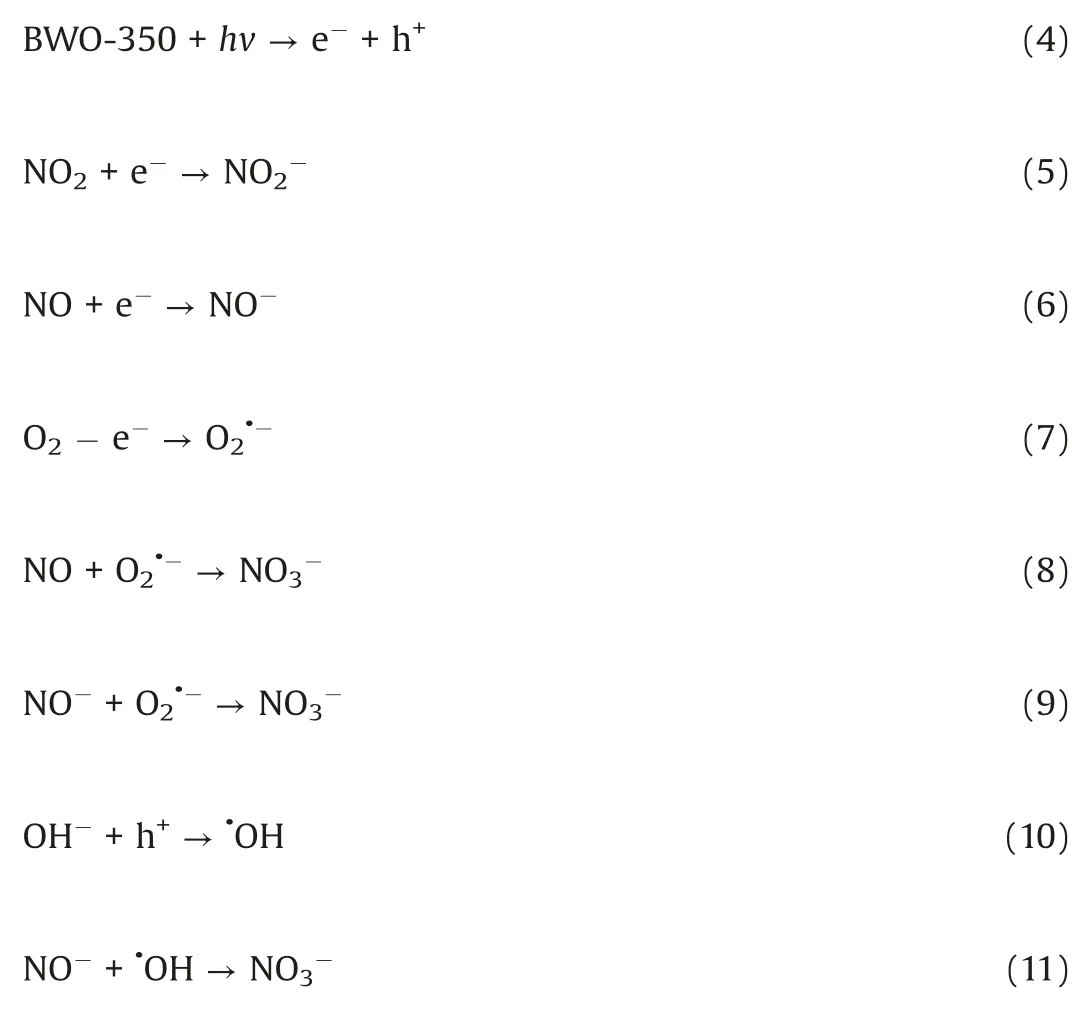
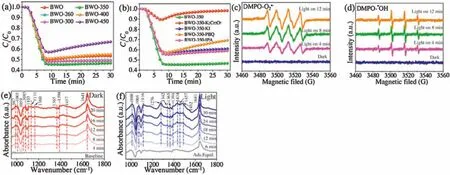
Fig.3.(a)Photocatalytic activity under visible light irradiation for the samples undergone at different thermal-treatment temperatures.(b)Photocatalytic activities of BWO-350 photocatalyst in the presence of various scavengers.(c)DMPO-spectra and(d)DMPO-•OH spectra of the BWO-350 sample under visible light irradiation.In situ FTIR spectra under dark condition(e)and visible light(f)reaction processes over BWO-350.
Based on the discussions made above,a probable mechanism diagram of the improved photocatalytic performance of Bi0@Bi2WO6-xis shown in Fig.4.Under visible light irradiation,the electrons in the valence band(VB)of Bi2WO6-xsemiconductor are excited to undergo energy level transitions,generating photogenerated carriers.Due to the SPR effect,Bi0metal nanoparticles also can generate hot electrons to participate in redox reactions.In this way,O2adsorbed on the photocatalyst surface can capture electrons and react to form the active species O2•-,and then NO and O2•-react more effectively to form nitrates.In addition,holes in the VB of Bi2WO6-xcan oxidize OH-to the active species•OH.In summary,the enhanced photocatalytic removal of NO by as-synthesized samples is mainly due to the synergistic effect of Bi0nanoparticles and oxygen vacancies.
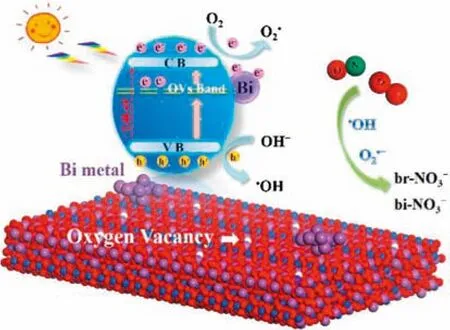
Fig.4.Mechanism of processes occurring during visible-light-driven photocatalytic NO removal over Bi0@Bi2WO6-x.
In summary,Bi2WO6microspheres were prepared by a simple hydrothermal method,and then Bi0nanoparticles loaded on Bi2WO6-xwith oxygen vacancies were prepared by thermal treatment method under N2atmosphere condition.The existence of OVs and Bi0nanoparticles not only increases the visible light utilization of the photocatalyst but also enhances the separation and transfer efficiency of photogenerated electron-hole pairs.The photocatalytic performance of Bi0@Bi2WO6-xsignificantly improved by the synergetic effect of the presence of appropriate Bi0nanoparticles content and surface oxygen vacancies.Our work employs a new approach towards controlling oxygen vacancies and Bi0metal nanoparticles loaded on the surface of semiconductor catalysts.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(No.51772183),the key Research and Development Program of Shaanxi Province(No.2018ZDCXL-SF-02-04),and the Fundamental Research Funds for the Central Universities(Nos.GK201903023 and GK201801005).
Appendix A.Supplementary data
Supplementary material related to this articlecanbe found, in the online version,at doi:https://doi.org/10.1016/j.cclet.2020.10.002.
杂志排行
Chinese Chemical Letters的其它文章
- Super-assembled highly compressible and flexible cellulose aerogels for methylene blue removal from water
- In-situ deposition of Pd/Pd4S heterostructure on hollow carbon spheres as efficient electrocatalysts for rechargeable Li-O2 batteries
- Sulfonic acid-functionalized core-shell Fe3O4@carbon microspheres as magnetically recyclable solid acid catalysts
- Boron-iron nanochains for selective electrocatalytic reduction of nitrate
- The capacity and mechanisms of various oxidants on regulating the redox function of ZVI
- Conducting polymer engineered covalent organic framework as a novel electrochemical amplifier for ultrasensitive detection of acetaminophen
