Recent advances in bismuth vanadate-based photocatalysts for photoelectrochemical water splitting
2021-08-26LinWngXioqingShiYuefJiHongfeiChengLeiWngQizhoWng
Lin Wng,Xioqing Shi,Yuef Ji,Hongfei Cheng,*,Lei Wng,Qizho Wng,,*
a School of Water and Environment,Key Laboratory of Subsurface Hydrology and Ecological Effects in Arid Region of Ministry of Education,Chang'an University,Xi'an 710054,China
b College of Chemistry and Chemical Engineering,Northwest Normal University,Lanzhou 730070,China
ABSTRACT Photoelectrochemical(PEC)technology is considered to be a promising approach for solar-driven hydrogen production with zero emissions.Bismuth vanadate(BiVO4)is a kind of photocatalytic material with strong photoactivity in the visible light region and appropriate band gap for PEC water splitting.However,the solar-to-hydrogen efficiency(STH)of BiVO4 is far away from the 10% target needed for practical application due to its poor charge separation ability.Therefore,this review attempts to summarize the strategies for improving the photocurrent density and especially hydrogen production of BiVO4 materials through PEC techniques in the last three years,such as doping nonmetal and metal elements,depositing noble metals,constructing heterojunctions,coupling with carbon and metalorganic framework(MOF)materials to further enhance the PEC performance of BiVO4 photoanode.This review aims to serve as a general guideline to fabricate highly efficient BiVO4-based materials for PEC water splitting.
Keywords:Bismuth vanadate(BiVO4)Photoelectrochemical Water splitting Hydrogen production Heterojunctions
1.Introduction
In view of fossil energy consumption that has been resulting in current global energy shortage and the accompanying environmental crisis,direct conversion of naturally abundant solar energy into chemical energy in a sustainable manner is one of the most promising solutions to solve the energy crisis and environmental pollution in the future[1].Since the pioneering work of photoelectrochemical(PEC)water splitting into oxygen and hydrogen using TiO2as the photoanode in 1972[2],PEC hydrogen production technology,a process to convert and store solar energy in chemical bonds,has attracted great attention[3].This may be due to external bias potential,which can help minimize electron-hole recombination rate and increase the generated electrons and holes that take part in the water splitting process.
A typical PEC cell is composed of two conductively connected electrodes immersed in an electrolyte with at least one electrode containing semiconductors(i.e.,a photoelectrode)for light harvesting,where the photoanode is made of an n-type semiconductor and the photocathode is made of a dark metal electrode[4].Generally,PEC water splitting involves four basic steps(Fig.1):Firstly,absorbs light to generate a pair of holeelectrons in a photoactive photoelectrode under visible sunlight illumination.Secondly,the holes on the surface of the photoanode cause water oxidation(OER)[5].Thirdly,the electrons generated by the photoanode transfer from the photoanode to the photocathode surface through external wires.Finally,the electrons on the surface of the photocathode reduce H+to form hydrogen(HER)[6].The mechanism of the whole reaction is shown in the following equations(Eqs.1-3):
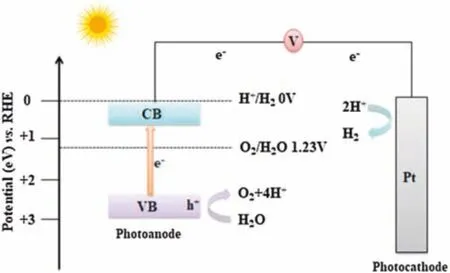
Fig.1.The basic mechanism of PEC water splitting.

From a thermodynamic point of view,a suitable semiconductor photoelectrode for PEC water splitting must fulfill the following several demands:(1)The minimum value of conduction band(CB)edge should be more negative than the reduction potential of hydrogen[7];(2)the maximum value of valence band(VB)edge should exceed the potential of oxidizing H2O to O2;(3)the photoelectrode should absorb photon energy equal to or greater than 1.23 eV to undergo a water splitting reaction[8].Therefore,the band gap energy(Eg)of the semiconductor photoelectrode should be in the range of 1.5-3.1 eV[9].To date,no single material completely fulfills all the thermodynamic requirements of PEC water splitting[10].Hence materials development remains the key issue for improving PEC performance.
As an important semiconductor photocatalyst,bismuth vanadate(BiVO4)has been widely examined because of its plentiful abundance,low cost,excellent(photo)-electrochemical stability in aqueous solutions and nontoxic.The BiVO4exists in three crystal structures:Scheelite structure with tetragonal phase and monoclinal phase and zircon structure with tetragonal phase.Among the three crystal structures,scheherite structure with monoclinal phase has higher thermodynamic stability and higher catalytic activity than other structures,and is most commonly used in photo-electrocatalysis.For the scheelite structure,BiVO4with tetragonal and monoclinic phases are dissimilar as the local environments of V and Bi ions are notably distorted in the latter.Hence,the V--O bonds in tetragonal scheelite BiVO4are all of equal length(1.72Å),while two different V--O bond lengths(1.77 Åand 1.69Å)are present in monoclinic scheelite BiVO4.In particular,the Bi3+lone electron on the Bi 6s orbital causes the partial deformation of the Bi--O ionic bond to enhance the distortion of the BiO8dodecahedron.The distortion of the VO4octahedron causes the center positions of the positive and negative charges to be separated.As a result,an internal electric field is generated,which is very conducive to the separation of photogenerated electron-hole pairs,so monoclinic BiVO4has higher photocatalytic performance than the other two structures.More recently,monoclinic BiVO4has received great attention as a photoanode material for PEC water splitting because BiVO4photoanode satisfy the necessary requirements listed above[11].This interest originates from an appropriate bandgap structure(Eg≈2.4 eV)of BiVO4that not only favors good light absorption but also provides a relatively negative CB edge(~0.02 V vs.RHE)to undergo water oxidation reaction driven by visible light.However,it has its own shortcomings of poor electron conductivity and sluggish water oxidation kinetics[12].The poor electron transport performance of BiVO4may be due to the disconnection of VO4tetrahedron units in the crystal structure of the material,indicating that that the photoexcited electrons in the V 3d conduction band have to hop between the VO4tetrahedra[5].In contrast,a theoretical study on the hole transport in BiVO4revealed a relatively weak hole localization in this material[6],again signifying that the charge transport in BiVO4is primarily limited by electron mobility.For example,the photocurrent densities achieved for unmodified BiVO4photoanodes(1.0 mA/cm2and 0.81 mA/cm2)are still far below its theoretical maximum photocurrent of 7.5 mA/cm2under standard AM 1.5 solar light irradiation[13].In addition,Subramanyam et al.reported that the solar-to-hydrogen conversion(STH)for pure BiVO4photoanode is 0.82%at 0.61 V vs.RHE,much lower than that the theoretical STH value of 9.2%[14].
Therefore,it is of great significance to overcome shortcomings of BiVO4for promoting its broad application in PEC water splitting.To this end,we summarize recent three years’reports(2017-2020)in the area of BiVO4-based materials for the PEC water splitting applications.A distinct emphasis of the review is that it aims to provide a better understanding of the fundamental design,fabrication,mechanisms and performance of BiVO4for PEC water splitting applications.Various strategies such as doping with noble metal,metal and nonmetal element,constructing a heterojunction,as well as combining with carbon and MOF materials will be discussed to enhance PEC performance of water splitting of BiVO4.Finally,further challenges to explore highly efficient BiVO4material systems are also presented.
2.Methods to enhance the PEC performance of BiVO4 for water splitting
The main drawback of BiVO4photoanode is their limited light absorption,poor charge separation and lower surface charge transfer efficiencies.To overcome those drawbacks,enormous efforts have been focused on how to optimize BiVO4photoelectrode,such as doping of metal/nonmetal atoms,depositing noble metals,constructing heterojunctions,coupling with carbon and metal-organic framework(MOF)materials.
2.1.Doping metals or nonmetals
Doping of BiVO4is considered as a process of introducing additional elements and impurities into the BiVO4framework to distinctly tune the optical,electronic,luminescent and other physical properties of BiVO4.There are two main types of elemental doping that will be discussed in this section,namely nonmetal doping and metal doping.In the field of photocatalysis,band gap engineering of BiVO4via the incorporation of anions,cations or the incorporation of both plays a predominant role to enhance the spectral responsive region and redox band potentials for targeted photocatalytic applications.Hitherto,numerous studies on the doping of metallic elements such as Fe,Co,Ni,Ga,Zn and Mn and some non-metallic elements including F,N and B elements into BiVO4have been extensively reported.
Recently,it was reported that the 7% In3+-doped BiVO4photoanode shows optimal PEC water oxidation activity(Figs.2a and b)[15].At 1.23 V vs.RHE,the 7% In3+-doped BiVO4photoanode exhibits a photocurrent density of 1.56 mA/cm2in 0.1 mol/L Na2SO4,which is over 200% greater than that of the undoped BiVO4photoanode.This change in water oxidation activity is attributed to the In3+-doping that passivated the surface states of BiVO4and thus inhibited the surface charge recombination.Similarly,Yang’s group synthesized Mo doped BiVO4photoanode on the fluorine-doped tin oxide substrate by electrodeposition method,the optimized Mo doped BiVO4(3AMo:BV,a substitute for ammonia-based precursor)shows~1.5 times higher activity in water oxidation kinetics efficiencies than that of undoped BiVO4at 1.23 V vs.RHE under AM 1.5 G illumination[16].The high oxidation kinetics efficiencies of the Mo doped BiVO4photoanode can be ascribed to the crystal deformation caused by larger tetrahedral ionic VO4and higher photovoltage generated by the interface of photoanode and electrolyte.
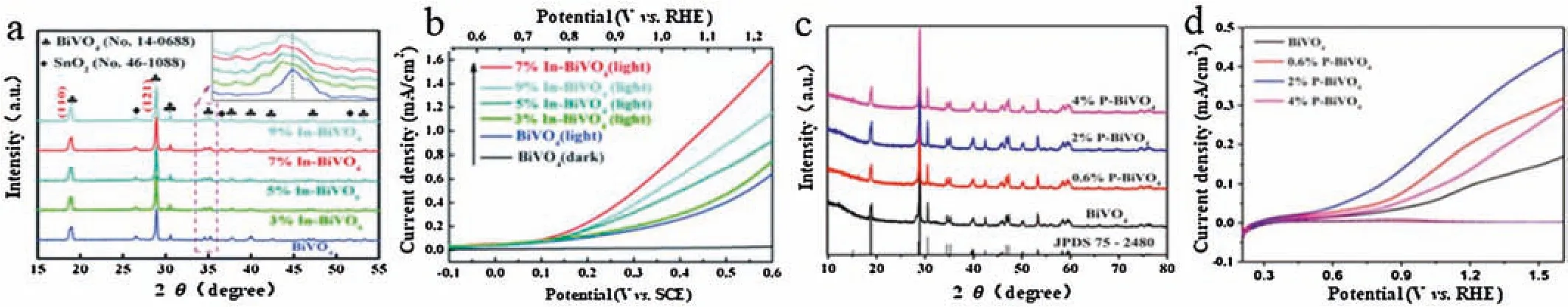
Fig.2.(a)XRD patterns of undoped BiVO4 and In3+-doped BiVO4 films(inset:the diffraction peak at 35.25°).(b)LSV scans for undoped BiVO4 and In3+-doped BiVO4 films.(a,b)Copied with permission[15]. Copyright 2018,Royal Society of Chemistry.(c)XRD patterns of BiVO4, 0.6%P-BiVO4,2%P-BiVO4 and 4%P-BiVO4.(d)Photocurrent-potential curves of pristine BiVO4, 0.6% P-BiVO4,2% P-BiVO4 and 4% P-BiVO4 in 0.5 mol/L Na2SO4 electrolyte with the AM 1.5 G simulated solar light at 100 mW/cm2(dashed lines represent dark currents).(c,d)Reporduced with permission ].Copyright 2018,Elsevier.
Meanwhile,Shi et al.also reported that 2% W-BiVO4showed a remarkable photocurrent increase with respect to the pristine BiVO4at 1.23 V vs.RHE under back side illumination.The improvement was attributed to the W doping that does have an effect on charge transfer at the semiconductor/electrolyte interface[17].Chakthranont et al.fabricated a core-shell black-Si/W-doped BiVO4photoanode structure for unassisted water splitting,which achieved an average STH of 0.45% over the course of 1 h at pH 7[18].Moreover,it is the first time to reported that Li doped nano-porous BiVO4photoanode showed an increase(>20 times)in the PEC water splitting compared to pristine BiVO4photoanode[19].The enhancement in PEC performance of Li:BiVO4is related to the formation of inter-band with band gap reduction due to interstitial Li doping in BiVO4through density functional theory calculations.
Apart from the metal doping,non-metallic elements doping has also been broadly employed to modify the optical and electronic properties of BiVO4by efficiently increasing the light absorption,reducing the band gap,accelerating the charge mobility,and prolonging the lifetime of charge carriers,all of which are necessary for pronounced PEC water splitting activity.Xia et al.synthesized P doped BiVO4by a dip-coating route and subsequent thermal treatment process(Figs.2c and d)[20].The incident photon-to-current conversion efficiency(IPCE)value for 2% PBiVO4photoelectrode is boosted to 37.24%(at 360 nm),which is higher than that of pristine BiVO4(13.17%).From the X-ray photoelectron spectra,the P-BiVO4possessed abundant oxygen vacancies,which can account for enhancing PEC water splitting performance of P-BiVO4.Apart from that,Irani et al.obtained N-modified BiVO4photoanode through chemical and physical methods[21].Due to the nitrogen bonded with two vanadium atoms in the lattice,a new empty state~0.1 eV below the conduction band minimum(CBM)was introduced.This state may act as an electron trap center,where photogenerated charge carriers are trapped and PEC water splitting performance is consequently increased.Anke et al.reported the preparation of fluorine-containing bismuth vanadate(F:BiVO4)powder using a new,clean,and simple solid-vapor reaction[22].Notably,F-incorporation significantly improves the performance of BiVO4photoanodes for PEC water oxidation.Namely,an increase of charge carrier density as well as a substantial suppression of charge-carrier recombination in the F:BiVO4result in higher PEC performance.
In addition to the above-mentioned metal and non-metallic elements doping,metal and non-metallic elements co-doping are also proved to enhance light absorption and charge separation,and thereby to improve the PEC water splitting performance of BiVO4.Rohloff’s group has reported that F and Mo co-doping can significantly improve the PEC water oxidation properties of BiVO4[23].They also found that Mo doping mainly improves lightharvesting,charge transport,and charge separation efficiencies,while F modification was demonstrated to primarily affect the charge transfer efficiency at the semiconductor-electrolyte interface.Besides that,Guan et al.demonstrated that the decorate BiVO4with Ag and N-doped carbon exhibits a maximum IPCE value of 30%at 1.23 V vs.RHE,which is~7.5 times to that of the bare BiVO4.This was attributed to the excellent light trapping capability,enhanced charge transport process as well as efficient photoexcited electron-hole separation of BiVO4-C/N-Ag.It is interesting to note that the BiVO4-C/N-Ag also showed outstanding photoelectric durability in comparison to the bare BiVO4[24].
2.2.Deposition of noble metals
Noble metal deposited on BiVO4is also effective in enhancing the photocatalytic properties of BiVO4materials.Significantly,the plasmonic noble metals such as Au,Ag and Pd act as charge sinks in the BiVO4to prevent the recombination of charge carriers,which evidently facilitate the redox reactions that enhance PEC performance.Furthermore,noble metal can improve the visible light harvesting of the BiVO4photocatalytic systems.
Among various plasmonic nanoparticles,Au nanoparticles deposited on various materials are the most investigated ones in the field of PEC water splitting.For example,BiVO4with Ag and N-doped carbon(BiVO4-C/N-Ag)was successfully developed via in-situ dip/redox capture for the first time by Guan et al.[24].The resulting composite was found to exhibit the following features,i.e.,the maximum photocurrent(2.42 mA/cm2at 1.23 V vs.RHE)and IPCE value(30%)were obtained,which is 4.32 times and 7.5 times higher than bare BiVO4(Fig.3a).The enhanced PEC activity can be due to the excellent light trapping capability and efficient photoexcited electron-hole separation(Fig.3b).Besides,the outstanding photoelectric durability for BiVO4-C/N-Ag was achieved in comparison to the bare BiVO4.

Fig.3.(a)The linear-sweep-voltammetry(LSV)under 100 mW/cm2 illumination(scan rate:50 mV/s).(b)Schematic mechanism of BiVO4-C/N-Ag photoanode for PEC water oxidation.(a,b)Reproduced with permission ].Copyright 2019,Elsevier.(c)Linear sweep voltammograms for BiVO4 film,hemispherical Au NP/BiVO4,and octahedral Au NP/BiVO4 photoelectrodes measured in 0.5 mol/L phosphate buffer with 1 mol/L Na2SO3 at a scan rate of 10 mV/s under 1.5 G solar light under the chopped light condition.(d)IPCE spectra of bare BiVO4 and octahedral Au NP/BiVO4 photoelectrodes at 1.23 V vs.RHE.(c,d)Reproduced with permission[25].Copyright 2017,Wiley VCH.
Similarly,the plasmonic effects of octahedral Au nanoparticles(NPs)on BiVO4for PEC water splitting was reported by Lee’s group[25].The investigations indicate that the octahedral Au NPs/BiVO4achieved an improved photocurrent of 2.4 mA/cm2at the 1.23 V vs.RHE and IPCE values approaching 50%in the wavelength of 450 nm(Figs.3c and d).The great enhancement is attributed to the effective absorption,charge generation and separation via localized surface plasmon resonance,direct electron transfer(DET)and plasmonic resonant energy transfer(PRET).Moreover,they suggested that PRET is the critical effect in enhancing the plasmonic water splitting efficiency of the octahedral Au NPs/BiVO4over DET.Later,Ghobadi et al.synthesized Au capped BiVO4(Au-BiVO4)photoanode by using oblique angle physical vapor deposition[26].Owing to the hot electron injection of plasmonic Au,the maximum photocurrent density of 1800 μA/cm2at 1.23 V vs.RHE was obtained(1.6 times than pure BiVO4).
Also,Yang’s group has found that the introduction of Pd nanoparticles(NPs)and nanorods(NRs)can effectively enhance PEC water splitting performance of BiVO4[27].The photoinduced electron-hole pair recombination might be depressed due to the hot electron injection upon SPR excitation of Pd NPs and NRs act as a sensitizer in both visible and NIR regions and thus it was possible for the charge carriers to endure effective PEC water splitting.The same conclusions were also observed in other literature[28-31].The above studies demonstrated that through rational design of noble metal/BiVO4nanostructures,both light absorption ability and charge separation efficiency can be enhanced,and the PEC activities of BiVO4are consequently improved.
2.3.Constructing heterojunctions
Constructing a heterojunction structure is an effective way to improve the PEC water decomposition ability of the BiVO4[32].Depending on their band structures of the coupled semiconductors,a built-in electric field was introduced in the interfaces,and thus a prolonged carrier lifetime and an enhancing interfacial charge transfer can be obtained from such heterostructures.Moreover,introducing second semiconductor often provides additional benefits,such as promoted light harvesting,advantageous surface states,and structural properties[33].At present,BiVO4-based heterojunction are the most widely studied photocatalysts in the field of PEC water splitting.Generally,the BiVO4-based heterojunction for PEC water splitting can be categorized into two different types depending on the charge carrier separation mechanism:1)Conventional type-II and p-n junction;2)direct Zscheme.In this section,the basic principles and applications of these three heterojunctions for PEC water splitting are discussed.
2.3.1.Conventional type-II heterojunction
The type II heterojunction is the most typical heterojunction system created to enhance the PEC performance of BiVO4[33].In type II systems,the CB position of semiconductor A is more negative than that of semiconductor B,and the VB position of semiconductor B is more positive than that of semiconductor A.Under light irradiation,the electrons in the CB of semiconductor A migrate to the CB of semiconductor B,while the holes in the VB of semiconductor B migrate to the VB of semiconductor A.Therefore,the photo-generated carriers effectively separate in the space.
Recently,Chae et al.has proved that WO3/BiVO4heterojunction showed promising PEC water splitting activity than the pure WO3and BiVO4,respectively[34].The type-II heterojunction can be produced between WO3and BiVO4.When the WO3/BiVO4heterojunction is radiated by visible light,the generated excited-electrons transfer from BiVO4CB to WO3CB,and the holes transfer from WO3VB to BiVO4VB,which can accelerate the separation of electron-hole pairs and also enhance the fascinating optical properties.Similarly,Zhou et al.prepared hierarchical WO3/BiVO4nano-porous sphere arrays(Fig.4a),and it revealed a high photocurrent of 5.5 mA/cm2at 1.23 V vs.RHE under one sun illumination(Fig.4b)[35].Meanwhile,W-doping,and oxygen vacancy creation can be directly incorporated into the as-obtained WO3/BiVO4type-II nanojunction,which can further increase charge separation and transfer efficiencies.
Other examples of type II BiVO4-based heterostructures are as follows.Zhang et al.reported that a three-dimensional FTO/TiO2/BiVO4core-shell was fabricated for PEC hydrogen production by combining atomic layer deposition and electro-deposition routes[36].It was found that the optimized FTO/TiO2/BiVO4exhibited a significantly improved PEC performance of 4.11 mA/cm2in the presence of holes scavenger(Fig.4c).The significantly enhanced PEC performance is attributed to the 3D continuous conductive FTO ordered inverse opals improving the charge transport and collection efficiency as well as the excellent contact between the TiO2and BiVO4with a large interface area facilitating the type II charge transfer process(Fig.4d).In this respect,very recently,Liu et al.reported that optimized TiO2/BiVO4/Co-Pi photoanode yielding a photocurrent density of~4.96 mA/cm2(0.63 V vs.Ag/AgCl),leading to a photo-to-energy conversion efficiency that was 9.0 times better than TiO2inverse opals and 2.3 times better than the TiO2/BiVO4photoanode[37].Likewise,CdS quantum dots sensitized olive-shaped mesoporous BiVO4was successfully synthesized by Zhou and co-workers[38].Apparently,an improved photo-electrical conversion efficiency of~1.29% is achieved for this photoelectrode material,which is ascribed to the formation of CdS/BiVO4type II heterostructure that can effectively promote the charge transfer behavior.
Similarly,Gao et al.synthesized NiMoO4/BiVO4photoanode for PEC water splitting via hydrothermal method,and cobalt phosphate(CoPi)as an oxygen evolution reaction(OER)cocatalyst was deposited on the above electrode[39].They achieved an improved photocurrent density of 5.3 mA/cm2at 1.23 V vs.RHE.This enhanced photocurrent density was originated from the development of a type II heterojunction at the NiMoO4/BiVO4interface.Other than this,Palaniselvam et al.reported the loading of NiCoO2nanoparticles on BiVO4thin film for PEC oxygen evolution reaction[40].The resulting BiVO4/NiCoO2composite electrode manifested a high charge separation efficiency than pure BiVO4owing to type II charge transfer mechanism between BiVO4and NiCoO2.
Our research group also prepared the MFe2O4(M=Ni,Co)/BiVO4composite photoelectrodes via electrophoretic deposition process,ensuing heating treatment and electrophoretic deposition technology[41].It was observed that MFe2O4(M=Ni,Co)/BiVO4showed higher photocurrent response values(at 1.23 V vs.RHE)and superior PEC hydrogen evolution performance than pure BiVO4electrode under visible light illumination.The improved PEC activity of water splitting was attributed to electrons in the CB of MFe2O4(M=Ni,Co)that transferred to CB of BiVO4and the holes in VB of BiVO4that transferred to the VB of MFe2O4(M=Ni,Co)(Fig.4e).Balamurugan et al.reported that the core-shell BiVO4/FeVO4inverse opal(IO)films presented higher activity for PEC hydrogen production than unmodified BiVO4,probably resulting froma favorable chargetransfer/transportphenomenon under type II band alignment[42].
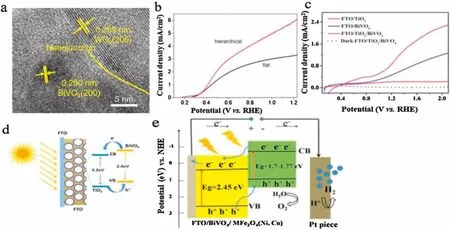
Fig.4.(a)HRTEM image of nanoparticle from hierarchical BiVO4 nanostructured film.(b)Photoelectrochemical activities for sulfite oxidation of nanoporous WO3/BiVO4 electrodes with hierarchical structure and flat structure,measured in a 0.5 mol/L phosphate buffer(pH 7)containing 0.5 mol/L Na2SO3 under AM 1.5 G,100 mW/cm2 illumination with a scan rate of 20 mV/s.(a,b)Reproduced with permission[35].Copyright 2017,American Chemical Society.(c)Linear sweep curve of the FTO/TiO2/BiVO4/Co-Pi and FTO/TiO2/BiVO4/Co-Pi composite inverse opals measured in 0.5 mol/L Na2SO4 solution.(d)The charge transfer mechanism of the FTO/TiO2/BiVO4 composite inverse opals.(c,d)Reproduced with permission[36].Copyright 2017,American Chemical Society.(e)The possible PEC hydrogen generation mechanism of MFe2O4(M=Ni,Co)/BiVO4 electrodes at 1.23 V vs.REH in 0.5 mol/L Na2SO4 under visible light.Reproduced with permission ].Copyright 2017,Elsevier.
These outcomes demonstrate that type II heterojunction engineering can effectively promote charge separation,increasing the conductivity and thereby enhance the photocurrent density and PEC water splitting properties.
2.3.2.P-N junction
As for the p-n junction,when p-and n-type semiconductor contacted,the p-n junction with a space charge area forms at the interface under the diffusion of electrons and holes,causing the internal electric field build up[33].Under light irradiation,the electrons in the CB of p-type semiconductor transferred to the CB of n-type semiconductor due to the internal electric field,while the holes finally gathered in the VB of the p-type semiconductor.The fast separation of electron-hole at the p-n heterojunction interface greatly reduces their recombination probability,and prolongs the life of photogenerated charges,thus improving the photocatalytic efficiency.
In the early study,Khoomortezaei et al.developed a triple heterojunction WO3/BiVO4/BiFeO3porous photoanode via a sol-gel method.It was found that formation of a p-n junction at BiVO4/BiFeO3can enormously enhance the charge separation and PEC water splitting performance[43].Recently,a p-n heterojunction of Cu2O/BiVO4was prepared for PEC water oxidation by a two-step electrodeposition method on an FTO substrate followed by annealing treatment[44],with a high photocurrent density of 1.72 mA/cm2at 1.23 V vs.RHE obtained(4.5 times higher than that of pristine BiVO4thin films).In addition,the cathodic shift of the onset potential of Cu2O/BiVO4was about 420 mV,which is the largest shift amongst all BiVO4based p-n hetero-junction photoanodes ever reported.The authors demonstrated that the enhancement of carrier density and negative shift of flat band potential are attributed to the p-n junction at the interface of semiconductors and an inner electronic field from n-type BiVO4to p-type Cu2O.Pan et al.fabricated Ag-embedded MoS2/BiVO4p-n heterojunction ternary composite(BiVO4-Ag-MoS2)electrode[45].Owing to the formation of a p-n heterojunction between MoS2and BiVO4to boost its poor electron-hole separation and transport properties by the built-in electrical potential,the photocurrent density of BiVO4-Ag-MoS2(2.72 mA/cm2at 0.6 V vs.RHE)can be enhanced 2.44 times compared with pure BiVO4electrode(0.79 mA/cm2).
Apart from the hybridization of metal oxide or sulfide semiconductor photocatalysts with BiVO4,some p-type VIII(Fe,Co,Ni)hydroxides such as FeOOH,NiOOH and CoOOH are deemed to be cost-effective and good cocatalysts for the modification of ntype BiVO4for enhanced PEC activity.For instance,Kim et al.modified BiVO4with FeOOH/NiOOH as an efficient cocatalyst for the PEC water oxidation[46].The presence of FeOOH/NiOOH reduces interface recombination at the BiVO4/OEC junction.The resulting BiVO4/FeOOH/NiOOH photoanode achieves a photocurrent density of 2.8 mA/cm2at 0.6 V vs.RHE.In another study,Zhang et al.demonstrated that the n-type BiVO4photoanodes modified by ultrathin p-type CoOOH structure(~2 nm)could significantly improve the PEC activities[47].
In a work by Wang et al.,they employed a facile electrodeposition and thermal treatment method to develop a novel BiVO4/FeOOH/NiOOH(BiVO/FeOOH/NiOOH)dual photoanodes[48].The successful development of the ternary hybrid photoanodes was demonstrated by the XRD,XPS and TEM.It was found that the BiVO/FeOOH/NiOOH photoanodes exhibit a remarkable and highly stable photocurrent density of 5.87 mA/cm2at 1.23 V vs.RHE under AM 1.5 G illumination.Similar to most of the previous reports,the formation of p-n junction photoanodes led to the rapid migration of photo-generated charge carriers to suppress the recombination rate.Most importantly,the as-obtained BiVO/FeOOH/NiOOH delivers a STH as high as 6.5% for unbiased water splitting.
In our previous work,the novel FeF2/BiVO4photoanode was successfully synthesized via electric deposition method for PEC generated hydrogen during water splitting process[49].The FeF2/BiVO4photoanode features greatly enhanced the performance for hydrogen evolution rate around 635 μmol within 3 h,three times more than pure BiVO4(Fig.5a).This is because of the formation of p-n heterojunctions between FeF2and BiVO4which can effectively prohibit carriers from recombining and enhance the efficiency of electrons and holes separation(Fig.5b).Subsequently,our group synthesized F-incorporated FeOOH(F:FeOOH)cocatalyst modified BiVO4photoanode by a one-step hydrothermal deposition method for PEC water oxidation[50].The resulting composite photoanode shows an impressive photocurrent density and a notable initial potential that is negatively shifted by 150 mV relative to the pure BiVO4electrode(Fig.5c).We also found that a p-n junction is formed between F:FeOOH and BiVO4,which is a promising property to spatially separate the charges to retard the recombination of charge carriers(Fig.5d).
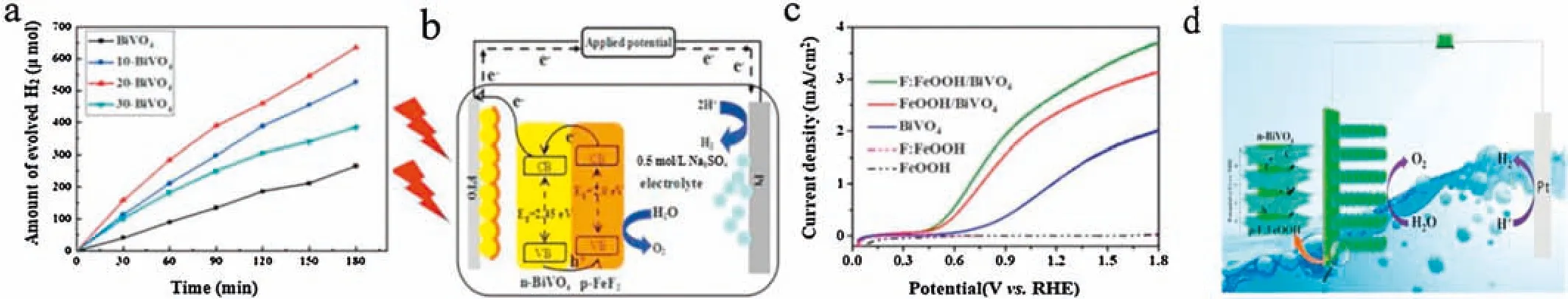
Fig.5.(a)Time course of produced H2 at 1.23 V vs.RHE in 0.5 mol/L Na2SO4 solution.(b)The possible PEC hydrogen generation mechanism of FeF2-BiVO4 electrode at 1.23 V vs.RHE in 0.5 mol/L Na2SO4 solution under visible light irradiation.(a,b)Reporduced with permission ].Copyright 2018,Elsevier.(c)LSV curves measured in 0.5 mol/L Na2SO4 with light.(d)The possible PEC hydrogen generation mechanism of F:FeOOH/BiVO4 electrode at 1.23 V vs.RHE in 0.5 mol/L Na2SO4 solution under visible light irradiation.(c,d)Reporduced with permission ].Copyright 2020,Elsevier.
All the above studies elucidate that the construction of p-n heterojunction is one of the most promising strategies to enhance the PEC water splitting activity of BiVO4by combining the effects of the built-in electric field and the band alignment between two semiconductors.
2.3.3.Z-scheme heterojunction
The Z-scheme heterojunction has both a high charge separation efficiency and a remarkably high redox ability by retaining a highly negative CB edge and a highly positive VB edge[51-53].Briefly,for the semiconductor-semiconductor Z-scheme heterojunction,the photogenerated electrons from the less negative CB edge of semiconductor 2 will interact with the less positive VB edge of semiconductor 1,leaving behind the electrons and holes in the CB of semiconductor 1 and the VB of semiconductor 2,respectively[54].Therefore,the Z-scheme heterojunction evidently has more advantages for achieving more efficient photocatalytic reduction compared with the conventional type-II heterojunction.
Recently,a Z-scheme g-C3N4/BiVO4heterojunction was prepared for PEC water splitting by Safaei’s group[55].It was found that PEC performance of g-C3N4/BiVO4heterojunction is 4 times than that of bare BiVO4at 1.23 V vs.RHE under visible light irradiation.There are three possible reasons for high PEC properties of g-C3N4/BiVO4:1)V4+was formed in the presence of hydrogen in the interplanar of g-C3N4,thus improving the mobility of charge carriers.2)The oxygen vacancy generated by V4+as the central role of catalytic sites and chemisorption substances,ultimately achieving higher photocurrent response.3)Experimental observations and Density Functional Theory simulations justified that the g-C3N4and BiVO4formed a Z-scheme heterojunction,where the electrons of CB of BiVO4recombined with the holes of VB of g-C3N4,resulting in the consequence that the photogenerated electrons directly reduce water from CB of g-C3N4while holes oxidize water at VB of BiVO4.Additionally,Nasir et al.synthesized a direct Z-scheme Se/BiVO4heterojunction by aerosol-assisted chemical vapor deposition method for PEC water splitting[56].An enhanced PEC activity is observed in case of Se/BiVO4heterojunction,as compared to Se and BiVO4,due to the Se makes a direct Z-scheme(band alignments)with BiVO4where the photoexcited electron of BiVO4recombines with the VB of Se,giving electron-hole separation at Se and BiVO4,respectively.Radzi et al.constructed TiO2/BiVO4photoelectrode using simple spin coating procedure for PEC water splitting[57].As a result,a highest photocurrent(35 μA/cm2,at 1.23 V vs.RHE)was obtained for TiO2/BiVO4photoelectrode,which is more than~3 times compared with bare TiO2and BiVO4.This enhanced performance was due to the Z-scheme charge transfer between TiO2and BiVO4,and thus efficiently improves the separation of the electron-hole pairs.
On the contrary,for the semiconductor-conductor-semiconductor Z-scheme heterojunction,a conductor as an intermediate is employed to bridge two semiconductors for the relative ease of electron transportation from semiconductor 2 to semiconductor 1.For example,an unconventional Z-scheme BiVO4-W-WO3photoanode is fabricated by Wang and co-workers for PEC water splitting[58],and they found that the resulting BiVO4-W-WO3photoanode showed a highest photocurrent density of 5 mA/cm2in 0.1 mol/L Na2SO4electrolyte under visible light illumination,as well as possessed highest IPCE values(>60% at wavelengths less than 460 nm)(Figs.6a-c).Meanwhile,the PEC stability of BiVO4was improved by restricting the electrolyte corrosion with coating WO3layers(Fig.6c).Interestingly,the remarkably enhanced PEC performance of BiVO4-W-WO3photoanode is attributed to the metal W acts as the recombination site of photogenerated electrons and holes,which are obtained from the CB of WO3and the VB of BiVO4,respectively(Fig.6d).Another group of researchers has also fabricated PEC cell with a Ru-loaded(CuGa)0.5ZnS2photocathode and a CoOx-modified BiVO4photoanode in order to enhance the PEC water splitting efficiency[59],and they found that the CoOxplayed an important role in the Zscheme system(Ru-loaded(CuGa)0.5ZnS2-BiVO4)to promote water oxidation on BiVO4,resulting in the enhancement of the electron injection from BiVO4to Ru-loaded(CuGa)0.5ZnS2through CoOx..
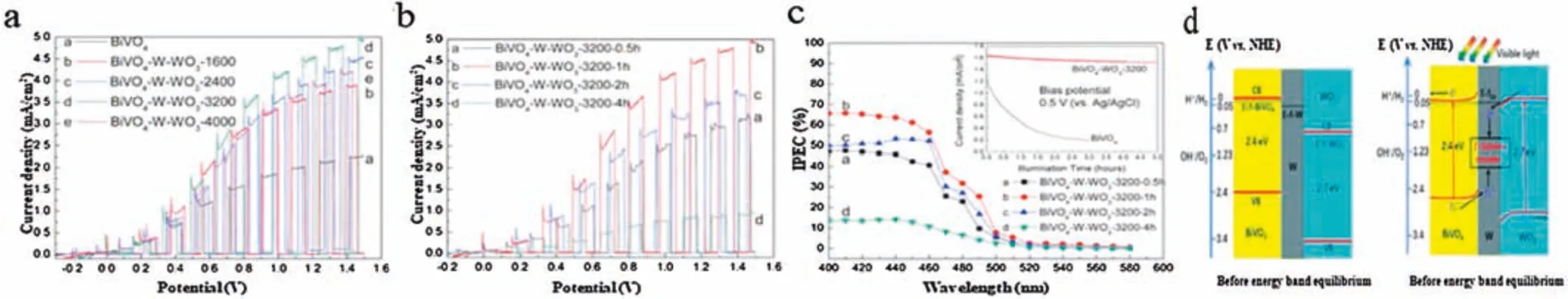
Fig.6.(a)Photo-induced linear sweep voltammetry(LSV)curves of BiVO4 and BiVO4-W-WO3 photoanodes with different deposition times.(b)LSV curves and(c)Incident photon-to-current conversion efficiency(IPCE)at bias potential of 1.5 V(vs.Ag/AgCl)of BiVO4-W-WO3-3200 photoanodes with different annealing times(0.5 h to 4 h)under 500°C in oxygen atmosphere;Photoelectrochemical stabilities of BiVO4 and BiVO4-W-WO3-3200 photoanodes are shown inset(c);Testing was conducted under a bias potential of 0.5 V(vs.Ag/AgCl).All tests were carried out in 0.1 mol/L Na2SO4 electrolyte.For LSV performance testing,100 mW/cm2 visible light(λ>420 nm)was employed.(d)Energy band structure of FTO-BiVO4-W-WO3 Z-scheme photoanode.Copied with permission[58].Copyright 2018,Royal Society of Chemistry.
The above results indicate that Z-scheme BiVO4-based constructions can not only favor the photogenerated charge separations in space but also promote the redox reactions during PEC water splitting processes.However,only few studies have focused on developing the BiVO4-based Z-scheme system in the photoanode or photocathode composite.Therefore,the development and design of Z-scheme BiVO4-based PEC water splitting systems with high stability,cyclability,high efficiency,and wide spectral response under sunlight irradiation has very critical significance in both theory and practice.
2.4.Coupling carbon materials
Carbon materials,such as graphitic carbon nitrides(g-C3N4),carbon nanotube(CNT),carbon quantum dots(CQDs),grapheme(G)and reduced graphene oxide(rGO),can be coupled with BiVO4,thus the PEC activity of BiVO4can be significantly improved.This enhancement can be ascribed to the fact that these carbon materials can act as a photoelectron reservoir,which stores the photogenerated electrons from BiVO4to substrates,or perform as a photosensitizer,which improves the light absorption of photocatalyst as an organic dye.
As a famous 2D mono/multilayered material,g-C3N4are also extensively applied as visible-light-driven catalyst for PEC water splitting due to its narrow bandgap of~2.70 eV with suitable CB/VB levels(ca.—1.10/1.60 eV).Some studies have proven that combining g-C3N4with BiVO4can strengthen the PEC performance of BiVO4.For example,an unprecedent heterojunctions of BiVO4quantum dots(QDs)/g-C3N4nanosheets(NSs)were synthesized by an in-situ growth strategy by Ye et al.[60].It was found that the BiVO4QDs/g-C3N4NSs exhibited enhancement in visible-lightdriven PEC performance.This was mainly due to the highly dispersed BiVO4nanocrystals,as well as the strong coupling and band alignment between BiVO4QDs and g-C3N4NSs.In another report,Samsudin et al.fabricated the micro-flower g-C3N4/BiVO4photocatalysts for PEC water splitting using lake water without sacrificial reagents[61].Fascinatingly,the 0.8 wt% g-C3N4/BiVO4evinced 21.4 mmol/h of hydrogen generated in comparison to other samples with an AQE of 4.27% at 420 nm.According to the experimental results and density functional theory(DFT)analysis of g-C3N4/BiVO4photocatalysts,the enhanced PEC property was attributed to better crystallinity,optical properties and efficient electron-hole separation corresponded to the synergistic effect of the band offset and built-in electric field.
In addition to g-C3N4,CNT and CQD are another carbon material with superior electronic properties and excellent conductivity.So far,there have been several reports to unite the advantages of BiVO4with the CNT or CQD to form the composite for promising catalytic applications.For example,Jessl et al.designed a honeycomb shaped 3D CNT/BiVO4as a photoelectrode for solar water splitting[62].Based on the experimental results,two main conclusions were drawn:1)The as-obtained honeycomb structure allows for an efficient transport of electrons through the electrode and enhanced light-electrode interaction;2)the developed CNT electrodes can survive harsh BiVO4synthesis conditions and subsequently be used as photoelectrodes for solar water splitting.In order to enhance the PEC water splitting efficiency,the NiOOH/FeOOH/CQD/BiVO4(NFCB)photoanodes were prepared successfully by Ye and co-workers[63].The LSV curves of the NFCB photoanodes showed a remarkable photocurrent density of 5.99 mA/cm2at 1.23 V vs.RHE under AM 1.5 G in KH2PO4aqueous solution without a hole scavenger(pH 7),which is about 4.4 times higher than the pristine BiVO4photoanode(1.35 mA/cm2at 1.23 V vs.RHE)(Fig.7a).Meanwhile,this novel NFCB photoanode could operate stably for 10 h with a Faraday efficiency of~95%,demonstrating the great potential of using CQDs for solar water splitting.Not only that,they also discovered that the CQDs in NFCB system can not only broaden the light response range and increase the separation of electrons and holes,but also maintain the hole transfer ability from BiVO4to the OEC layer(Fig.7b).
In addition to the above-mentioned carbon materials,G and rGO are also coupled with BiVO4to reinforce the PEC performance of BiVO4due to its excellent electric conductivity and a high surface area that increases the charge carrier.Pan and co-workers fabricated Zn-BiVO4/graphene quantum dots(GQDs)/Co-Pi film through a modified electrodeposition process[64].As a result,the Zn-BiVO4/(GQDs)/Co-Pi electrodes exhibited a photocurrent of 3.01 mA/cm2and an IPCE of 57%(at 0.6 V vs.RHE),which is 8.6 times of the pristine BiVO4.This finding vividly implies that the introduction of GQDs extends the light absorption and facilitates the charge transfer.
Most recently,Chen et al.prepared BiVO4/rGO/NiFe-layered double hydroxide(LDH)photoanode for PEC water splitting through spin-coating rGO nanosheets on BiVO4[65].As compared to pristine BiVO4,BiVO4/rGO,and BiVO4/NiFe-LDH counterparts,the BiVO4/rGO/NiFe-LDH photoanode showed a significantly higher photocurrent density(3.26 mA/cm2at 1.23 V vs.RHE)under AM 1.5 G illumination and excellent stability.It was demonstrated that rGO sheets act as an efficient electron shuttling mediator for suppressing the electron-hole recombination on the surfaces of BiVO4by quickly extracting the photogenerated holes from BiVO4and promoting the formation of NiFe-LDH at a mild potential.Additionally,upon the drop-casting method,Bi-rGO/BiVO4photoanode was successfully achieved[14].The Bi-rGO/BiVO4photoanode benefited a low charge transfer resistance.As a result,improved charge transfer and separation efficiency were attained in the Bi-rGO/BiVO4photoanode as supported by an increased photocurrent(6.05 mA/cm2at 1.23 V)by 1.7 times compared to Bi-BiVO4(3.56 mA/cm2at 1.23 V).Moreover,a better STH(2.34% at 0.61 V)was displayed for the BirGO/BiVO4photoanode(Fig.7c).This enhancement in PEC activity is due to the combined action of Bi NPs in improving the charge carrier density and rGO in increasing the charge carrier mobility(Fig.7d).

Fig.7.(a) J-V curves recorded at a scan rate of 25 mV/s under AM 1.5 G irradiation in potassium phosphate solution with no hole scavenger(pH 7).(b)Illustration of the PEC water oxidation at the NFCB photoanode;(a,b)Copied with permission[63].Copyright 2017,Royal Society of Chemistry.(c)H2 evolution of Bi-rGO/BiVO4 under light illumination under 2 h in comparison with pure BiVO4 and Bi-BiVO4.(d)Schematic diagrams of energy level alignment and charge transfer in Bi-rGO/BiVO4 photoanode under solar light illumination.(c,d)Copied with permission[14].Copyright 2020,Elsevier.
The roles of various carbon nanomaterials such as CNT,rGO,or g-C3N4(GCN)in BiVO4 photoanode were investigated for PEC water oxidation by Prakash et al.[66].It was observed that PEC performance toward water splitting follows the order,BiVO4<BiVO4/CNT<BiVO4/rGO<BiVO4/GCN.The enhanced PEC performance could be due to the nanojunction formation between BiVO4and CNT or rGO or GCN minimizing the chances of charge recombination.As for BiVO4/GCN,the lower bulk and interfacial resistance,high charge donor,and efficient charge transfer led to the better performance in comparison to other photoanodes.
To sum up,it is anticipated that the formation of BiVO4/carbon materials heterojunctions is a promising pathway for the development of BiVO4-based photoanode with high quality to achieve large specific surface areas,prolonged lifetime of charge carriers,enhanced electron transport property,improved charge transfer and separation efficiency and reduced recombination rate of charge carriers for favorable PEC water splitting.
2.5.Coupling metal-organic framework(MOF)materials
Metal organic frameworks(MOFs)are a class of crystalline organic-inorganic compounds,which are covalently linked by the inorganic building block and also organic linkers.Similar to other semiconductors,MOF photocatalysts can photo-generate holes and electrons upon visible-light irradiation with redox activity,leading to the transfer of holes and electrons to the desired reactant molecules to initiate the light driven photo-redox processes.In view of band structures of MOFs,the outer orbitals of organic linkers construct the VB,whereas the empty outer orbitals of metal contribute to the CB of MOFs.Thus far,the incorporation of MOFs with BiVO4has widely attracted increasing attention due to a variety of structural and species diversities as well as tunable cavities,porosity and high surface areas of MOFs.
For instance,Liu et al.synthesized MIL-101(Fe)/Mo:BiVO4photoanode through hydrothermal method to overcome the poor electron-hole separation and utilization efficiency of BiVO4[67].Notably,a remarkable stable photocurrent density of 4.01 mA/cm2was observed for optimized MIL-101(Fe)/Mo:BiVO4anode at 1.23 V vs.RHE in Na2SO4aqueous,about 4 times higher than that of pristine BiVO4photoanode(Fig.8a).Moreover,the MIL-101(Fe)/Mo:BiVO4photoanode possessed excellent stability for fast light off-on cycles.The greatly improved PEC performance of the photoanode is due to the ultrathin MIL-101(Fe)layer as an oxygen evolution cocatalyst can effectively extend the visible-response region,accelerate the charge separation,and provide more accessible active sites for water oxidation(Fig.8b).

Fig.8.(a)Photoelectrochemical performance tests of different photoanodes:LSV curves recorded under illumination and dark condition.(b)Schematic presentation of band level positions for Mo:BiVO4 and MIL-101(Fe)showing the charge transfer path.(a,b)Reproduced with permission ].Copyright 2019,Elsevier.(c)LSV curves of BiVO4 and BiVO4@Co-MIm electrodes under illumination(the dotted line is LSV curve of BiVO4@Co-MIm without illumination for comparison).(d)The proposed photogenerated charges transporting process and the process of water oxidation on the BiVO4@Co-MIm anode.(c,d)Reproduced with permission ].Copyright 2019,Elsevier.
Recently,our research group synthesized BiVO4@Co-MIm film by a simple water bath deposition method for PEC water splitting[68].Three noteworthy phenomena were observed for the BiVO4@Co-MIm electrode:1)A higher photocurrent of 3.16 mA/cm2at a potential of 1.23 V vs.RHE under simulated sunlight,which is 2.4 times that of BiVO4photoanode(Fig.8c);2)a higher charge injection efficiency and charge separation efficiency than that of BiVO4photoanode;3)a stable H2producing Faradic efficiency of 91.8%can be gained.Additionally,the enhancement of PEC properties of BiVO4@Co-MIm electrode due to Co-MIm could accept photogenerated holes transferred from BiVO4to catalyze water oxidation reaction and accelerate surface reaction kinetics(Fig.8d).In our other work,CoNi-MOFs/BiVO4photoanode was successfully prepared for PEC water oxidation via a facile hydrothermal deposition method[69].In particular,the CoNi-MOFs/BiVO4exhibited an excellent photocurrent density of 3.2 mA/cm2at 1.23 V vs.RHE,which is 3 times higher than that of pristine BiVO4.
As a result,the ratio of evolved O2and H2on the surface of CoNi-MOFs/BiVO4photoanode is close to the theoretical value.In addition,the Faradaic efficiencies of the H2and O2evolution reaction were about 90%.Finally,it is worth mentioning that the CoNi-MOFs layer not only greatly increases the surface reaction sites but also remarkably enhances the charge separation of BiVO4photoanode.
Apart from coupling MOFs with BiVO4directly,MOFs are also frequently used as precursor materials to synthesize high surface area effective cocatalysts to decorate the BiVO4 photoanode.For example,Cardenas-Morcoso et al.coupled BiVO4with MOFderived,highly porous cobalt oxide(CoOx)via calcination in air[70].After the introduction of CoOx,the resulting BiVO4-CoOxrevealed 4-times higher photocurrents compared to the bare BiVO4.Furthermore,The LSV and EIS analysis confirmed that the ZIF-67 MOF-converted CoOxco-catalysts significantly accelerate the kinetics of water oxidation,due to a large amount of catalytically-active water oxidation sites were exposed.Similarly,Co3O4/BiVO4photoelectrode was obtained via annealed Co-MOF/BiVO4photoanode in air by Xu’s group[71].There was no doubt that the Co3O4/BiVO4photoanode exhibited the highest photocurrent density(2.35 mA/cm2at 1.23 V vs.RHE)and PEC hydrogen production rate(0.61 mmol/cm2in 5 h),which was about 3.2 times higher than that of bare BiVO4.The significant enhancement of PEC performance over Co3O4/BiVO4could be attributed to suppression of charge recombination.
In short,numerous MOFs/BiVO4heterojunction systems have been developed for the past few years.As anticipated,a greater PEC water splitting activity over the bare BiVO4photoanode was achieved on the hybrid nanocomposites,signifying the advancements of heterojunction.Capitalizing on the current phase forward,it is expected that the development of more MOFs/BiVO4PEC systems for multifarious energy and environmental applications.
3.Conclusion and outlook
Over the past three years,numerous efforts have been made to improve solar hydrogen production via PEC water splitting under solar irradiation.In this work,we reviewed recent reports using engineering techniques to fabricate efficient and enhanced photoactive BiVO4materials for PEC water splitting.These engineering techniques include:doping nonmetal and metal elements,depositing of noble metals,constructing heterojunctions,as well as coupling with carbon and MOF materials.To this end,the enhancement of the PEC water splitting performance of BiVO4-based nano-structures was large recognized by two main far-reaching criteria,such as harvesting visible light and hindering the recombination rate of photoinduced charge carriers.In spite of some promising results reported thus far,the studies in this field are still in preliminary stages and further developments are prominently required.Notably,the works still suffer from the relatively low efficiency and low stability of the BiVO4-based nanocomposites,which are far from the requirements of industrial needs(Table 1).

Table 1 Overview of BiVO4-based photoanodes for PEC water splitting.
Therefore,for exploring more highly efficient and stable BiVO4-based photoelectrodes that can efficiently retard the charge recombination and capture the solar spectrum is of paramount importance in the field of PEC water splitting.Until now,most of the BiVO4-based nanomaterials possess a type II heterojunction system,which have been proven to be outstanding for harvesting visible light and hindering the recombination rate of photoinduced charge carriers.However,few studies have focused on the BiVO4-based Z-scheme heterojunction for PEC water splitting.Therefore,exploring more active BiVO4-based Z-scheme photocatalytic systems with additional properties such as earth abundance,low cost,nontoxicity,and scalability will be more attractive in the future.Besides,the mechanisms of Z-scheme BiVO4-based photocatalysts remain largely unclear and must be extensively studied.Various characterization methods such as,radical-species trapping test,time-resolved photoluminescence emission decay spectra,X-ray photoelectron spectroscopy characterization can be an effective strategy for providing a full image of the PEC water splitting reaction for a direct Z-scheme.
Apart from that,BiVO4act as matrix material for the decoration of nanoparticles to form a hybrid system.However,it is challenging to control the particle size on the BiVO4substrate at the molecular level,resulting in severe aggregation.Consequently,the wellcontacted heterojunction interface between BiVO4and the nanoparticles is difficult to accomplish.This markedly affects the intimate interfacial interaction between BiVO4and the other composite for effective charge transport and separation.To address this shortfall,surface functionalization of BiVO4to tune the surface charge with specific surface groups will be a positive point to strengthen the anchoring ability of BiVO4.
Last but not least,some key issues that account for the high PEC activity,i.e.,optical absorption,electronic band structure and interfacial charge transfer across the BiVO4-based nanocomposites,should be exhaustively investigated to gain theoretical insights by means of first principles DFT calculations.A combination of experimental and theoretical results from fundamental studies and computer simulations should be preferred as it is the best approach to advance the current state of knowledge on PEC water splitting mechanisms of BiVO4-based photoelectrodes.
In general,PEC water splitting is a promising route for the production of H2applying for free solar energy.Therefore,the development and design of BiVO4-based systems for PEC water splitting with high stability,cyclability,nontoxicity,high efficiency,and wide spectral response under sunlight irradiation has very critical significance in both theory and practice.
Declaration of competing interest
All authors declared that they do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China(Nos.21663027,21808189),the Fundamental Research Funds for the Central Universities of Chang’an University(No.300102299304)and the Natural Science Basic Research Fund of Shaanxi Province(No.2020JZ20).
杂志排行
Chinese Chemical Letters的其它文章
- Super-assembled highly compressible and flexible cellulose aerogels for methylene blue removal from water
- In-situ deposition of Pd/Pd4S heterostructure on hollow carbon spheres as efficient electrocatalysts for rechargeable Li-O2 batteries
- Sulfonic acid-functionalized core-shell Fe3O4@carbon microspheres as magnetically recyclable solid acid catalysts
- Boron-iron nanochains for selective electrocatalytic reduction of nitrate
- The capacity and mechanisms of various oxidants on regulating the redox function of ZVI
- Conducting polymer engineered covalent organic framework as a novel electrochemical amplifier for ultrasensitive detection of acetaminophen
