Evaluation on Uncertainty of Detection Results of Aerobic Plate Count
2021-08-24YunxiaWANGLijuanJINGCuizhiLIZhiyongLULijunLIU
Yunxia WANG, Lijuan JING, Cuizhi LI, Zhiyong LU, Lijun LIU
Quality Management Department, Inner Mongolia Yili Industrial Group Co., Ltd., Hohhot 010110, China
Abstract [Objectives]To determine the aerobic plate count(APC)in the milk samples, evaluate the uncertainty of the test results, and to provide a scientific basis for the quality control of the testing process.[Methods]In compliance with the national food safety standard Food Microbiological Examination: Aerobic Plate Count(GB 4789.2-2016), the aerobic plate count in the milk samples was detected.The source of the uncertainty of the test result was analyzed and a mathematical model was established in accordance with Evaluation and Expression of Uncertainty in Measurement(JJF 1059.1-2012).Then, the introduced uncertainty components were evaluated to determine the uncertainty of the final combined aerobic plate count.[Results]The expanded uncertainty of the test result of the aerobic plate count in the milk samples was 0.043 4, and the logarithmic value interval of the results was(3.924, 4.010), and the antilogarithm was taken to get the aerobic plate count in the sample to be 8 395-10 233 CFU/mL.[Conclusions]This method can effectively evaluate the uncertainty of the aerobic plate count, and ensure the accurate and scientific laboratory test data.
Key words Aerobic plate count(APC), Uncertainty, Quality assurance, Food safety
1 Introduction
In recent years, food safety issue is attracting wider and wider attention.The aerobic plate count(APC)is an important indicator for evaluating food hygiene.The aerobic plate count can directly reflect the condition of food hygiene and provide data support for the hygiene evaluation.At present, the aerobic plate count is always determined in accordance with the national food safety standardFood
Microbiological
Examination
:Aerobic
Plate
Count
(GB 4789.2-2016).Although the test steps are not complicated, the accuracy of the test results is affected by many factors such as sample uniformity, instrument accuracy, sample preparation, dilution process, culture conditions, colony count, and experimental environment.These factors alone or in combination may cause certain errors in the test results.Therefore, it is necessary to analyze and evaluate the uncertainty induced by these factors to further reduce the impact of errors.Uncertainty is an evaluation that characterizes the range of the true value of the measured value.It gives the range of values within which the true value is asserted to lie according to a certain confidence probability.It can make the test data more objective and scientific, especially when the test result of the tested sample is close to the limit value.In order to ensure that the test results are more objective and accurate, it is particularly important and necessary to carry out uncertainty analysis and evaluation.This is of great significance for providing accurate data and issuing compliance judgments based on this data.
BothGuidance
on
the
Application
of
Testing
and
Calibration
Laboratory
Competence
Accreditation
Criteria
in
the
Field
of
Microbiological
Testing
(CNAS-CL01-A001)andRequirements
for
Measurement
Uncertainty
(CNAS-CL07)set forth clear requirements for the uncertainty evaluation of microbial test results: the microbiological testing laboratory should take into account the various main uncertainty components in the test, and have the ability to evaluate the uncertainty of each quantified measurement result; in some cases, considering the importance of the test results, it is necessary to list the main uncertainty components and make a reasonable assessment.Therefore, the testing laboratory that has passed the accreditation must strictly implement the analysis and evaluation of the uncertainty of the microbiological measurement results.In compliance with the national food safety standardFood
Microbiological
Examination
:Aerobic
Plate
Count
(GB 4789.2-2016), we measured the aerobic plate count in the milk samples.In accordance withEvaluation
and
Expression
of
Uncertainty
in
Measurement
(JJF 1059.1-2012), we analyzed the source of the uncertainty of the test result and established a mathematical model.Then, we evaluated the introduced uncertainty components to determine the uncertainty of the final combined aerobic plate count, to further improve the accuracy and reliability of laboratory test data.2 Materials and methods
2.1 Materials and reagents
The milk samples were purchased from the market; plate counting agar(PCA)and sterile saline were purchased from Beijing Land Bridge Technology Co., Ltd.2.2 Instruments and equipment
ML1602 electronic analytical balance(Mettler-Toledo Instruments Co., Ltd., Switzerland); 0400/001/EU sterile homogenizer(Seward limited, UK); BPX-162 electric heating thermostat incubator(Shanghai Boxun Industry & Commerce Co., Ltd., China); GI54DW autoclave(Zealway Instrument Inc.(Xiamen), China); 88880018 vortex oscillator(Thermo Fisher Scientific Corporation, USA); SW-CJ-2F vertical flow clean bench(Suzhou Antai AirTech Co., Ltd., China).2.3 Methods
2.3.1
Measurement of the aerobic plate count.We prepared and measured milk samples in accordance with with the national food safety standardFood
Microbiological
Examination
:Aerobic
Plate
Count
(GB 4789.2-2016): aseptically measured 25 mL milk sample and put into a sterile homogenization bag, added 225 mL sterile normal saline, and slapped with a slap type homogenizer for 1 min to prepare a 1∶10 uniform sample solution; selected 2 to 3 serial dilutions according to the estimation of the contamination degree of the sample.For each dilution, pipetted 1 mL of the diluted sample solution to inoculate 2 plates, with sterile saline as a blank control.Poured 15-20 mL of PCA medium at about 46 ℃ into each plate.After the medium was solidified, turned the plate over and placed at(36±1)℃ for(48±2)h, calculated the aerobic plate count on each plate and reported the result.2.3.2
Evaluation of uncertainty.In accordance with the specifications ofEvaluation
and
Expression
of
Uncertainty
in
Measurement
(JJF 1059.1-2012), we analyzed and evaluated the uncertainty components generated during the determination process, and finally evaluated the uncertainty of the aerobic plate count.3 Results and analysis
3.1 Establishment of the mathematical model
Based on the calculation formula of the aerobic plate count in the national food safety standardFood
Microbiological
Examination
:Aerobic
Plate
Count
(GB 4789.2-2016), we established the following mathematical model, selected plates with 30-300 CFU colonies for counting, and determined the aerobic plate count, as shown in formula(1).Y
=nN/V
(1)
whereY
denotes the aerobic plate count in a single determination/(CFU/mL),n
is the dilution factor of the sample,N
is the number of colonies in 1 mL sample diluent(CFU),V
is the volume of the sample diluent(mL).3.2 Analysis of uncertainty sources
Although the steps of the determination of the aerobic plate count are not complicated, many factors may influence the results of the determination of the aerobic plate count, such as the accuracy of sample weighing, culture conditions, homogenization time, sample uniformity, multiple dilution, colony count, and repeated measurement,etc
.In this study, we mainly analyzed and evaluated the uncertainty from sample weighing, dilution process, sample volume, and repeated measurement.This experiment was performed by the same experimenter in strict accordance with the national standard method to complete the detection of a single milk sample, therefore the influence of factors such as homogenization, culture and environment on the uncertainty of the aerobic plate count was unified into the repeatability determination for analysis and evaluation.3.3 Evaluation of uncertainty components
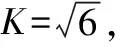
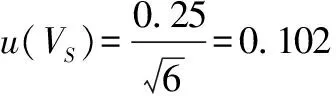
(2)
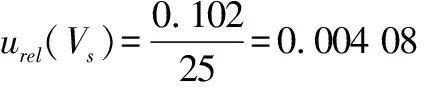
(3)
whereu
(V
)denotes the standard uncertainty introduced when weighing the 25 mL milk sample, andu
(V
)is the corresponding relative standard uncertainty.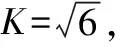
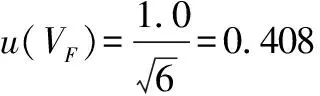
(4)
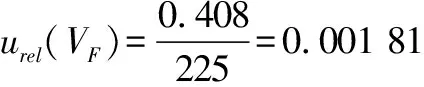
(5)
whereu
(V
)denotes the standard uncertainty introduced when weighing 225 mL of sterile normal saline using a 250 mL graduated cylinder to prepare a 1∶10 dilution sample solution, andu
(V
)is the corresponding relative uncertainty.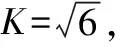
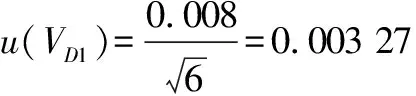
(6)
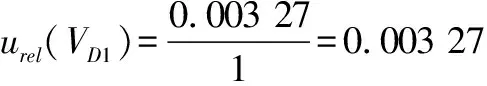
(7)
We calculated the standard uncertaintyu
(V
10)and the corresponding relative standard uncertainty urel(V
10)introduced by the 10 mL graduated pipette(Grade A)during the stepwise dilution of the sample according to formula(8)and formula(9).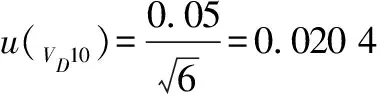
(8)
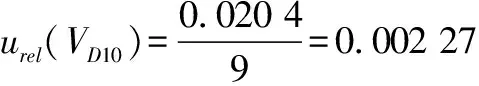
(9)
From the results of colony count, it can be seen that the aerobic plate count on the 1∶1 000 dilution plate of the milk sample did not meet the count range, while the aerobic plate count on the 1∶10 and 1∶100 dilution plate met the count range and could be used for calculation of the aerobic plate count.We calculated the relative standard uncertaintyu
(N
)introduced during the stepwise dilution of the milk sample at 1∶10 times and 1∶100 times according to formula(10).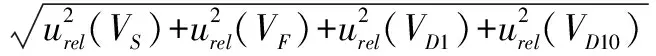
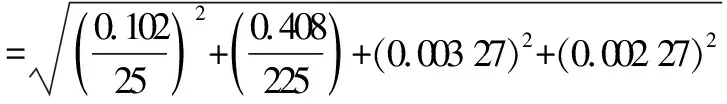
=0.005 98
(10)
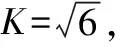

(11)
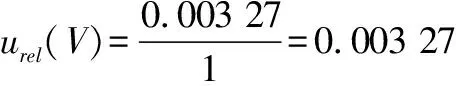
(12)
whereu
(V
)denotes the standard uncertainty introduced by the 1 mL graduated pipette(Grade A)during the stepwise dilution of the sample, andu
(V
)is the corresponding relative standard uncertainty.3.3.5
Relative standard uncertainty for determination of the aerobic plate count.From the relative standard uncertaintyu
(N
)introduced by the stepwise dilution obtained above and the relative standard uncertaintyu
(V
)of the sample volume , we obtained the relative standard uncertaintyu
(Y
)for the measurement of the aerobic plate count, which can be calculated according to formula(13).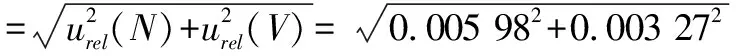
=0.006 82
(13)
whereu
(Y
)denotes the relative standard uncertainty of the determination of the aerobic plate count obtained from the analysis and evaluation of the sample weighing, sample preparation process, dilution process, and sample volume process.3.3.6
Standard uncertainty for the sample repeated determination.We measured the aerobic plate count in the milk sample by 6 times, calculated the aerobic plate count on each dilution plate, took the logarithm of the measured value of the aerobic plate count, and calculated the average and standard deviation.According to the type A evaluation method of measurement uncertainty, we made a statistical analysis and evaluation, and obtained the repeated measurement of the standard uncertainty.The results are shown in Table 1.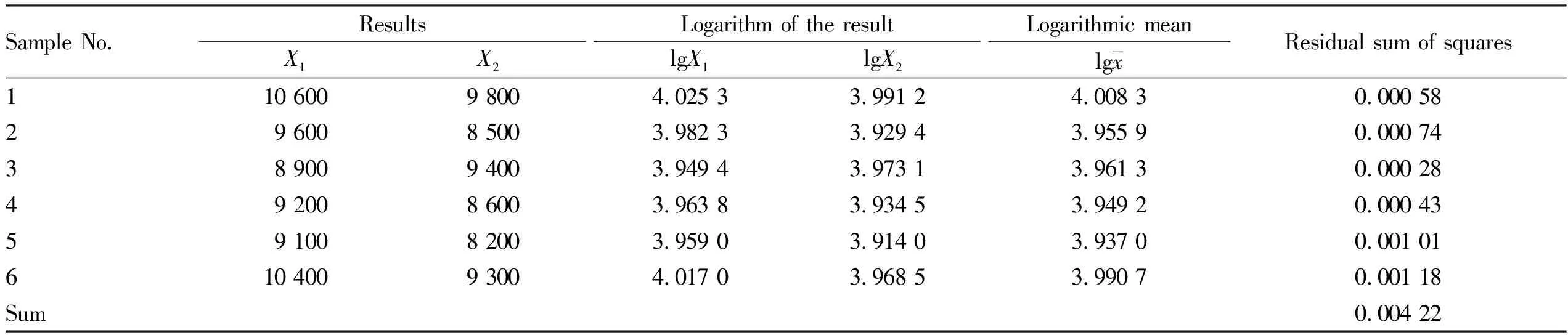
Table 1 Results of aerobic plate count
Based on the data in Table 1, we used the Bessel formula to calculate the standard deviation of the aerobic plate count in milk samples according to formula(14).

(14)
whereS
is the standard deviation of the measured sample,a
denotes the logarithmic value of the counting result,a
is the logarithmic mean of the counting result,n
is the number of parallel samples.In this experiment, the number of parallel detection was 2 times, so when calculated according to the following formula, we obtained the standard uncertaintyu
(R
)introduced by repeated measurement of the samples, using the formula(15).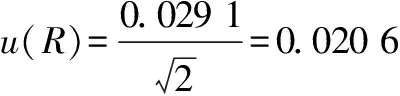
(15)
3.4 Combined standard uncertainty
Comprehensively considering calculation results of the above uncertainty components, we could obtain the combined standard uncertaintyu
(Y
)for the determination of the aerobic plate count in the milk samples according to formula(16).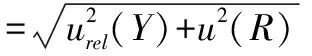
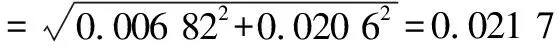
(16)
3.5 Expanded standard uncertainty
In accordance with the requirements ofEvaluation
and
Expression
of
Uncertainty
in
Measurement
(JJF 1059.1-2012), when the inclusion probabilityp
=95% andK
=2, we could calculate the expanded uncertaintyU
according to formula(17).U
=K
×u
(Y
)=2×0.
021 7=0.
043 4(17)
3.6 Report of results
The logarithmic mean of the determination result of the aerobic plate count was 3.967.Whenp
=95%,K
=2, the expanded uncertaintyU
of the detection result of the aerobic plate count in the milk samples was 0.043 4, and the logarithmic value interval of the results was(3.967 0-0.043 4, 3.967 0+0.043 4).Taking the antilog of this interval value, we could get the aerobic plate count in the milk samples: 8 395-10 233 CFU/mL.4 Discussion
4.1 Type of uncertainty
The uncertainty in daily testing and analysis laboratories are divided into two types.(i)Type A uncertainty evaluation.This type is using statistical analysis method to evaluate the uncertainty.It is the measurement uncertainty obtained by repeating the measurement several times, then calculating the average value and standard deviation.Type A evaluation is carried out on the basis of repeatability measurement, taking account of the role of each influence, so it is more realistic, objective and convincing.(ii)Type B uncertainty evaluation.In actual measurement, it is impossible or unnecessary to perform multiple repeated measurements.The uncertainty can only be evaluated by non-statistical analysis methods, called Type B evaluation.It is approximated based on experimental information, relevant experience or other information.Generally, it is necessary to first estimate the range of possible values to be measured, assume the probability distribution of the measured value, and then calculate the uncertainty based on the possible distribution of this range.4.2 Sources of uncertainty
Sources of the uncertainty of microbial determination results are very complicated.There are many influencing factors.It is necessary to focus on the consideration and analysis of the process and experimental environment of the detection experiment, such as the sample to be tested, the equipment used, the experimental environment, and the operation personnel and testing methods,etc.
It is necessary to determine the main and key sources of uncertainty after analysis one by one, and then calculate and evaluate these main uncertainty components.4.3 Evaluation of uncertainty
In the process of evaluating the uncertainty of the measurement results, it is necessary to first determine the measured and measurement method, and then establish a mathematical model to analyze the functional relationship between the measured and each input; consider and analyze the sources of uncertainty in accordance with the various factors described in Section3
.2
; evaluate the uncertainty of type A and type B in accordance with Section3
.1
; form the combined standard uncertainty by calculating each uncertainty component; then calculate the expanded uncertainty based on the measured probability distribution, confidence level and coverage factor; finally report the uncertainty of the measurement result.Now, it completes the evaluation of the uncertainty of the measurement result.5 Conclusions
In the process of determination of the aerobic plate count and data statistics, it is first required to be as comprehensive as possible and fully consider the impact of various factors on the determination results.It is necessary to focus on the analysis of the uncertainty introduced by factors such as weighing, sample addition, dilution, counting, and repeated determination, and calculate the uncertainty introduced by each uncertainty component based on the mathematical model.Besides, the data of the aerobic plate count detection result is divergent and does not conform to the normal distribution, it is not suitable for direct calculation of its standard deviation, thus it is necessary to take the logarithm before performing statistical analysis of the data.It is feasible to use the Bessel formula to calculate the standard uncertainty, and finally form the combined uncertainty of the aerobic plate count, in order to further reduce the errors introduced by various factors, and make the detection result closer to the true value.Using the uncertainty analysis and evaluation method can effectively ensure the accuracy, reliability, objectivity and scientificity of the detection data, and it is expected to provide strong support for the actual detection work of the microbiology laboratory.
杂志排行
Asian Agricultural Research的其它文章
- Realization Path for Inclusive Finance to Support Rural Revitalization in Poverty-stricken Areas
- Competitiveness and Complementarity of Cocoon Silk Trade between China and Southeast Asia
- Analysis of Topographic Heterogeneity in the Sanjiangyuan
- New Insights of Neuromedin B and Its Receptor NMBR Involvement in Immunity
- Analysis of Factors Influencing Residents’ Satisfaction with Pairing Aid Policy in Xinjiang: Based on the Data from Three Prefectures in Southern Xinjiang
- A Study on the "Quality" and "Quantity" of Financial Ecology in Henan Province
