Electric Field:A Key Signal in Wound Healing
2021-08-04NaixinJIAJinruiYANGJieLIUJiapingZHANG
Naixin JIA ,Jinrui YANG ,Jie LIU ,Jiaping ZHANG,*
1 Department of Plastic and Aesthetic Surgery,State Key Laboratory of Trauma,Burns and Combined Injury,Southwest Hospital,The Third Military Medical University (Army Medical University),Chongqing 400038,China
# These authors contributed equally to this study and share first authorship.
KEY WORDS
INTRODUCTION
After an injury,the movement of electric charges is key in beginning wound repair[1].The movement of electric charges leads to the generation of a naturally occurring endogenous electric field (EF) and electric current,which were discovered over 150 years ago.Modern technologies,such as vibrating probes,have been used to further confirm the field’s spatial and temporal characterizations[2].Electrical signals modulate cell behavior within the range of 500 μm to 1 mm from the edge of the wound,which may last for several days until the epithelium completely covers the wound.Increasing evidence has demonstrated that naturally occurring endogenous EFs guide the orientation and migration of fibroblasts,keratinocytes,and macrophages and may also interfere with inflammation,angiogenesis,and tissue remodeling.In addition to human skin wounds,EFs are also measured in other types of wounds,such as stumps of amputated newt limbs and amputated frog tadpole tail wounds and are proposed to be essential for regeneration[3-6].Many important molecules have been identified to mediate the cellular response to EFs,which have been covered in recent reviews[7-10].In this review,we focus on the role of EFs in wound healing and their current therapeutic applications through conductive electrical stimulation (ES) equipment.
TRANSEPITHELIAL ELECTRICAL POTENTIAL
The epidermis generates a voltage across the body,called the transepithelial potential (TEP) or“skin battery”.In humans,the average voltage gradient between the stratum corneum (the outermost layer of the skin) related to the basal side ranges from 10 mV to 60 mV depending on the region measured[11-12].The TEP is generated by actively pumping Na+to the basal side and Cl-out apically (Fig. 1)[13].Epithelial cells are linked by tight junctions and constitute the main electrical resistive barrier.Thus,directional ion transport coupled with the barrier establishes the TEP[14].Similar TEPs exist in other epithelial tissues such as the cornea,gastrointestinal duct,urinary duct,and respiratory duct.In the corneal epithelium,the TEP is maintained at approximately 40 mV by ion pumping in an asymmetric manner[15].TEP is the basis for the generation of endogenous EFs after wounding.

Fig.1 TEP generated by actively pumping Na+ from its apical to its basal side and Cl- from its basal to apical side (adapted with permission [13].Copyright 2003,Elsevier Ltd.)
WOUND ENDOGENOUS ELECTRIC FIELD
Generation of Endogenous EF
After wounding,the epithelial barrier is destroyed,and the TEP is short-circuited.The potential at the wound site thus becomes negative in relation to the potential underneath the unwounded epidermis.This potential gradient drives the positive charge flow toward the wound,thus forming laterally orientated EFs (Fig.2)[16].The wound EFs exist and continue driving the electric currents until the wound is healed,and the barrier is restored.Wound EFs are mainly distributed near the wound edge.In guinea pig skin wounds,the lateral EFs fall from 140 mV/ mm at the wound edge to 0 mV/mm just 3 mm lateral to the wound edge.Similar endogenous EFs have been detected in human skin wounds.Significantly,compared with acute wounds,chronic wounds possess a weaker EF,which may be implicated in delayed healing[17].
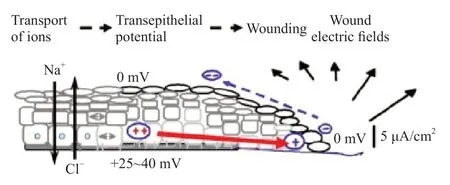
Fig.2 Schematic diagram illustrating the generation of endogenous EF (adapted with permission [15].Copyright 2009,Elsevier Ltd.)
The diagram in Fig.3A depicts the pattern of the endogenous EFs (red arrows) and the electric current closed loop (black loop with arrows) in a skin wound.The EF intensity was approximately 0 V/m at the wound center and gradually increased away from the wound center,reaching a maximum of 100 V/m at the wound edges (Fig.3B).In the wounded region,these long-lasting,large electric currents and EF are shown to be active responses,which are indispensable for wound healing[18].
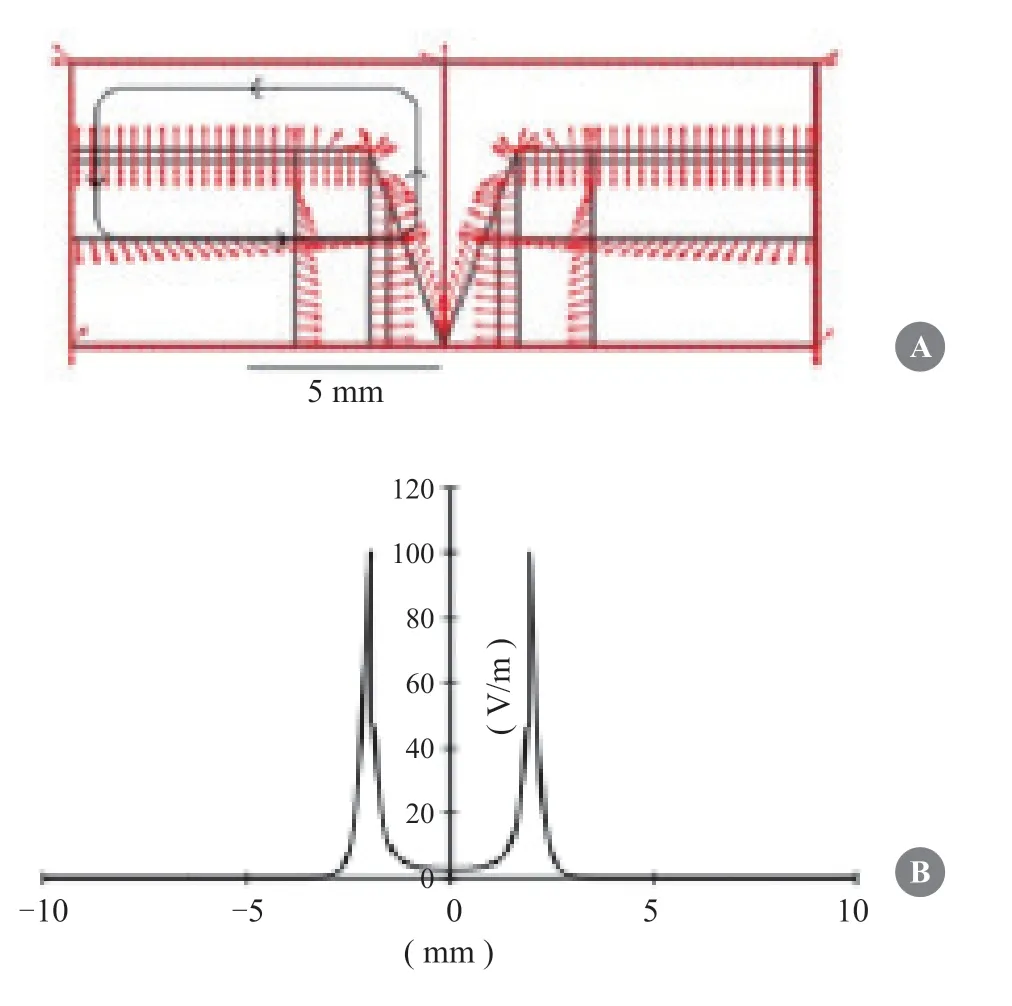
Fig.3 Distribution of EFs and currents at wound surface.(A) Direction of EFs and currents in a wound.(B) The EF intensity changes with distance from the wound edges.The“0”point means the center of wound.Adapted with permission [18].Copyright 2017,Hindawi Ltd.
Ion Flux
EFs and electric currents are generated by the flow of charged ions that exist in the cell cytoplasm and intercellular substances.After wounding,an increase in ion flux promotes an increase in electric currents[15].Previous studies have confirmed that the primary components required for generating electric currents are sodium (Na+) and chloride (Cl-).Other ions such as potassium (K+) and calcium (Ca2+) may also be involved in this process.Using an ion-selective microelectrode,Vieira et al.[19]have shown a remarkable sodium influx in mouse corneal wounds,reaching a maximum within 5 min after wounding and then remaining at a high equal level.Moreover,chloride influx was observed to have the largest negative ion flux in rat corneal wounds.In contrast to sodium and chloride,the effluxes of calcium and potassium were small,which increased sharply and then dropped quickly to a low,stable level[20].
ROLE OF ELECTRIC FIELDS IN WOUND HEALING
Skin wound healing is regarded as a conservative evolutionary process that includes four consecutive phases:hemostasis,inflammation,proliferation,and remodeling[21-22].Many studies have demonstrated that EFs play a role in wound healing by directing and accelerating cell migration,angiogenesis,and nerve growth[23-27].In addition,ES has been shown to increase blood circulation and protein synthesis as well as exhibit bactericidal effects[28].
Hemostasis Phase
The first phase of wound healing is hemostasis (0 h after injury).While few studies have examined the effects of EFs on this process,EFs have been shown to promote the production of platelet-derived microparticles and the activation of platelet surface procoagulant markers[29].After platelet degranulation,complement cascades are activated,which in turn provokes inflammatory cells to eliminate bacteria[30].
Inflammation Phase
The second phase of wound healing is inflammation (1-3 days after injury).This process involves immune cells such as neutrophils and macrophages,which serve as first-line defenses[30].Within hours after injury,neutrophils and macrophages are recruited to the wound area.They are induced by chemokines such as intercellular adhesion molecule 1,vascular cell adhesion molecule 1,and E-selectin and then migrate toward the wound site to execute phagocytosis to remove biological invasions and foreign debris[31].
Although biochemical factors play a major role in this phase,EF may also be an important participant.It has been shown that endogenous EFs strengthen cell phagocytosis and autolysis by increasing the electrical chemotaxis of neutrophils and macrophages in an EF strength-dependent manner[32].More importantly,EFs can significantly enhance the ability of macrophages to uptake targets and the production of cytokines such as TNF-α,NT3,IL-1,and IL-23[33-34].In addition,clinical evidence has shown that EF therapy significantly reduces pain and inflammatory biomarkers at the surgical incision site,with self-acceptance improved as a consequence[35-36].These results indicate that EFs participate in the inflammatory phase during wound healing.
Proliferative Phase
The third phase of wound healing is proliferation (4-21 days after injury),which is characterized by angiogenesis,granulation regeneration,and re-epithelialization.Angiogenesis and granulation regeneration are the basis of reepithelialization,which begins through the migration of keratinocytes at the edge of the wound[37].Many types of cells respond to EFs via directional cell migration,a phenomenon called galvanotaxis or electrotaxis.In an applied EF,keratinocytes migrate directionally toward the cathode in the same direction as the endogenous wound EFs.More importantly,studies have indicated that EFs might be an overriding guidance cue that directs cell migration during wound healing.Using anin vitromodel of monolayer wound healing,Zhao et al.[2]found that applied EFs in physiological strength could completely override other co-existing guidance cues and direct cells to migrate into or away from the wound.In their study,when EF was applied with polarity in the default healing direction,cells at the wound edge migrating into the wound were significantly increased;however,when the EF was applied with the field vector against the default healing direction,a field as low as 25 mV/ mm could stop the cells migrating into the wound,and a field above 25 mV/mm had clear and significant overriding effects in guiding cells to migrate away from the wound.Similar results were also observed in the EFguided migration of stratified epithelium[18].This study suggests that the role of EFs in wound healing may be far more important than expected because cell migration into wounds is an essential part of all wound healing in mammals.Interestingly,we found that the electrotactic migration of keratinocytes could be stimulated in the presence of tissue hypoxia,suggesting a synergistic effect of the wound microenvironment on keratinocyte migration[38].Hypoxia usually occurs immediately after an injury to the skin,representing an initial stimulus that triggers the wound healing process.Considering that oxygen tension in thein vivowound microenvironment changes spatially and temporally,the synergistic effects of wound hypoxia with endogenous EFs on keratinocyte migration would be more complexin vivothan that observedin vitro.
EFs also have significant effects on vascular endothelial cells and fibroblasts[24,26].Wang et al.[24]found that EFs enhanced the proliferation and migration of human umbilical vein endothelial cells to accelerate angiogenesis by activating PI3K/Akt and ERK1/2 signaling.Guo et al.[26]demonstrated the electrotactic migration of dermal fibroblasts in an applied EF.However,endothelial cells and fibroblasts are induced by chemical kinetics rather than electrical chemotaxis[39-40].Therefore,the effect of EFs seems to be more significant on keratinocytes than on other cells because the impairment of epidermal migration is known to be the main reason causing healing delay,and endogenous EFs are likely disrupted in chronic wounds[2,41-42].
Remodeling Phase
The fourth stage of wound healing is remodeling (occurring approximately 21 days after injury),which may extend to a year or more.In this process,fibroblasts regulate wound matrix breakdown by matrix metalloproteinases and synthesis of a new extracellular matrix.This slow process increased the tensile strength of the wound.EFs have been shown to guide the migration of myofibroblasts to accelerate wound remodeling[40]and may reduce scar formation by inhibiting TGF-β/Smad signaling[39].Our previous study demonstrated that biphasic pulse direct current electric fields (DCEFs) induced fibroblast alignment and elicited a directional contraction of the gels in a model of fibroblast-populated collagen lattice[43].Furthermore,we found that EF-induced fibroblast alignment was mediated by RhoA-regulated F-actin redistribution[43].This study provides a possible approach to scar contracture,as the size and duration of applied biphasic pulse DCEFs can be easily controlled.
WOUND HEALING PROMOTED BY ELECTRIC THERAPY
Direct Current
The direct current is similar to the endogenous current.Low-intensity direct current (LIDC) can avoid damaging healthy tissues during skin repair[44-48].In a study by Gault et al.,76 patients underwent continuous LIDC,6 of whom had bilateral ulcers;therefore,one side of ulcers was set as a control.They received 200-800 μA LIDC three times per day for 2 h at the wound site.Among the 6 patients,the healing rate of current-applied ulcers was 30%,while that of the control was 14.7%[49].
Pulsed Current
Pulsed current (PC) is the unidirectional or bidirectional flow of charged particles that periodically stops for a limited period of time before the next event[43].The PC is characterized by waveform,amplitude,duration,and frequency.PC has two types of waveforms:monophasic and biphasic.Monophasic PC is usually described as a low-voltage pulsed current a high-voltage pulsed current (HVPC),and the treatment electrodes are usually placed on the damaged skin surface.Biphasic PC is bidirectional,and its waveform is described as asymmetric or symmetric,with both electrodes usually placed on the wound edge[50].PC is widely used in clinical trials.Peter et al.[51]conducted a randomized controlled trial in which 40 patients with diabetic foot ulcers randomly received HPVC (20 min per hour for 8 h every day) or sham therapy for 12 weeks.The healing rate of the treatment group was 65% compared to 35% for the sham group,and the patients treated three times per week had the best healing effect.We found that pulsed EF (EF=150 mV/mm;duty cycle=60%;frequency=0.1 Hz) can trigger electrotaxis in keratinocytes,comparable to that induced by constant DCEF,with fewer electrochemical side effects[52].Therefore,PC may be particularly suitable as a means of electrotherapy in patients,while minimizing side effects.
Bioelectric Dressing
Bioelectric dressing is an effective method for delivering ES to wounds.Kim et al.[53]demonstrated the antibacterial efficacy of bioelectric dressings,including zinc and silver,against diverse wound pathogens.In this study,they inferred that wound pathogens were effectively killed by ESin vitro.The wound pathogens included extendedspectrum β-lactamase bacteria,multidrug-resistant bacteria,and methicillin-resistantStaphylococcus aureus.
Directional Electric Field
There are substantial differences between the currently used ES and endogenous EFs.ES usually has no polarity in the current,whereas the current in endogenous EFs flows from the edge to the center of the wounds.As a result,endogenous EFs can guide epidermal cell migration into wounds directionally.Theoretically,wound healing would be accelerated if exogenous EF is applied in a direction similar to that of endogenous EFs.However,exogenously applying a sustained EF to the wound surface is difficult.We have successfully established a model that could be used for safe and stable application of exogenous EF on the wound surface in which the positive electrode is placed on the wound edge,while the negative electrode is placed on the wound center[54].This model has broken several technical bottlenecks:Ⅰ) the electrode is made up of carbon fiber that has no obvious side effects such as electrothermal effects and electrolysis;Ⅱ) electrodes are tightly fixed on wounds by vacuum sealing,which makes a stable and continuous application of the exogenous EF to the wound;Ⅲ) vacuum sealing,at the same time,produces an excellent wound microenvironment that is essential for keratinocyte proliferation,migration,and re-epithelialization by drainage and infection control (Fig.4).In this model,we found that an exogenous EF of 100 mV/mm applied in the same direction as the endogenous EF (+100 mV/mm) significantly accelerated wound healing.At day 7,the length of newly formed epidermis in the +100 mV/mm-applied wounds was 1.57 longer than that of the control.Microscopically,the newly formed epidermis migrated in a regular,uniform,and organized pattern.These findings offer important insights into the roles of directional EFs in wound healing and provide theoretical and empirical foundations for the clinical application of EFs.
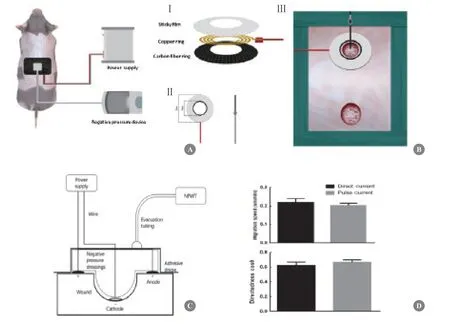
Fig.4 Application of exogenous EFs on wound surface.(A) Schematic diagram of the exogenous EFs application on pig skin wound surface with vacuum sealing.(B) Carbon fiber was used to form ring (positive) and needle (negative) electrodes (Ⅰ and Ⅱ).The ring electrode was placed on wound circumference,while the needle electrode was placed vertically at the wound center (Ⅲ).(C) Entire device was depicted by conceptual diagram of longitudinal section.(D) It was confirmed by cellular experiments that pulsed current with 70% duty ratio and direct current had the similar effects on guiding keratinocytes migration.Adapted with permission [54].Copyright 2020,Elsevier Ltd.
CONCLUSIONS
Naturally occurring endogenous EFs are an intrinsic property of wounds that are known to serve a crucial role in wound healing and regeneration.While different ES or EF devices have been used in clinical trials,the mechanisms by which the electric signals improve healing have not been well clarified,making it difficult to substantiate using any specific form of ES or EF and optimize electric therapy.This may be the reason the clinical application of EFs for wounds has been at the trial stage for over half of a century and that results are still very discrepant.Another fundamental question that must be answered is how cells sense electric signals.Although a variety of asymmetrically distributed membrane receptors,such as epidermal growth factor receptors,concanavalin A receptors,acetylcholine receptors,and integrins,have been demonstrated to be involved in the electrotactic response[55-60],the role of specific sensor molecules remains to be elucidated.Therefore,a thorough understanding of the responding or sensing mechanisms will enable clinicians and engineers to apply a better or wider degree of EF modulation for rebuilding tissues and organsin vivoandin vitro.
FUNDING
This work was supported by the National Nature Science Foundation of China (NSFC no.81873936).
ETHICS DECLARATIONS
Ethics Approval and Consent to Participate
N/A
Consent for Publication
All the authors have consented to the publication of this article.
Competing Interests
The authors declare that they have no competing interests.The authors state that the views expressed in the article are their own and not the official position of the institution or funder.
AUTHORS’ CONTRIBUTIONS
NX J,J L,and JR Y wrote the manuscript.JP Z critically reviewed the manuscript.All authors have read and approved the final manuscript.
杂志排行
Chinese Journal of Plastic and Reconstructive Surgery的其它文章
- The Practice of China’s Cosmetic Medicine Dated Back to 3 800-4 800 Years Ago
- Progress in Implant-Based Breast Reconstruction:What Do We Know?
- Mechanisms and Management of Postparalysis Facial Synkinesis
- Looped,Broad,and Deep Buried Suturing Technique for Wound Closure
- A Novel Composite Skin Graft Technique with Fat Derivatives
- A Case of Congenital Syringocystadenoma Papilliferum
