Therapeutic potential of thymoquinone in combination therapy against cancer and cancer stem cells
2021-08-02ZaynabFatfatMaamounFatfatHalaGaliMuhtasib
Zaynab Fatfat, Maamoun Fatfat, Hala Gali-Muhtasib
Zaynab Fatfat, Maamoun Fatfat, Hala Gali-Muhtasib, Department of Biology, American University of Beirut, Beirut 1107 2020, Lebanon
Hala Gali-Muhtasib, Center for Drug Discovery, American University of Beirut, Beirut 1107 2020, Lebanon
Abstract The long-term success of standard anticancer monotherapeutic strategies has been hampered by intolerable side effects, resistance to treatment and cancer relapse.These monotherapeutic strategies shrink the tumor bulk but do not effectively eliminate the population of self-renewing cancer stem cells (CSCs) that are normally present within the tumor. These surviving CSCs develop mechanisms of resistance to treatment and refuel the tumor, thus causing cancer relapse. To ensure durable tumor control, research has moved away from adopting the monotreatment paradigm towards developing and using combination therapy.Combining different therapeutic modalities has demonstrated significant therapeutic outcomes by strengthening the anti-tumor potential of monotreatment against cancer and cancer stem cells, mitigating their toxic adverse effects, and ultimately overcoming resistance. Recently, there has been growing interest in combining natural products from different sources or with clinically used chemotherapeutics to further improve treatment efficacy and tolerability. Thymoquinone (TQ), the main bioactive constituent of Nigella sativa, has gained great attention in combination therapy research after demonstrating its low toxicity to normal cells and remarkable anticancer efficacy in extensive preclinical studies in addition to its ability to target chemoresistant CSCs. Here, we provide an overview of the therapeutic responses resulting from combining TQ with conventional therapeutic agents such as alkylating agents, antimetabolites and antimicrotubules as well as with topoisomerase inhibitors and non-coding RNA. We also review data on anticancer effects of TQ when combined with ionizing radiation and several natural products such as vitamin D3, melatonin and other compounds derived from Chinese medicinal plants. The focus of this review is on two outcomes of TQ combination therapy, namely eradicating CSCs and treating various types of cancers. In conclusion, the ability of TQ to potentiate the anticancer activity of many chemotherapeutic agents and sensitize cancer cells to radiotherapy makes it a promising molecule that could be used in combination therapy to overcome resistance to standard chemotherapeutic agents and reduce their associated toxicities.
Key Words: Thymoquinone; Combination therapy; Cancer cells; Cancer stem cells; Conventional cancer therapy; Natural products
INTRODUCTION
Cancer incidence and mortality are still growing worldwide despite the monumental efforts and the significant progress made in developing therapeutic strategies and improving detection techniques for combatting this disease. Around 19 million new cases and nearly 10 million deaths are estimated globally in 2020[1]. The conventional therapeutic strategies used to treat cancer are surgery, radiotherapy and chemotherapy, in addition to targeted and hormonal therapy. The effectiveness of these approaches has been found to be limited when used in monotherapy strategies due to cancer resistance, tumor relapse and treatment-induced toxicities[2-6]. Ample evidence has demonstrated that the intratumoral heterogeneity is a prominent contributor to cancer resistance to monotherapy and tumor recurrence[7]. The tumor consists of a heterogeneous population of cells that show distinct genetic, epigenetic, and phenotypic features in addition to different sensitivity to the standard therapeutic modalities[8-11]. A growing body of literature has supported the role of cancer stem cells(CSCs) in generating this intratumoral heterogeneity. These CSCs are characterized by their ability to self- renew and to differentiate into various lineages of cancer cells composing the tumor. They are also resistant to the widely used therapeutics measures[12].
Over the last few decades, there has been increased interest in combining cancer treatments rather than using single therapeutic agents. A monotherapeutic strategy having one mode of action eradicates only one subpopulation of tumor cells. Other subpopulations which are less sensitive can escape the treatment and reform a resistant tumor, thus resulting in cancer relapse and treatment failure. In contrast,combined therapeutic agents act simultaneously on multiple targets and eradicate several subpopulations of tumor cells. This results in improving their therapeutic efficacy, limiting their toxicity by lowering the effective therapeutic dose of each agent,preventing the development of resistance and consequently ensuring an effective eradication of the complex heterogeneous nature of the tumor[13]. Bioactive natural products are attracting considerable attention in cancer therapy because they are less toxic and more available and cost effective when compared to synthetic monotargeted drugs[14]. Natural therapeutics have been found to exert effective antineoplastic activity and to potentiate the anticancer effect of conventional therapeutics against CSCs and cancer cells[15,16]. Around 38% of the anticancer drugs approved during the last 40 years are either natural productsper se, their derivatives or have a pharmacophore derived from a natural product[17].
Thymoquinone (TQ), the major bioactive compound extracted fromNigella sativaessential oil, has shown promising antitumor activityin vitroandin vivoagainst a wide range of cancer types[18]. What makes TQ an attractive therapeutic agent is its safe profile. It was found to be non-toxic to several normal cells including normal mouse kidney cells[19], normal human lung fibroblasts[20] and normal human intestinal cells[21]. TQ exerts its antineoplastic effects through several modes of action, and its exact molecular target is not known yet. It inhibits cancer cell proliferation and blocks the cell cycle progression. In addition, TQ induces apoptosis by generating reactive oxygen species (ROS), causing DNA damage, upregulating pro-apoptotic factors,activating caspases and causing poly (ADP-ribose) polymerases (PARP) cleavage,disrupting mitochondrial membrane integrity besides modulating several pathways such as p53, wingless/integrated (Wnt), mitogen-activated protein kinase, signal transducer and activator of transcription 3 (STAT3)[22]. It also interrupts metastasis by downregulating the epithelial to mesenchymal transition transcription factors twistrelated protein 1 (TWIST1) and E-Cadherin, and inhibits angiogenesis by suppressing the nuclear factor kappa B (NFkB) pathway[22]. Interestingly, TQ was found to inhibit the proliferation of several chemoresistant cancer cells and induce apoptosis in colon CSCs that are resistant to the conventional chemotherapeutic drug 5-fluorouracil (5-FU)[23,24].
These effective anticancer properties of TQ made it an interesting therapeutic candidate for combination therapy with standard therapeutic agents or other natural products to improve cancer treatment efficacy and safety (Figure 1). Here, we shed light on the combinatorial effects of TQ on the activity of these therapeutic agents used in treating CSCs and cancer cells.

Figure 1 Thymoquinone in combination therapy against different types of cancer. A: Thymoquinone in combination with conventional chemotherapeutic drugs; B: Thymoquinone in combination with natural products. TQ: Thymoquinone; CYC: Cyclophosphamide; TMZ: Temozolomide; CDDP: Cisplatin; BTZ:Bortezomib; 5-FU: 5-Fluorouracil; GCB: Gemcitabine; PAC: Paclitaxel; DTX: Docetaxel; CBZ: Cabazitaxel; TP: Topotecan; DOX: Doxorubicin; ZA: Zoledronic acid;TAM: Tamoxifen; As: Arsenic trioxide; IM: Imatinib; Vit D3: Vitamin D3; Mel: Melatonin; Res: Resveratrol; Pip: Piperine; Ams: Artemisinin; Art: Artesunic acid; Dio:Diosgenin; Gen: Genistein; I3M: Indirubin3monoxime; FA: Ferulic acid; Emo: Emodin; Sel: Selenium.
TQ EFFECTS AGAINST CANCER CELLS
TQ in combination with conventional chemotherapeutic agents
The mode of action of each chemotherapeutic agent as well as the cellular and molecular mechanisms of action of the combination treatment are presented in Table 1.
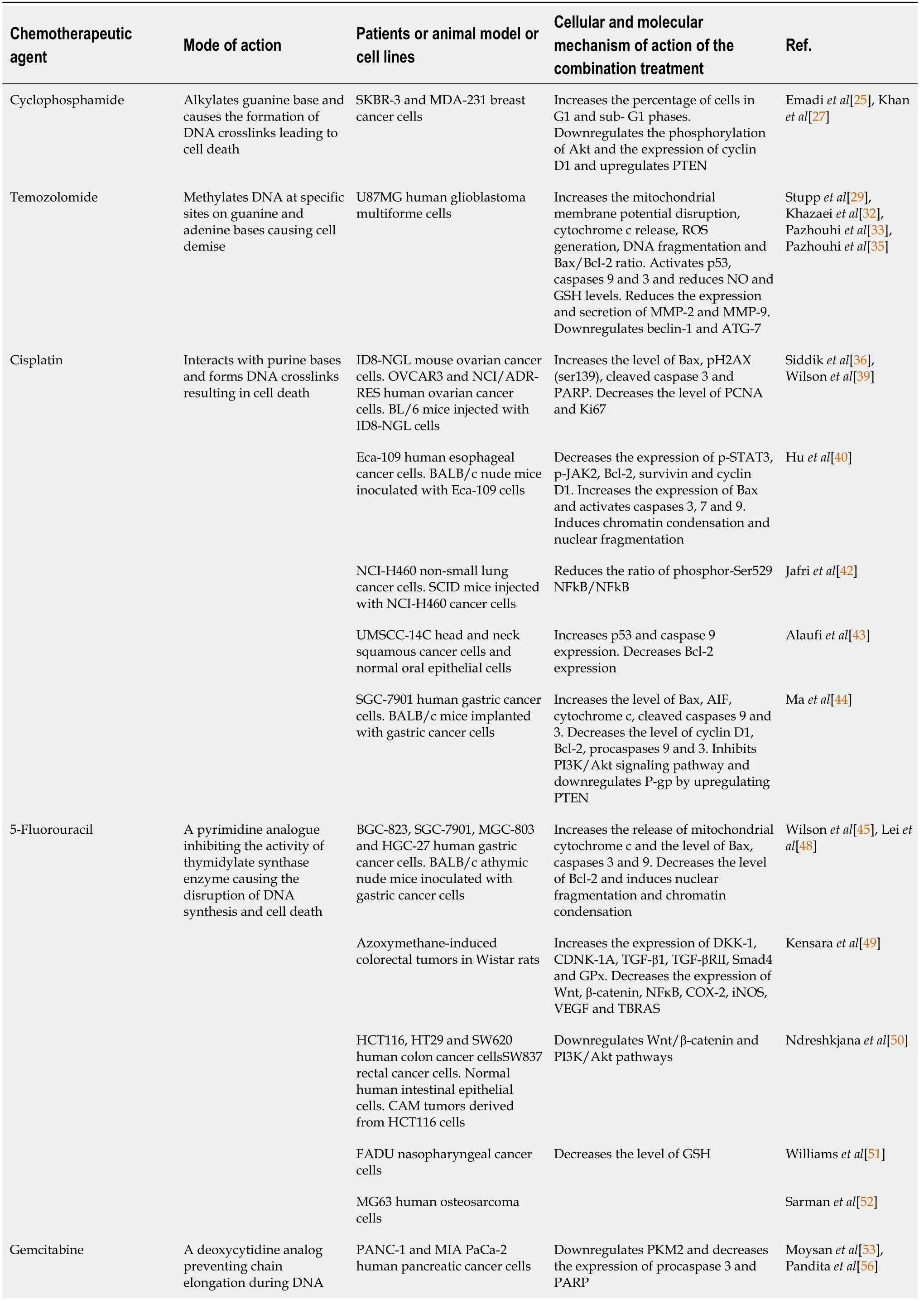
Table 1 Mode of action of the chemotherapeutics agents and cellular and molecular mechanism of action of the combination treatment in preclinical and clinical studies

causing cell death PANC-1, BxPC-3, and AsPC-1 human pancreatic cancer cell lines. BALB/c nude mice injected with PANC-1 cells Downregulates Notch1, NICD, Bcl-2,Bcl-xL and XIAP. Inactivates Akt/mTOR/S6 signaling pathway and decreases the phosphorylation and nuclear translocation of p65.Upregulates PTEN, caspases 3 and 9 and Bax and increases cytochrome c release Mu et al[57]MCF-7 and T47D human breast cancer cells Increases pre-G1 cell population Bashmail et al[58]Paclitaxel Inhibits microtubules disassembly and induces mitotic arrest 4T1 mouse breast cancer cells.Ehrlich tumor cells. Balb/c mice injected with Ehrlich tumor ascites cells Increases the level of full length and cleaved caspases 3, 7 and 12 and PARP. Reduces phosphorylated p65 and Akt1. Modulates genes involved in apoptosis, cytokine -cytokine receptor interaction, Fas signaling,p53 signaling and JAK/STAT signaling Ojima et al[59],Şakalar et al[63]MCF-7 and T47D human breast cancer cells Increases pre-G1 cell population.Increases the level of cleaved caspase 3 and PARP and the expression of beclin-1 and LC3-II Bashmail et al[64]MCF-7 human breast cancer cells Soni et al[65]Docetaxel Inhibits microtubules disassembly and induces mitotic arrest DU-145 human prostate cancer cells Blocks PI3K/Akt signaling pathway and induces DNA fragmentation Ojima et al[59],Dirican et al[69]DU-145 and C4-2B human prostate cancer cells Inhibits PI3K/Akt signaling pathway. Increases the expression of Bax, Bid, caspase 3 and PARP and decreases the expression of Bcl-xL Singh et al[70]MCF-7 and MDA-MB-231 human breast cancer cells Induces DNA damage, cells shrinkage, nuclear fragments,apoptotic bodies and cytoplasmic vacuolation Alkhatib et al[71]MCF-7 and MDA-MB-231 human breast cancer cells Zafar et al[72]MCF-7 and MDA-MB-231 human breast cancer cells.Balb/c mice healthy or injected with Ehrlich ascites carcinoma cells Induces nuclear fragmentation and restores the levels of oxidative stress parameters MDA, SOD and GSH.Prevents the alteration of blood cell count and serum biochemical parameters AST, ALT, creatinine and BUN Zafar et al[73]MCF-7 breast cancer cells Odeh et al[74]Cabazitaxel Inhibits microtubules disassembly and induces mitotic arrest MCF-7 and MDA-MB-231 human breast cancer cells Induces DNA fragmentation and increases the sub-G1 population Ojima et al[59],Kommineni et al[78]Doxorubicin Intercalates DNA, inhibits topoisomerase II, forms free radicals when reduced leading to cell cycle arrest and cell death Human HTLV-1 positive (HuT-102) and HTLV-1 negative(Jurkat). CD4+ malignant T-cell lines. NOD/SCID mice inoculated with HuT-102 tumor cells Increases the sub-G1 population and induces ROS production. Disrupts the mitochondrial membrane potential. Downregulates the expression of NFkΒ and Ki67 and increases the phosphorylation of p53 Meredith et al[79],Fatfat et al[83]HL-60 acute myeloid leukemia cells. Dox resistant HT-29 colon carcinoma cells. MCF-7/TOPO multi-drug resistant breast cancer cells Induces caspases 3 and 8 activity and ROS generation. Disrupts the mitochondrial membrane potential Effenberger-Neidnicht et al[84]BALB/c OlaHsd-foxn1 nude mice injected with MDA-MB-231 breast cancer cells Induces p38 MAPK phosphorylation and inhibit the expression of XIAP,survivin, Bcl-xL and Bcl-2 Woo et al[85]SMMC-7721 and HepG2 hepatocarcinoma cells and human normal liver cells HL-7702 Increases caspase 3 and PARP cleavage Jehan et al[86]
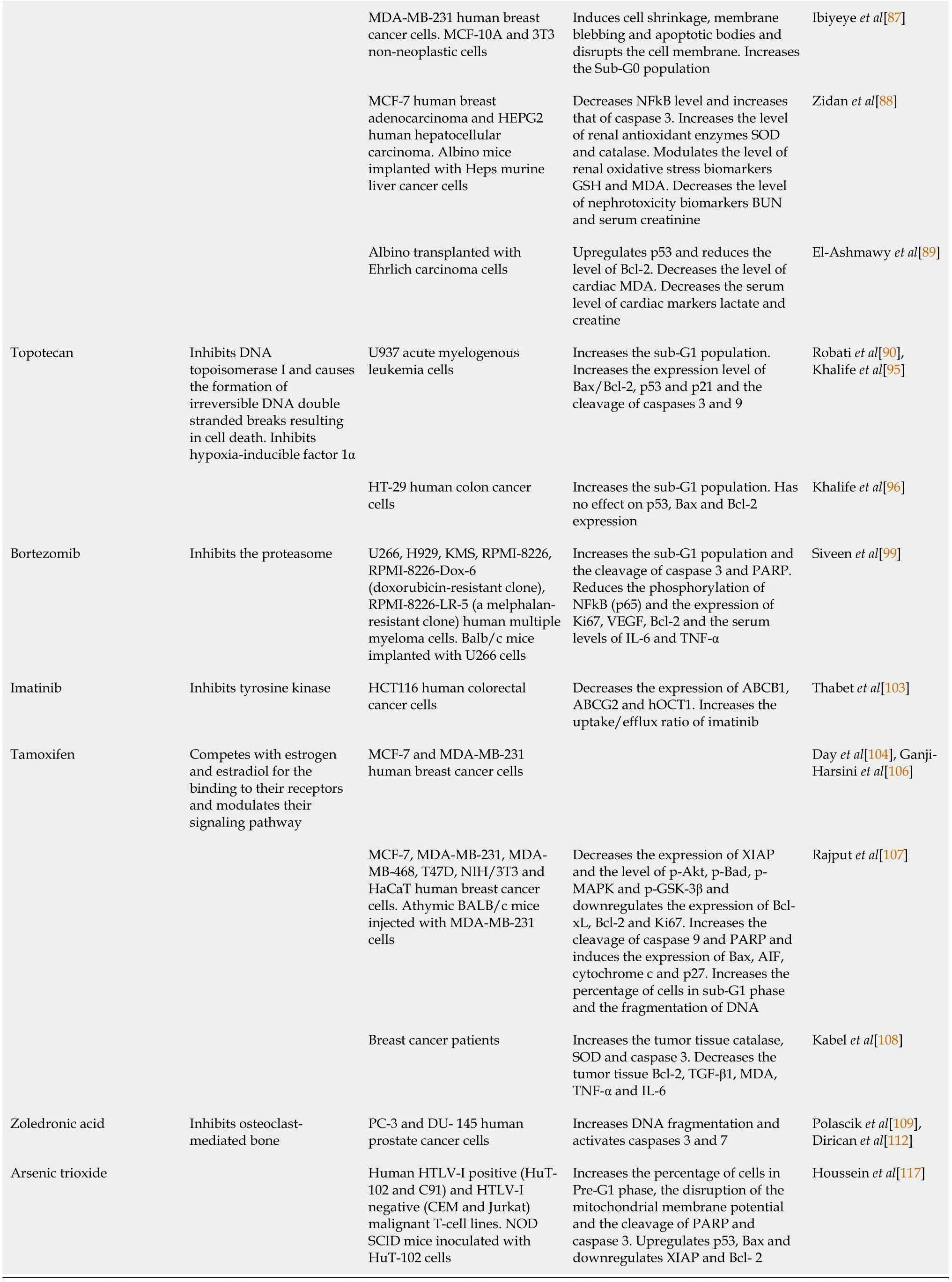
PTEN: Phosphatase and tensin homolog; ROS: Reactive oxygen species; Bax: Bcl-2-associated X protein; NO: Nitric oxide; GSH: Glutathione; MMP: Matrix metalloproteinase; ATG-7: Autophagy-related 7; pH2AX: Phospho-histone 2AX; PCNA: Proliferating cell nuclear antigen; JAK2: Janus kinase 2; STAT3:Signal transducer and activator of transcription 3; NFkB: Nuclear factor kappa B; PI3K: Phosphatidylinositol-3-kinase; AIF: Apoptosis inducing factor;PARP: Poly (ADP-ribose) polymerases; CAM: Chorioallantoic membrane; MAPK: Mitogen-activated protein kinase; COX-2: Cyclooxygenase 2; iNOS:Inducible nitric oxide synthase; VEGF: Vascular endothelial growth factor; TBRAS: Thiobarbituric acid reactive substances; DKK-1: Dickkopf-related protein-1; CDNK-1A: Cyclin-dependent kinase inhibitor 1A; TGF-β1: Tak transforming growth factor beta 1; TGF-βRII: Transforming growth factor, beta receptor II; GPx: Glutathione peroxidase; GSH: Glutathione; XIAP: X-linked inhibitor of apoptosis protein; mTOR: Mammalian target of rapamycin; PKM2:Pyruvate kinase M2; Bid: BH3 interacting-domain death agonist; AST: Aspartate transaminase; ALT: Alanine transaminase; MDA: Malondialdehyde; SOD:Superoxide dismutase; BUN: Blood urea nitrogen; IL-6: Interleukin 6; TNF-α: Tumor necrosis factor alpha; GSK-3β: Glycogen synthase kinase 3 beta;ABCB1A: ATP-binding cassette subfamily B member 1; ABCG2: ATP-binding cassette subfamily G member 2; hOCT1: Human organic cation transporter 1.
Alkylating agents
Cyclophosphamide[25]:Cyclophosphamide has been used in treating a broad spectrum of cancers including leukemia, lymphoma, breast and ovarian cancers[26]. In a study conducted by Khanet al[27], TQ was found to amplify the growth inhibitory effects of low doses of cyclophosphamide in breast cancer cells. This combination upregulated the expression of phosphatase and tensin homolog (PTEN) and downregulated the phosphorylation of its downstream signaling molecule Akt in addition to decreasing the expression of cyclin D1. The PTEN/phosphatidylinositol-3-kinase(PI3K)/Akt pathway is known to be an important tumorigenic pathway responsible for cell cycle progression, survival, and migration of malignant cells[28].
Temozolomide[29]:Temozolomide (TMZ) has been approved by the Food and Drug Administration for the treatment of glioblastoma multiforme[30]. However, the anticancer efficacy of TMZ has been limited by cancer resistance[31]. TQ was found to be a potent enhancer of the anti-proliferative and apoptotic activity of TMZ in glioblastoma cells. The modulation of the apoptotic players including ROS generation, disruption of mitochondrial membrane potential, activation of p53, caspases 9 and 3 was more pronounced in combination treatment compared to separate treatments[32]. Moreover,combining TQ and TMZ caused a stronger inhibitory effect on glioblastoma cells migration, invasion and adhesion than each drug alone. This synergistic inhibitory effect was found to be associated with a decrease in the expression and secretion of matrix metalloproteinases MMP-2 and MMP-9[33] known to promote metastatic spread and to contribute to angiogenesis[34]. Interestingly, TQ was found to block TMZ-induced autophagy, which was suggested to be a prosurvival mechanism of cell resistance to TMZ. TQ suppressed TMZ-induced expression of key players in the autophagy pathway beclin-1 and autophagy-related 7[35].
Cisplatin[36]:Cisplatin (CDDP) is one of the most used chemotherapeutic drugs in the treatment of a wide range of cancer types[37]. The primary dose-limiting side effect of CDDP is the dose- dependent nephrotoxicity, which restricts the use of high doses of CDDP to increase its anticancer activity[38]. Numerous studies have demonstrated the anti-neoplastic efficacy of combining TQ with CDDP in different types of cancers as an alternative way to increase CDDP potency. In ovarian cancer, these two agents werefound to synergize to induce apoptosisin vitroand in a mouse syngeneic model. The combination was more effective in increasing the levels of Bcl-2-associated X protein(Bax), phospho-histone 2AX on serine 139, cleaved caspase 3 and PARP and in downregulating proliferating cell nuclear antigen compared to CDDP alone[39]. A study conducted by Huet al[40] found that TQ enhanced the apoptotic effect of CDDP in esophageal carcinomain vitroandin vivothrough downregulating JAK2/STAT3 pathway known to be involved in cancer cell proliferation, survival, angiogenesis and metastasis[41]. Another study showed the synergistic inhibitory effects of the combination of TQ and CDDP on the proliferation of non-small lung cancer cells and on the growth of lung cancer xenografts through the suppression of NFkB[42]. The improvement of CDDP-induced apoptosis by TQ was also demonstrated in oral squamous carcinoma cells. The combination was more potent in upregulating p53 and caspase 9 and downregulating Bcl-2 than CDDP alone[43]. In addition, combining TQ with CDDP resulted in a superior anti-neoplastic activity in gastric cancerin vitroandin vivoby further upregulating PTEN expression compared to CDDP alone[44].
Antimetabolites
5-FU[45]:5-FU is the third most frequently used chemotherapeutic drug in the treatment of a variety of solid cancers, but its clinical efficacy is hampered by drug resistance and treatment-associated toxicities[46,47]. It is the second most frequent chemotherapeutic agent that causes cardiotoxicity symptoms[46]. The potential chemomodulatory effects of TQ on 5-FU anticancer activity have been investigated in various cancer types. TQ was reported to chemosensitize gastric cancer cells to 5-FUinduced apoptosis by upregulating Bax, caspases 3 and 9 and downregulating Bcl-2[48]. Moreover, the combination of TQ with 5-FU synergistically suppressed azoxymethane-induced colorectal tumors initiation and development in rats without causing nephro- and hepato-toxicities. The dual combination enhanced the decrease in the expression level of pro-oncogenic genes [Wnt, β-catenin, NFkB, cyclooxygenase 2(COX-2), inducible nitric oxide synthase (iNOS), vascular endothelial growth factor(VEGF), and thiobarbituric acid reactive substances] and the increase in the expression level of anti-oncogenic genes [dickkopf-related protein-1 (DKK-1), cyclin-dependent kinase inhibitor 1A (CDNK-1A), transforming growth factor beta 1 (TGF-β1), transforming growth factor, beta receptor II (TGF-βRII), Smad4, and glutathione peroxidase]compared to separate treatments[49]. In another study, Ndreshkjanaet al[50] linked 5-FU with TQ by esterification to form a new hybrid molecule SARB and tested it on colon cancer cells. Both combination and hybrid treatments enhanced the cytotoxic effects of single agentsin vitro, while SARB was more effective in suppressing the growth of chorioallantoic membrane xenograftsin vivo. The cytotoxic effects of 5-FU,TQ and the natural product epigallocatechin-3-gallate in triple and double combinations were evaluated in nasopharyngeal cancer cells. The results revealed that the triple combination had the most potent effect in reducing the total number of cancer cells, and the dual combination of TQ and 5-FU was more effective than the combination of TQ and epigallocatechin-3-gallate[51]. In addition, TQ augmented the apoptotic effects of each of 5-FU and the alkylating agent oxaliplatin in osteosarcoma cells. Interestingly, combining TQ with low doses of each of these drugs was found to produce the same anticancer efficacy as higher doses of these agents[52]. Therefore,this treatment strategy may help in alleviating 5-FU and oxaliplatin undesired adverse effects.
Gemcitabine[53]:Gemcitabine (GCB) has been approved for treating different types of cancer including pancreatic and breast cancers[54]. The therapeutic application of GCB was compromised by several drawbacks including its short half-life in the blood circulation, poor membrane permeability in addition to the development of chemoresistance[55]. TQ and GCB were found to induce synergistic apoptosis in GCB sensitive and resistant pancreatic cancer cells by downregulating pyruvate kinase M2 expression[56]. In another study, pretreatment of pancreatic cancer cells with TQ followed by low doses of GCB resulted in a synergistic apoptotic and growth inhibitory responsesin vitroandin vivoby downregulating Notch1/PTEN, PI3K/Akt/mammalian target of rapamycin and NFkB mediated signaling pathways[57]. In the context of breast cancer, TQ boosted the apoptotic activity of GCB against T47D cells. While in the apoptosis defective MCF-7 cells, the combination of TQ with GCB induced significant cell death by autophagy[58].
Antimicrotubules
Paclitaxel[59]:Paclitaxel (PAC) is widely used for the treatment of several cancer types including breast, ovary, colorectal and lung cancers[60]. The major challenges that restrict its curative effect are chemoresistance and adverse effects that are mainly caused by the polyethylated castor oil that is usually added to its formulation to increase its solubility[61,62]. Three studies have evaluated the potential of the combinatorial effect of TQ and PAC in breast cancer. TQ-PAC combination produced a synergistic anticancer activity through the modulation of genes involved in apoptosis,cytokine-cytokine receptor interaction, Fas signaling, p53 signaling and JAK/STAT signaling[63]. In another study, combining TQ with PAC augmented the necrotic and caspase dependent- apoptotic responses in T47D breast cancer cells compared to PAC alone. While in the apoptosis defective MCF-7 cells, both individual and combined treatments induced significant cell death by autophagy[64]. The co-encapsulation of TQ and PAC in polymeric biodegradable poly(lactide-co-glycolide) nanoparticles lowered PAC effective anticancer dose and reduced cancer cell viability more effectively than PAC loaded nanoparticles or its free counterpart[65]. Therefore, this therapeutic approach may help in hijacking the toxicities associated with the clinical use of PAC.
Docetaxel:Docetaxel (DTX) has been approved for the treatment of different type of tumors including prostate cancer and breast cancer[66]. However, low water solubility, treatment related toxicities and drug resistance limit its application in clinical practice[61,67,68]. TQ was found to potentiate the apoptotic activity of DTX in prostate cancer cells by inducing a more prominent suppression of the signaling pathway PI3K/Akt compared to DTX alone[69]. Co-treatment of prostate cancer cells with these two agents resulted in a greater upregulation of Bax, BH3 interacting-domain death agonist (Bid), caspase 3 and PARP and a higher downregulation of Bcl-xL compared to individual treatments[70]. To enhance drug solubility, increase their efficacy and reduce DTX toxicities, multiple nanoparticle drug delivery systems for the co-delivery of TQ and DTX have been developed and evaluated on breast cancer cells. Loading TQ and DTX into a borage nanoemulsion delivery system allowed the lowering of the required effective dose of DTX and enhanced cell death in cancer cells through simultaneous stimulation of apoptosis and autophagy[71]. In another study, coencapsulating TQ and DTX in low-molecular-weight chitosan coated lipid nanocapsules was found to exhibit stronger cytotoxic and anti-angiogenic responses in cancer cells compared to the free single treatments[72]. The co-delivery of TQ and DTX in Pegylated lipid nanocapsules produced more effective apoptotic and anti-migratory effects in cancer cells in addition to a higher tumor growth inhibition in mice bearing Ehrlich ascites carcinoma compared to free single treatments. Interestingly, these dual drugs loaded lipid nanocapsules prevented the development of DTX-induced hematological, hepato- and nephro- toxicities, an indicator of their protective potential[73].Moreover, TQ and DTX were co-encapsulated into pegylated liposomes and tested against MCF-7 breast cancer cells. The half maximal inhibitor concentration of each of TQ and DTX co-loaded into liposomes were lower than those of the free individual drugs[74].
Cabazitaxel:Cabazitaxel (CBZ) was approved as the second line therapy for metastatic castration-resistant prostate cancer[75]. However, its low aqueous solubility, poor membrane permeability, and severe side effects like neutropenia and anemia are the challenging drawbacks for successful cancer management[76,77]. Combining TQ with CBZ caused synergistic apoptotic effects in breast cancer cells. To address the drug delivery challenge, TQ and CBZ were co-loaded in lipospheres. The combined drugs loaded lipospheres had enhanced apoptotic effects compared to the drug combination in solution[78].
Cytotoxic antibiotics
Doxorubicin[79]:Doxorubicin (DOX) is a primarily adopted chemotherapeutic agent for treating a wide spectrum of solid and liquid tumors[80]. Despite the robust anticancer activity of DOX, chemoresistance and severe side effects especially cardiotoxicity weakened its potency[81]. Nearly 11% of the patients treated with this agent develop acute cardiotoxicity[82]. Several studies demonstrated the powerful combinatorial effect of TQ on the anticancer efficacy of DOX. Combining TQ with DOX allowed the lowering of DOX dose by up to 2-fold while maintaining its anticancer potential against adult T cell leukemia (ATL). TQ and DOX synergized to induce caspases and ROS mediated apoptosis in human T-lymphotropic virus-1 positive and human T-lymphotropic virus-1 negative CD4+ malignant T cell linesin vitroin addition to suppressing the growth of an ATL xenograft in mice[83]. In addition, cotreatment of HL-60 acute myeloid leukemia cells with TQ and DOX induced two consecutives waves of caspase 3 activity in addition to more than 7-fold increase in ROS generation compared to DOX alone[84]. In breast cancer, TQ potentiated the antitumor activity of DOXin vivoby inducing apoptosis and inhibiting tumor cell proliferation to a larger extent than separate treatments[85]. Recently, TQ was shown to improve the apoptotic effect of subtoxic doses of DOX in hepatocarcinoma cells by further increasing the cleavage of caspase 3 and PARP in addition to reducing DOXinduced cytotoxicity to normal liver cells[86]. This synergistic inhibitory effect of TQ and DOX combination was also observed in chemoresistant cancer cells. TQ augmented DOX cell growth inhibitory effect by 2- and 1.2-fold in multi-drug resistant breast cancer cells and in DOX resistant colorectal cancer cells, respectively[84]. To enhance the synergistic effect of these two agents, two nanodrug delivery systems have been developed. Loading TQ and DOX in cockle shell-derived aragonite calcium carbonate nanoparticles (ACNP) showed higher efficacy in inducing apoptosis and reducing migration and invasion in breast cancer cells than the free drugs or the single drug loaded ACNP while being non-toxic to non-neoplastic cells[87]. In addition,incorporating TQ and DOX into F2 gel (poly-N-acetyl glucosamine) nanofibers exhibited superior cellular growth inhibition and apoptosis in breast and liver cancer cells compared to free drugs and single drug loaded nanoparticles. The anticancer potency of this nanodrug co-delivery system was further demonstrated in twoin vivocancer models. The dual loading TQ and DOX nanoparticles enhanced tumor suppressionviaapoptosis in mice bearing liver carcinoma by decreasing NFkB level and increasing caspase 3 as well as in mice bearing solid Ehrlich carcinoma by attenuating Bcl-2 level and up-regulating p53. Interestingly, this treatment also reduced the nephro- and cardio-toxicities induced by DOX through the attenuation of the oxidative stress[88,89].
Topoisomerase inhibitor
Topotecan[90]:Topotecan (TP) was approved for the second-line treatment of small cell lung cancer and was recommended to treat platinum resistant ovarian cancer[91,92]. The instability of the chemical structure of TP in aqueous solutions and in the plasma reduces its anticancer efficacy and causes side effects[93,94]. TQ was found to boost the anti-proliferative and apoptotic effects of non-cytotoxic doses of TP in acute myelogenous leukemia and in colon cancer cells. This effect was exerted by upregulation of p53 and Bax, downregulation of Bcl-2, increase in the cleavage of caspases 9 and 3 in leukemia cells and through p53- and Bax/Bcl-2-independent mechanisms in colon cancer cells. In addition, pretreatment of leukemia cells with TQ followed by TP was found to be more effective than the simultaneous application of both therapeutic agents[95,96].
Proteasome inhibitor
Bortezomib:Bortezomib (BTZ) was approved for the treatment of multiple myeloma[97]. It acts by inhibiting NFkB pathway known to be constitutively activated in multiple myeloma due to genetic aberrations in its components[98]. TQ was found to augment the apoptotic activity of BTZ in multiple myeloma cellsin vitroby enhancing caspase 3 activation and PARP cleavage. In a xenograft multiple myeloma mouse model, TQ potentiated the anti-neoplastic effects of BTZ by further suppressing NFkB and consequently downregulating the proliferative (Ki67), anti-apoptotic (Bcl-2),angiogenic (VEGF) and inflammatory (interleukin-6 and tumor necrosis factor-α)effectors. The authors further showed that TQ reduced the proliferation of BTZ resistant multiple myeloma cells[99].
Tyrosine kinase inhibitor
Imatinib:Imatinib (IM) is a potent tyrosine kinase inhibitor that was approved for treating chronic myeloid leukemia and gastrointestinal stromal tumors[100]. Resistance to IM was reported to develop in cancer patients through several mechanisms including the modulation of the expression of drug efflux and influx transporters[101,102]. In a study conducted by Thabetet al[103], TQ was found to improve the antiproliferative and apoptotic effects of IM in colorectal cancer cellsin vitro. Interestingly,this was accompanied by a significant decrease in the expression of the drug transporters ATP-binding cassette (ABC) subfamily B member (ABCB) 1, ABCG2 and human organic cation transporter 1 leading to a significant increase in IM uptake/efflux ratio compared to IM alone.
Hormone receptor modulator
Tamoxifen[104]:Tamoxifen (TAM) is one of the first-line therapies for hormone receptor-positive breast cancer patients[105]. A synergistic apoptotic effect was observed by combining TQ and TAM in breast cancer cellsin vitroregardless of hormone receptor status[106]. Apoptosis was induced through synergistic inhibition of X-linked inhibitor of apoptosis protein (XIAP) resulting in caspase 9 activation and PARP cleavage along with PI3K/Akt pathway inhibition, which caused the downregulation of Bcl-xL, Bcl-2, and upregulation of Bax, apoptosis inducing factor, cytochrome c and p27. TQ was also found to enhance TAM anti-angiogenic, anti-migratory and anti-invasive effects in breast cancer[107]. In addition, treating breast cancer patients with a combination of TQ and TAM resulted in greater increase in 5-year survival rate and decrease in relapse rate of patients compared to single treatments. At the molecular level, the dual treatment induced a higher increase in tumor tissue antioxidant enzymes (catalase and superoxide dismutase) and increased caspase 3 expression compared to individual treatments. Moreover, the combination of TQ and TAM enhanced the decrease in tumor tissue Bcl-2, TGF-β1, lipid peroxidation product malondialdehyde and pro-inflammatory cytokines tumor necrosis factor-α and interleukin-6 compared to each treatment alone[108].
Biphosphonate
Zoledronic acid:Zoledronic acid is a nitrogen-containing bisphosphonate that inhibits osteoclast-mediated bone resorption. It was approved to prevent and reduce the progression of skeletal complications associated with bone metastasis from solid tumors including prostate cancer[109]. Besides its anti-resorption activity, preclinical and clinical data demonstrated its anti-tumor effects in different types of cancer[110,111]. TQ intensified the apoptotic activity of zoledronic acid in PC-3 (hormone resistant and chemotherapy sensitive) and DU-145 (hormone and chemotherapy resistant) prostate cancer cell lines through a synergistic increase in DNA fragmentation in both cell lines and a synergistic activation of caspases 3 and 7 in PC-3 cells[112].
Arsenic trioxide
Arsenic trioxide was approved for the treatment of acute promyelocytic leukemia[113]. The combination of arsenic trioxide (As) with interferon alpha (IFN-α) was found to have an effective anti-neoplastic activity in ATL. As and IFN-α synergistically induced apoptosis in ATL leukemia cellsin vitroand cured murine ATL[114,115]. A phase II trial involving patients with relapsed/refractory adult T-cell leukemia/lymphoma showed that the combination of As and IFN-α exhibited anticancer effects but caused significant toxicity[116]. Combining TQ with As and IFN-α induced synergistic apoptotic activityin vitroandin vivoand allowed the reduction of the toxic doses of As. TQ alone or TQ/As/IFN-α combination downregulated XIAP and Bcl-2, upregulated Bax and induced cleavage of PARP and caspase 3[117], ultimately leading to enhanced apoptosis.
TQ in combination with ionizing radiation
Radiotherapy is a mainstay therapeutic modality for the treatment of early and advanced solid cancers. Nearly 50% of cancer patients receive radiotherapy during their treatment course[118]. However, its therapeutic potency was found to be compromised by the damage of the surrounding healthy tissue in addition to the development of radioresistance[119]. To overcome these challenges and enhance radiotherapy efficacy, exploring radiosensitizers, molecules that make cancer cells more susceptible to radiations, has attracted great attention[120]. Several studies demonstrated the radiosensitizing role of TQ on cancer cellsin vitro. TQ augmented the anti-proliferative and apoptotic effects of ionizing radiation and further enriched the sub-G1 population in breast cancer cells[121]. In addition, sensitization with TQ prevented the radiation-induced metastatic progression of breast cancer cells through the restoration of the levels of TGF-β and its downstream effectors in addition to epithelial and mesenchymal markers[122]. In melanoma, TQ enhanced the apoptotic responses of low doses of gamma knife irradiation by further inhibiting the phosphorylation of STAT3, which is known to play a key role in cancer cell proliferation,survival, angiogenesis and metastasis[41]. It also improved the gamma knife irradiation-induced immune response by further attenuating the secretion of tumor-related inflammatory cytokines[123]. The cellular and molecular mechanisms of action of TQ in combination with radiation and other therapeutic agents discussed in this review are presented in Table 2.

Table 2 Cellular and molecular mechanism of action of the combination treatment in preclinical studies

PTEN: Phosphatase and tensin homolog; ROS: Reactive oxygen species; Bax: Bcl-2-associated X protein; GSH: Glutathione; MMP: Matrix metalloproteinases; ɣ-H2AX: Gamma-histone 2AX; JAK2: Janus kinase 2; STAT3: Signal transducer and activator of transcription 3; NFkB: Nuclear factor kappa B; COX-2: Cyclooxygenase 2; iNOS: Inducible nitric oxide synthase; VEGF: Vascular endothelial growth factor; DKK-1: Dickkopf-related protein-1;CDNK-1A: Cyclin-dependent kinase inhibitor 1A; TGF-β1: Transforming growth factor beta 1; TGF-βRII: Transforming growth factor beta receptor II; GSH:Glutathione; mTOR: Mammalian target of rapamycin; AST: Aspartate transaminase; ALT: Alanine transaminase; MDA: Malondialdehyde; IL: Interleukin;INF: Interferon; TNF-α: Tumor necrosis factor alpha; MCP-1: Monocyte chemoattractant protein-1; RANTES: Regulated on activation normal T cell expressed sequence; TWIST1: Twist-related protein 1; ZEB1: Zinc finger E-box binding homeobox 1; MDM-2: Mouse double minute 2; NADPH:Nicotinamide-adenine dinucleotide phosphate; CAM: Chorioallantoic membrane.
TQ in combination with non-coding RNA
Gene therapy is a modern therapeutic approach that demonstrated immense and impressive potential against cancer. It consists of delivering therapeutic genetic materials such as small interfering RNA (siRNA), microRNA, and anti-sense oligonucleotides into cancer cells to restore target gene expression, which is modulated and associated with tumorigenesis[124]. miR-34a is a tumor-suppressive microRNA foundto be downregulated in numerous human cancers including breast cancer[125]. Reintroducing miR-34a in metastatic breast cancer cells targeted and inhibited the expression of epithelial to mesenchymal transition-associated proteins TWIST1, zinc finger E-box binding homeobox 1 and NOTCH1 and suppressed breast cancer cell migration and invasion. Moreover, combining TQ with miR-34a synergistically downregulated TWIST1 and zinc finger E-box binding homeobox 1, suggesting the promising therapeutic potential of this combination against breast cancer metastasis[126]. In another study, multilamellar gold niosomes were developed for the codelivery of therapeutic Akt-siRNA and TQ to overcome chemotherapeutic resistance induced by Akt overexpression in breast cancer. TQ-siRNA dual loaded niosomes produced stronger anti-proliferative and apoptotic effects in breast cancerin vitroandin vivocompared to free TQ and TQ loaded neiosomes. The mechanism of the combination treatment involved an effective decrease of the cellular level of Akt which sensitized breast cancer cells to TQ toxicity leading to inhibition of mouse double minute 2 and therefore induction of p53-dependent apoptosis[127].
TQ in combination with natural molecules
Vitamins:Vitamin D3, the active metabolite of vitamin D, was reported to have potent chemopreventive effects against colorectal cancerin vitroandin vivo[128,129]. In addition, vitamin D supplementation was demonstrated to have clinically positive effects on survival outcomes in patients with colorectal cancer[130]. TQ was found to enhance the chemopreventive effect of vitamin D3 in suppressing the initiation and progression of colon tumors in an azoxymethane-induced rat model of colon cancer.The combination treatment significantly attenuated the number of grown tumors and large aberrant crypts foci. In addition, it decreased the level of pro-oncogenic (Wnt, βcatenin, NFkB, heat shock protein 90 HSP-90) and angiogenic (VEGF, iNOS and COX2)biomarkers and increased the expression of anti-oncogenic (DKK-1, CDNK-1A, TGF-β 1, TGF-β/RII and Smad4) biomarkers compared with individual treatments[131].
Hormones
Melatonin:Melatonin is a natural hormone involved in different biological activities including regulating the circadian rhythm[132]. Ample evidence revealed that melatonin exerts powerful anti-tumor effects through different modes of action including the activation of anticancer immune responses[133]. The combination of TQ with melatonin in breast cancer bearing mice resulted in 60% of cure in treated mice and produced a stronger apoptotic, necrotic and anti-angiogenetic response in addition to a more potent activation of T helper 1 mediated anticancer immune response compared to separate treatments[134].
Plant-derived molecules
Numerous studies have tested the anti-neoplastic efficacy of combining TQ with other plant-derived molecules in different types of cancer. Artemisinin is a sesquiterpene lactone extracted from the Chinese medicinal plantArtemisia annua[135]. Fröhlichet al[136,137] linked each of Artemisinin and its semisynthetic derivative artesunic acid with TQviacovalent bonds and tested the anticancer efficacy of the formed hybrid moleculesin vitro. They found that the ether-linked artemisinin-TQ hybrid exhibited a potent and selective anti-proliferative activity that was superior to that of the conventional drug DOX against sensitive and multidrug-resistant leukemia cells without being toxic to normal human foreskin fibroblasts[136]. They also found that the esterlinked artesunic acid-TQ hybrid promoted apoptosis mediated by ROS-induced DNA damage in colon cancer cells while being non-toxic to normal colon epithelial cells. The hybrid’s effect was found superior to each of the conventional drug 5-FU, the dual and individual treatments[137]. In another study, Daset al[138] demonstrated the synergistic anti-proliferative and apoptotic potential of combining TQ with diosgenin, a natural steroidal saponin isolated from several plants such asTrigonella foenumgraecum[139], in squamous cell carcinomain vitroand in a sarcoma 180-induced mouse model. Recently, Bhattacharjeeet al[140] investigated the combined effect of TQ with emodin, which is a natural anthraquinone obtained from various herbs includingRheum palmatum[141]. The results revealed that the dual treatment triggered a synergistic apoptotic response in breast cancer cells and enhanced the reduction of cancer cell migration compared to monotreatment by downregulating two important molecular players, namely focal adhesion kinase and integrin β1. In anex ovochorioallantoic membrane xenograft model, TQ and emodin were found to suppress the tumor growth and limit the migration of tumor cells to the liver and lung of the chick embryo[140]. Combining low doses of TQ and ferulic acid, obtained fromFerula asafetidaplant[142], potently inhibited the proliferation of breast cancer cells, while single treatments did not exhibit any inhibitory effects[143]. Moreover, it has been found that the combination of TQ and genistein, a flavonoid found in soybeans[144], resulted in a higher induction of apoptosis in thyroid cancer cells than treatment with either agent alone[145]. In lung cancer, the combination of TQ and indirubin-3-monoxime, a drug derived from the traditional Chinese herbal remedy Danggui Longhui Wan[146],resulted in synergistic apoptotic and anti-migratory effectsin vitroand synergistic tumor growth suppressionin vivo[147]. At the molecular level, the dual treatment decreased the phosphorylation of survival-regulatory proteins Akt, mammalian target of rapamycin and NFkB and activated caspase 3 and p53 in animal tumors[147].Furthermore, combining TQ and piperine, the major alkaloid found inPiper nigrum L[148], resulted in a synergistic inhibition of breast cancerin vitroandin vivo[149]. It induced a high degree of apoptosis and extensive necrosis, inhibited angiogenesis, and stimulated T helper 1 anticancer immune response with no liver and kidney toxicities.Interestingly, TQ was found to play the major role in inducing the caspase-mediated apoptosis[149].
In another study, the encapsulation of TQ and piperine in micro-vehicles made of a natural polymer guar gum extracted from the seeds ofCymompsis tetraganolobusplant[150] synergistically reduced the viability of hepatocellular carcinoma cells[151]. This was associated with ROS generation as indicated by an enhanced decrease in the level of intracellular antioxidant glutathione and nicotinamide-adenine dinucleotide phosphate[151]. Two studies assessed the anticancer effectiveness of combining TQ with resveratrol, a stilbene polyphenolic compound extracted from over 70 plants including Polygonum cuspidatum[152]. TQ and resveratrol combination resulted in a greater cytotoxic effect on hepatocellular carcinoma cells compared to single treatments[153]. In addition, TQ and resveratrol synergized to effectively inhibit breast cancerin vitroandin vivo. The combined drugs induced apoptosis and necrosis,inhibited angiogenesis and stimulated the anticancer immune response without causing liver and kidney toxicities[154]. Co-treating osteosarcoma cells with TQ and selenium, a micronutrient/trace element found abundantly in Astragalus bisulcatus[155], was found to be effective in decreasing cell viability, inducing cellular damage,and attenuating the levels of alkaline phosphatase and glutathione[156].
TQ EFFECTS AGAINST CANCER STEM CELLS
TQ in combination with chemotherapeutics agents
TQ was found to potentiate the effects of each of GCB and PAC in depleting the CD44+/CD24-CSCs population within MCF-7 and T47D breast cancer cells[58,64]. In another study, the co-delivery of DOX and TQ in ACNP effectively eradicated breast CSCs enriched from MDA-MB-231 cells cultured in 3D compared to single drug loaded ACNP and drug combinations in solution. The combined drugs loaded ACNP efficiently attenuated the self-renewal potential of breast CSCs as evidenced by the decrease of their mammospheres forming efficiency. This was accompanied by the reduction of breast CSCs markers CD44 and CD24 expression and aldehyde dehydrogenase 1 activity. In addition, the dual drugs loaded ACNP suppressed breast CSCs migration and invasion[157]. In colorectal cancer, combination of TQ and 5-FU as well as their hybrid SARB downregulated two major stem cell regulatory pathways Wnt/βcatenin and PI3K/Akt. In addition, they were found to effectively reduce the selfrenewal potential of colorectal CSCs and eradicate CD133+colorectal CSC population[50].
TQ in combination with natural products
The combined treatment of TQ and emodin improved the elimination of breast CSCs as demonstrated by the enhanced reduction in mammospheres forming efficiency and in CD44+/CD24-CSCS population compared to single treatments. Moreover, it downregulated the stemness promoting transcription factors Oct 4 and SOX2[140].
CONCLUSION
We have emphasized the tremendous potential of TQ in augmenting the antineoplastic effects of different therapeutic modalities against a wide range of cancer cells. TQ sensitized cancer cells to radiotherapy and improved outcomes of cancer resistance to conventional chemotherapeutic agents. The use of TQ in combination therapy also lowered the effective doses of standard chemotherapies which helped reduce their associated toxicities while maintaining their therapeutic effectiveness. The combination of TQ with other plant-derived molecules has shown interesting results and merits further investigation to introduce them as potential candidates for treating cancer. Although the studies investigating TQ potency in eliminating CSC in combination therapy are scarce, their results demonstrated great promise. Involving TQ in combination therapy could possibly further eliminate CSCs from tumors and prevent regrowth of neoplasms.
Despite its remarkable anticancer activity, studies reporting TQ anticancer therapeutic potential in clinical settings are still limited due mainly to its hydrophobicity and poor bioavailability. Few studies have supported combined therapies of TQ with nanoparticle formulations to circumvent the drug delivery challenges. These nanoparticles further enhanced the inhibitory effects of the combined agents against cancer or CSC in preclinical studies. Future efforts should be devoted to developing and testing these effective targeted nanoformulations of the combined agents including TQ for potential clinical translation.
杂志排行
World Journal of Clinical Oncology的其它文章
- BRCA mutations and gastrointestinal cancers: When to expect the unexpected?
- Esophagogastric junction adenocarcinoma: Preoperative chemoradiation or perioperative chemotherapy?
- Mechanisms of acquired resistance of BRCA1/2-driven tumors to platinum compounds and PARP inhibitors
- Proteoglycans and their functions in esophageal squamous cell carcinoma
