Highly accurate theoretical study on spectroscopic properties of SH including spin-orbit coupling*
2021-07-30ShuTaoZhao赵书涛XinPengLiu刘鑫鹏RuiLi李瑞HuiJieGuo国慧杰andBingYan闫冰
Shu-Tao Zhao(赵书涛) Xin-Peng Liu(刘鑫鹏) Rui Li(李瑞) Hui-Jie Guo(国慧杰) and Bing Yan(闫冰)
1Key Laboratory of Functional Materials and Devices for Informatics of Anhui Higher Education Institutes,School of Physics and Electronic Science,Fuyang Normal University,Fuyang 236037,China
2Institute of Atomic and Molecular Physics,Jilin University,Changchun 130012,China
3Department of Physics,College of Science,Qiqihar University,Qiqihar 161006,China
Keywords: SH,MRCI+Q,potential energy curves,spin-orbit coupling
1. Introduction
The SH molecule plays a crucial role in the fields of environmental sciences, life sciences, and astrophysics. The SH is of great importance in fossil fuel combustion processes and naturally occurring reactions generating sulfurous pollutants.[1,2]There are a large number of SH functional proteins in the human body, which play an important role in stabilizing protein conformation and maintaining protein activity.[3]In astrophysics, due to the relatively high content of sulfur in the universe, some researchers have carried out experimental observations on SH in space.[4,5]Therefore, the study of molecular structure and molecular spectrum of SH has important reference significance for future research.
In the experiment,numerous scholars have measured the spectrum of SH. In 1950, the SH was first spectroscopically detected,and the dissociation energies and vibration frequencies of the molecule were obtained by Porter.[6]Ramsay[7]reported the dissociation energy of ground state X2Π and an initial set of spectroscopic constants by using flash photolysis.Later, based on the results of Ramsay’s research, the (2, 0)bands of the A2Σ+-X2Π system of SH were recorded.[8]The absorption spectrum of SH was observed by the flash photolysis in the vacuum ultraviolet. Morrow[9]observed the transition from the X2Π to seven excited states in the vacuum ultraviolet absorption spectrum,and four of these excited states were associated with a Rydberg series.After that,Schniederet al.[10]improved the spectrum technology and obtained the accurate dissociation energy(3.71±0.07 eV)of the ground state X2Π. Pathaket al.[11]again photographed the (0, 0) band of SH by the emission spectrum of the low-pressure diffusion flame of the mixed gas burning in potassium vapor, and also detected the(0, 0)and(0, 1)band systems of the isotope SD for the first time. The molecular beam laser-induced fluorescence (LIF) experiment of SH was carried out by Ubachset al.,[12]and rotationally dependent predissociation was analyzed from linewidth measurements. In 1995, Ramet al.[13]obtained a more precise set of molecular constants, including the SOC effect through Fourier transform infrared spectroscopy. Subsequently,Milan[14]assigned high excited states below the lowest ionization limit via the two-photon excitation spectra, and Rydberg-valence interaction was used to identify the finer detail of electronic structures. Later,Wheeleret al.[15,16]investigated the predissociation mechanism of A2Σ+state of the SH by employing the technique of cavity ringdown spectroscopy. For SH,linewidth measurements for rovibrational levels of the A2Σ+were determined, and the variation of predissociation rates with respect to rovibrational quantum numbers were illuminated.
Along with experimental studies, a number of theoretical studies were carried out to investigate the electronic structure and spectroscopy of SH. Hirstet al.[17]used the multireference single and double excitation configuration interaction (MRD-CI) method to calculate the electronic structure of X2Π and A2Σ+of SH. Based on the calculated vertical excitation energy, Hirst pointed out that the B2Σ state is2Σ-,which is also verified by Park’s computational results.[18]Later,Senekowitschet al.[19]obtained the transition probability of SH fromab initiocalculations and gave the dipole moments.It was also pointed out that the PECs of a4Σ-and A2Σ+intersect atR=3.1 a.u. Bruna[20]carried out MRD-CI studies on SH and obtained the spectroscopic constants and dipole moments(DM)of bound X2Π and A2Σ+.In 2001,Lee[21]utilized the effective valence shell Hamiltonian method to calculate the transition dipole moments (TDMs) between the X2Π and the excited states A2Σ+, B2Σ-, C2Δ and D2Π. And Resendeet al.[22]calculated the low-lying states of the SH associated with the three lowest dissociation limits H(2S)+S(3P,1D,1S)at the CASSCF-MRCI level. In 2008,the PECs of A2Σ+,a4Σ-,B2Σ-and b4Π were calculated by Briteset al.[23]by using MRCI method with aug-cc-pV6Z basis set,and their mutual SOC integrals were also determined.
So far,most of the studies of SH concentrate on the lowlying states. So,in this study,the electronic structure of lowlying states and Rydberg states (c4Σ-and F2Σ-) of SH are calculated by the MRCI method.In order to improve the accuracy, Davidson correction (+Q), scalar relativistic effect, CV electron correlation,and SOC effect are considered in the calculations. The above physical effects would affect the electronic structure of small molecules.[24-31]The PECs of 10 ΛS states related to the 5 lowest asymptotes are determined.The spectroscopic constants and the DMs are calculated. The phenomenon of curve crossing and avoided crossing are studied, and the predissociation mechanism is analyzed by using the SO matrix elements. The radiative lifetimes of A2Σ+and F2Σ-states are determined.
2. Calculation method
In this study, we use the MOLPRO program package[32]to calculate the electronic structure of the 10 Λ-S states corresponding to the 5 lowest asymptotes of the SH molecule.Before starting the calculation of the electronic structure of the SH molecule,we test the basis sets of S and H atoms. Finally,the Gaussian-type basis sets,aug-cc-pV5Z-DK[33],and aug-cc-pwCV5Z-DK[34,35]are chosen for H and S atoms, respectively.At a series of bond lengths in the range ofR=0.9-10.0 ˚A, we use the multi-reference configuration interaction method (MRCI) to calculate the eigenvalue energy of 10 ΛS states. Utilizing the reference wavefunction determined by the complete active-space self-consistent field (CASSCF)[36]method,the eigenvalues of the 10 Λ-S states are calculated by MRCI[37]method. In CASSCF calculations, four a1, one b1,and one b2molecular orbitals are selected as active spaces.The energy calculated by MRCI does not include the sizeconsistency error. We introduce Davidson(+Q)[38]correction to consider the effect of higher electron excitation and eliminate size inconsistency error. In the MRCI calculation,the ten electrons in 1s2s2p shells are put into the closed shell,which are correlated via single and double excitations. That is to say,all the 17 electrons of SH are correlated in MRCI calculation,which is denoted as MRCI+CV result in this work.
In order to reveal the relativistic effect on the modification of the electronic structure, the scalar relativistic effect is included through third-order Douglas-Kroll[39]and Hess electronic integral.[40]The SOC effect is calculated by the state interaction method utilizing the full Breit-Pauli operator(HBP),so the eigenvalues of Ω states are determined by diagonalizing ˆHBP+ ˆHelin the basis of Λ-S wavefunction. With the help of the avoided crossing principle,the PECs of Λ-S states and Ω states are plotted. Based on the calculated PECs,the spectroscopic constants of the bound states are determined by LEVEL program.[41]
3. Results and discussion
3.1. PECs of the 10 Λ-S states
The 10 Λ-S states of SH are computed by MRCI+Q method. Figure 1 shows the PECs of those states correlating to the S(3P)+H(2S), S(1D)+H(2S), S(1S)+H(2S),S(5S)+H(2S) and S(3S)+H(2S) dissociation limits. The energy splits of1D-3P,1S-3P,5S-3P and3S-3P of S atom are 8818, 21814, 53523 and 57003 cm-1, which are in good agreement with the observed experimental results of 9043,21984, 52428 and 55135 cm-1.[42]From Fig. 1, it can be found that the potential wells of the 3 bound states A2Σ+,c4Σ-and F2Σ-are all located in the high-density areas of states, which make their PECs intersect with multiple repulsive states. The interaction between these electronic states may lead to predissociation.
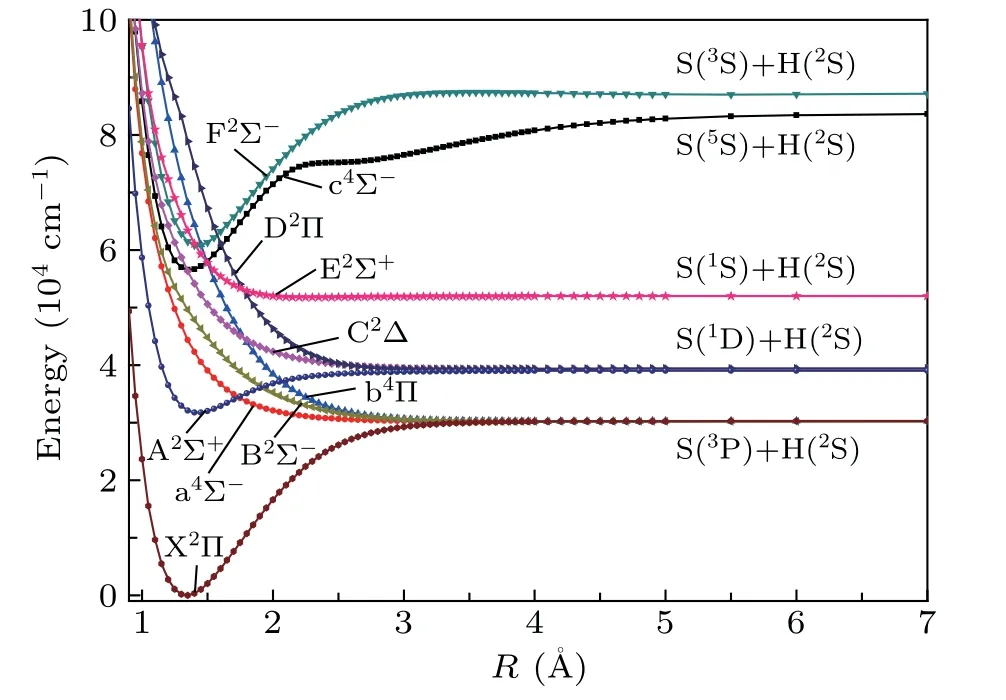
Fig.1. Potential energy curves of Λ-S states of SH.
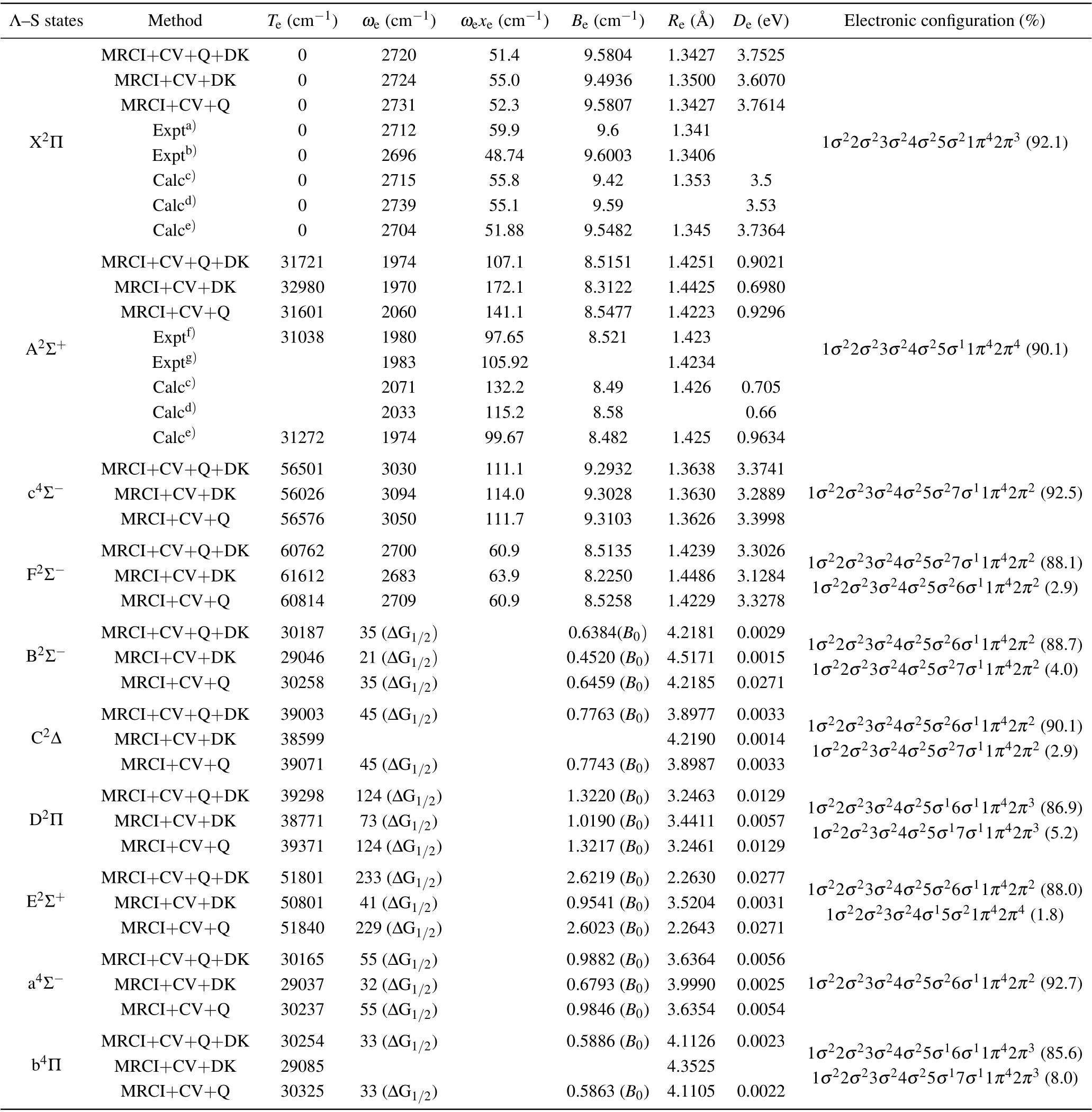
Table 1. Spectroscopic constants of Λ-S states of SH.
The spectroscopic constants of SH are determined and displayed in Table 1. In order to illuminate the influence of various effects on the electronic structure, the electronic structure calculations are performed at different levels of MRCI+CV+Q+DK, MRCI+CV+DK and MRCI+CV+Q,and these calculation results are summarized in Table 1. The X2Π is mainly composed of the open-shell electronic configuration 1σ22σ23σ24σ25σ21π42π3(92.1%). Comparing the spectroscopic constants obtained by MRCI+CV+Q+DK and MRCI+CV+Q method, the values ofωe,ωeχeandDeat MRCI+CV+Q+DK level are reduced by 11 cm-1,0.9 cm-1and 0.0089 eV. When the Davidson correction and the DK effect are included,ωe,ωeχe,Be, andReare more close to the latest experimental results[13]of 2696 cm-1,48.74 cm-1, 9.6003 cm-1, and 1.3406 ˚A. The dominant electronic configuration of the first excited state A2Σ+is 1σ22σ23σ24σ25σ11π42π4(90.1%), which corresponds to the single-electron excitation (5σ →2π) with respect to the electronic configuration of X2Π. Without the Davidson correction,Te,ωeandωeχeare computed to be 32980,1970 and 172.1 cm-1, differing from the corresponding measurements[8]by 1942, 10 and 74.5 cm-1. The results ofTe,ωeandωeχeexcluding DK effect were 31601, 2060 and 141.1 cm-1,differing from the experimental results[8]by 563, 80, 43.5 cm-1. After considering the Davidson correction and DK effect, the values ofTe,ωe,ωeχe,BeandReare offset from the experimental results by 683 cm-1(2.20%),6 cm-1(0.30%), 9.5 cm-1(9.73%), 0.0059 cm-1(0.07%)and 0.0021 ˚A (0.15%), respectively. Furthermore, the spectroscopic parameters of the Rydberg states F2Σ-of SH are also calculated, whose values ofTe,ωe,ωexe,Be,ReandDeat MRCI+CV+Q+DK level are 60762 cm-1, 2700 cm-1,60.9 cm-1, 8.5135 cm-1, 1.4239 ˚A and 3.3026 eV, respectively. As shown in Fig. 1, the PEC of c4Σ-exhibits a potential barrier atR= 2.2 ˚A. The potential barrier of c4Σ-is due to the avoided crossing of higher excited states with4Σ-symmetry. The avoided crossing causes an obvious change of leading electronic configuration of c4Σ-. At the equilibrium distance, the leading electronic configuration of c4Σ-is 1σ22σ23σ24σ25σ16σ21π42π2(92.5%). AtR= 2.6 ˚A, the leading electronic configuration of c4Σ-is changed into 1σ22σ23σ24σ25σ16σ21π42π2(34.6%), 1σ22σ23σ24σ25σ16σ17σ11π42π2(25.0%), and 1σ22σ23σ24σ25σ27σ11π42π2(19.8%),which exhibit obvious multi-configuration character.
The DMs of the 10 Λ-S states are calculated by MRCI method, which are plotted in Fig. 2. The DM of X2Π is 0.2797 a.u., which is consistent with the experimental result 0.2439 a.u.[43]The DM of A2Σ+has a peak value of 0.5631 a.u. atR= 1.6 ˚A. In addition, the DM of a4Σ-,C2Δ,B2Σ-and E2Σ+have roughly the same changing trend,because their main electronic configurations are the same,all of them are 1σ22σ23σ24σ25σ26σ11π42π2. Because the main electronic configurations of b4Π and D2Π are both 1σ22σ23σ24σ25σ16σ11π42π3, their DMs also have a similar changing trend. As shown in Fig.2,the DM curves of the 10 Λ-S states have a common characteristic. The DM curves all return to zero at infinity,which indicates that the dissociation products of the molecule are neutral atoms. It should be noted that the DM curves of c4Σ-and F2Σ-tend to zero more slowly than those of the other eight electronic states, which may be caused by the Rydberg character of c4Σ-and F2Σstates.
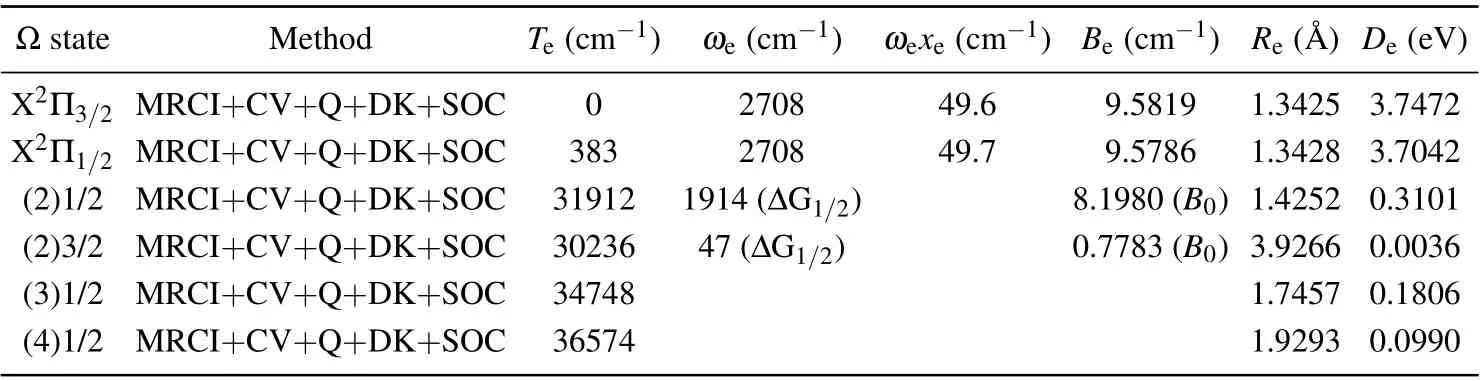
Table 2. The spectroscopic constants of low-lying Ω states of SH.
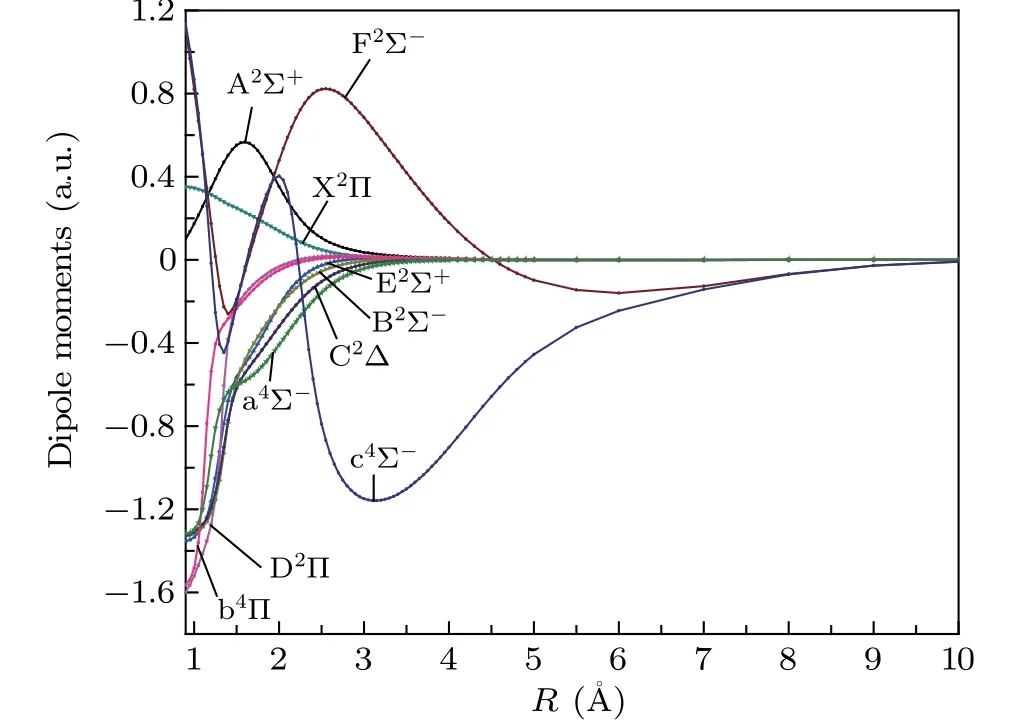
Fig. 2. The dipole moments of Λ-S states of SH determined by the MRCI method.
3.2. Spin-orbit coupling and predissociation
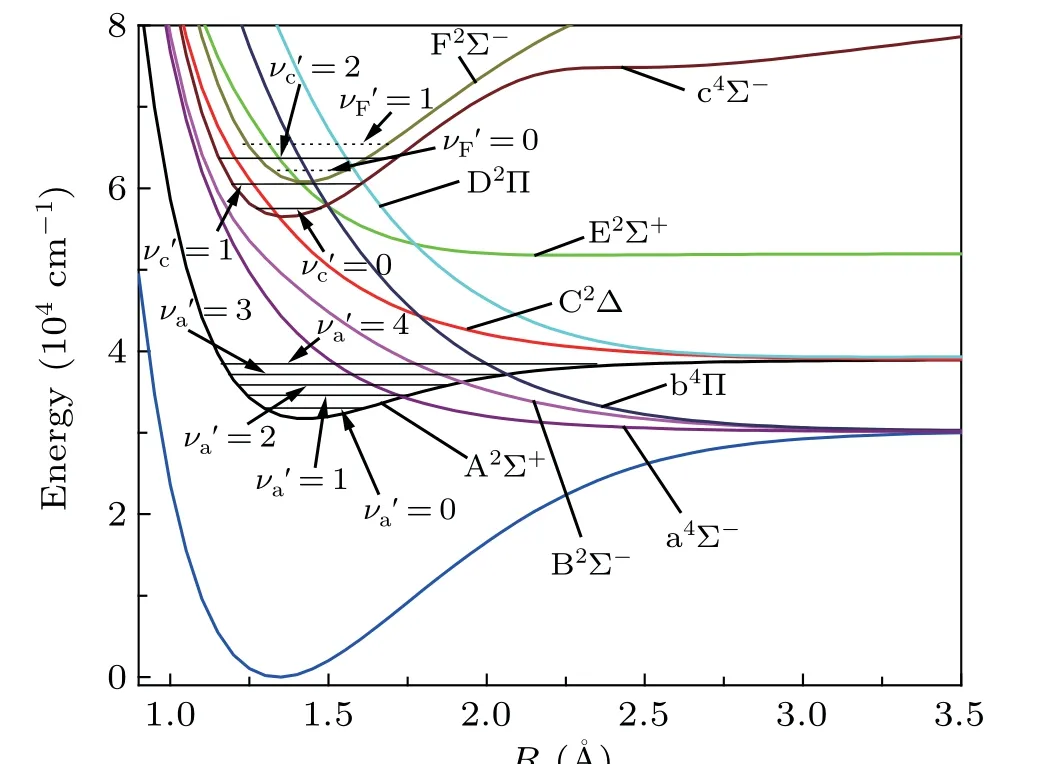
Fig.3. An amplified view of the crossing region and the vibrational levels of A2Σ+,c4Σ- and F2Σ- states.
As indicated above,the curves of A2Σ+,c4Σ-and F2Σstates cross with multiple repulsive states.The curve crossings and predissociation mechanisms of the SH system are studied.The curve crossings are spread over two areas for the PECs of Λ-S states. As shown in Fig. 3, the A2Σ+PEC crosses with the PECs of a4Σ-,B2Σ-and b4Π atR=1.7-2.1 ˚A,with the energy range of 30000-40000 cm-1. And the second crossing region is located atR=1.3-1.7 ˚A, with an energy range of 56000-66000 cm-1. In this area, the intersecting curves are c4Σ-with repulsive states E2Σ+,b4Π and D2Π,as well as F2Σ-states with repulsive states E2Σ+,b4Π and D2Π. These intersecting curves can provide many possible predissociation pathways.As shown in Fig. 3, in the first crossing area, the intercrossing points are located atν′≥1 of A2Σ+state;hence,theν′=0 of A2Σ+is unperturbed by nearby states. In the second crossing area, the intercrossing points are situated at the bottom of the potential well of c4Σ-and F2Σ-states, so all theν′≥0 vibrational states of the two states may be predissociated via crossed states. The predissociation pathways can be studied by means of SOC integral. Hence,the SO matrix elements between the interacting electronic states in the crossing area are calculated. The definitions of the schematic representation for the spin-orbit matrix elements are listed in Table 3.The variation of absolute values of the SO matrix elements with the internuclear distances is plotted in Fig.4.
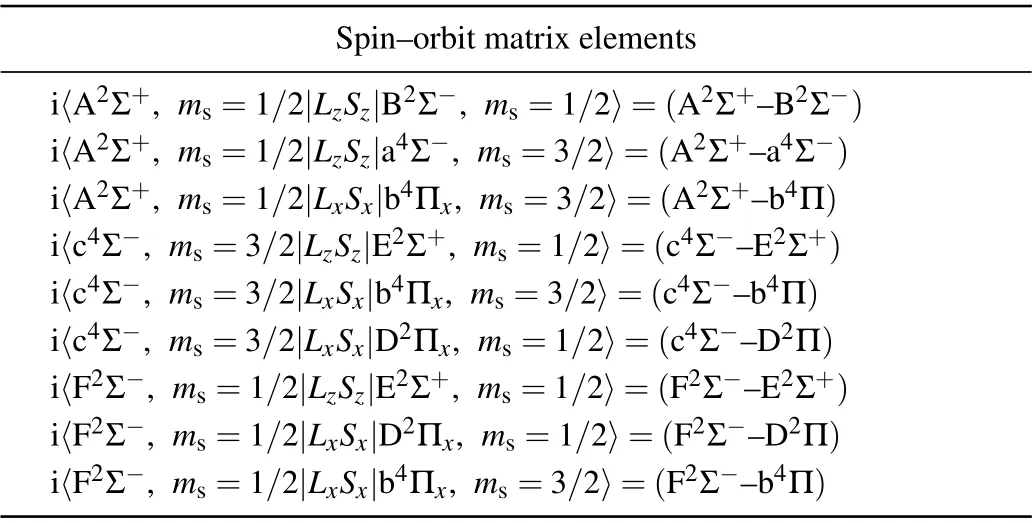
Table 3. Spin-orbit matrix elements between different Λ-S states of SHa).
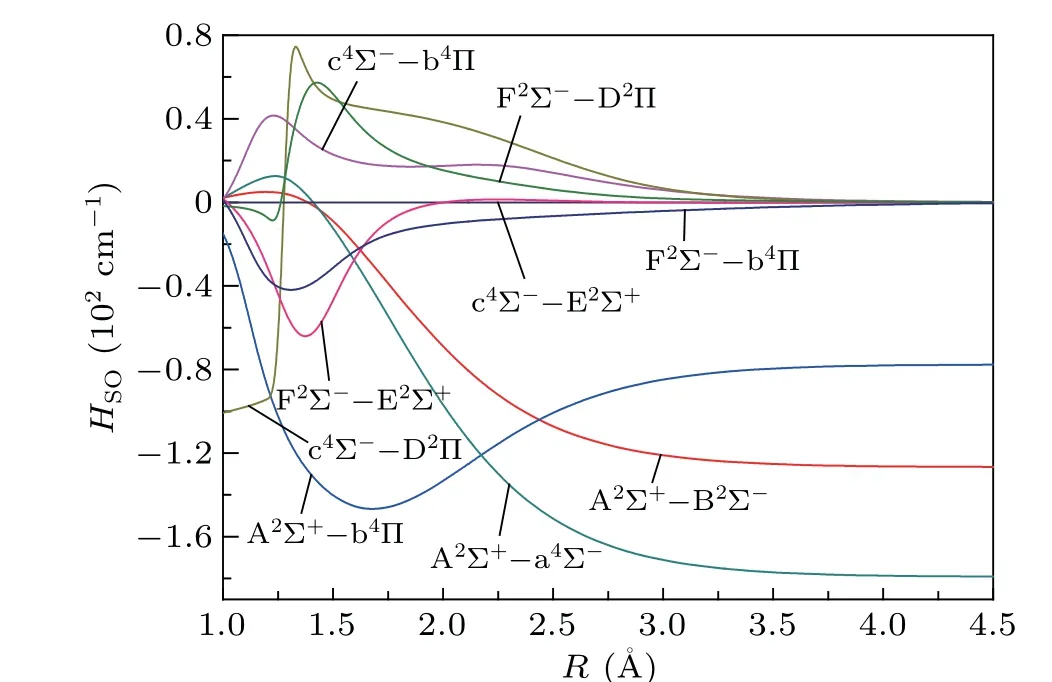
Fig. 4. Evolution of the values of spin-orbit matrix elements related to the A2Σ+,c4Σ- and F2Σ- states with the internuclear distance.
For A2Σ+state, the values of SO matrix elements of A2Σ+→a4Σ-and A2Σ+→B2Σ-at the crossing point are 54 cm-1and 57 cm-1. For the values of A2Σ+→a4Σ-and A2Σ+→B2Σ-, which are sufficient to induce certain predissociation. The repulsive state b4Π intersects with A2Σ+atR=2.06 ˚A,in betweenν′=3 andν′=4. The crossing position between b4Π and A2Σ+is in good agreement with the result of Wheeleret al.[15]At the intersection point, the SO value of A2Σ+→b4Π is 130 cm-1, which can cause strong predissociation. Our conclusion is consistent with the experimental results of Bruna and Lee.[20,21]
For the c4Σ-state, the SO matrix element of c4Σ-→E2Σ+at the crossing point is close to zero, which means that these predissociation pathways are forbidden. And the absolute values of SO matrix elements of c4Σ-→D2Π and c4Σ-→b4Π at the intersection point are 46 cm-1and 23 cm-1,so the pathway c4Σ-→D2Π can lead to weak predissociation, and the channel c4Σ-→b4Π is negligible. For the F2Σ-state, the absolute values of SO matrix elements of F2Σ-→E2Σ+, F2Σ-→D2Π and F2Σ-→b4Π at the curve crossing are 64,36 and 45 cm-1. From these,we can find that F2Σ-→E2Σ+could produce predissociation. However, the SOC of F2Σ-with D2Π states and F2Σ-with b4Π states are weakly,which can cause some disturbance to F2Σ-state.
3.3. PECs of the 18 Ω states
Considering the SOC effect,different Λ-S states with the same Ω components will be recombined, and 18 Ω states are generated from the 10 Λ-S states of SH.With the help of the avoided crossing principle,the PECs of 18 Ω states are drawn in Fig. 5. For the sake of clarity, we represent the states of Ω=1/2 and Ω=3/2,5/2 in Figs.5(a)and 5(b),respectively.In Fig.5,we can see that X2Π splits into X2Π1/2and X2Π3/2,the energy gap of which is 383 cm-1. The dissociation energiesDeof X2Π3/2and X2Π1/2are 3.7472 eV and 3.7042 eV,which are 0.0053 eV and 0.0483 eV below the value of X2Π,respectively. In addition, since the PEC of X2Π does not intersect with other electronic states,the values ofωe,ωexe,BeandReof X2Π3/2and X2Π1/2are basically the same as those of pure X2Π. Compared with the values of ground state X2Π,we can find theωe,ωeχe,BeandReof X2Π3/2are modified by 12 cm-1,1.8 cm-1,0.0015 cm-1and 0.0002 ˚A.
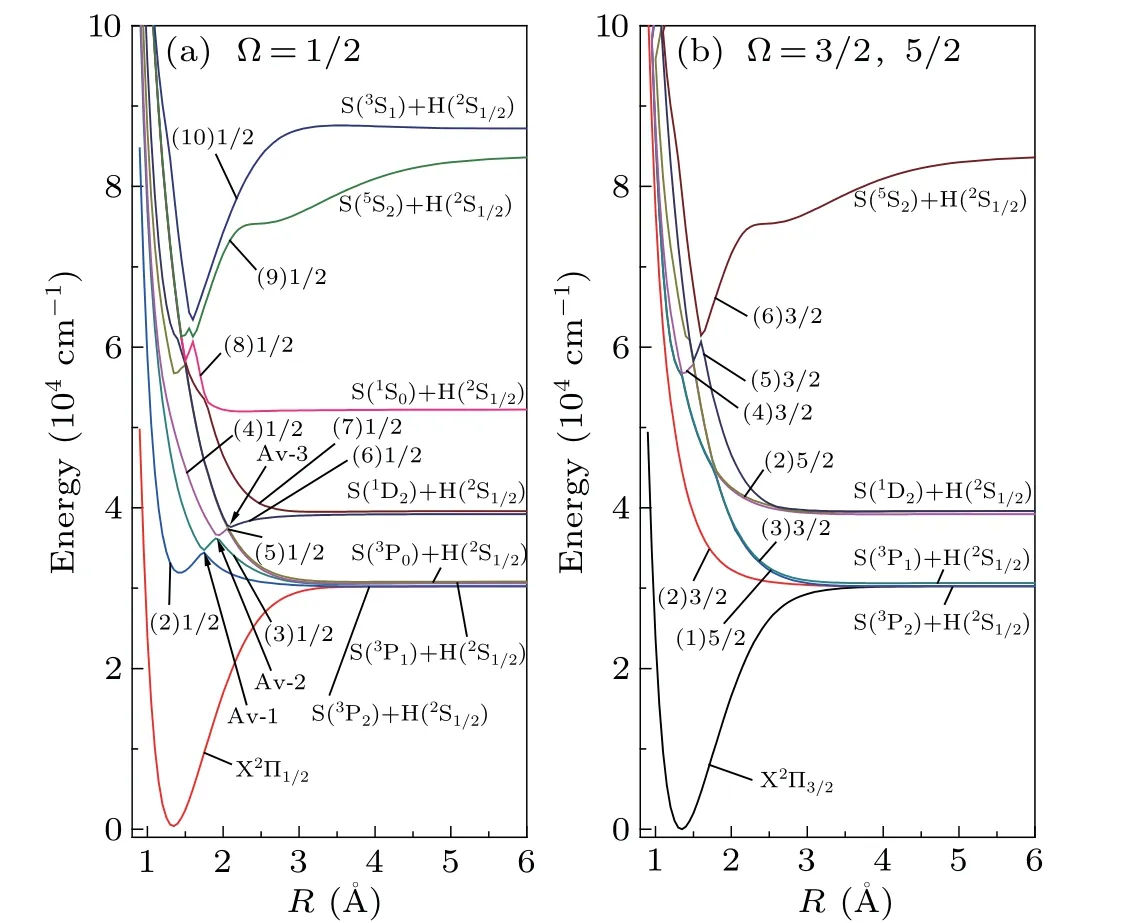
Fig.5. The PECs of the Ω states: (a)Ω=1/2,(b)Ω=3/2 and 5/2.
The bound state A2Σ+crosses with the a4Σ-state nearR=1.75 ˚A. Both the two states have Ω=1/2 component,so the PEC of Ω=(2)1/2 exhibits an avoided crossing point atR= 1.75 ˚A. The main configuration of the Ω = (2)1/2 state before avoided crossing point (R ≤1.75 ˚A) is A2Σ+,while the main configuration of it after avoided crossing point(R ≥1.80 ˚A) is a4Σ-. Compared with pure A2Σ+, the values ofTe,ωe,BeandReof(2)1/2 do not change much,only 191 cm-1,60 cm-1,0.3171 cm-1and 0.0001 ˚A,respectively.
After including the SOC effect, the PECs of Ω = 1/2 states in the energy range of 34000-40000 cm-1form three avoided crossing points atR=1.75, 1.90 and 2.05 ˚A, which are identified as Av-1, Av-2 and Av-3 in Fig.5. At the Av-1,the dominant Λ-S state of(2)1/2 changes from A2Σ+to a4Σ-.Similar to that,the main Λ-S state of(3)1/2 and(4)1/2 varies from A2Σ+to B2Σ-at the Av-2 and transform from A2Σ+to b4Π at the Av-3.
3.4. Transition properties and radiative lifetimes
The Einstein coefficient of spontaneous emission between the vibrational levelsν′andν′′is determined by the following equation:
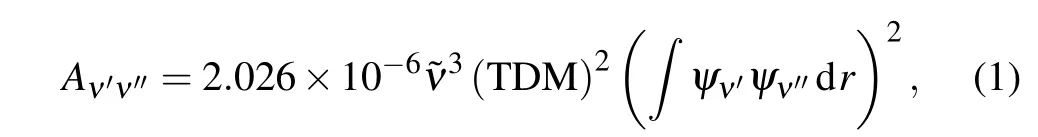
where ˜νis the transition energy in cm-1,TDM is the averaged transition dipole moment in atomic unit,and(�ψν′ψν′′dr)2is the FCFs between vibrational levelsν′andν′′. And the radiative lifetimes of the vibrational levelν′for a given state are obtained by reciprocal of total Einstein coefficientAν′ν′′,whose unit is seconds,
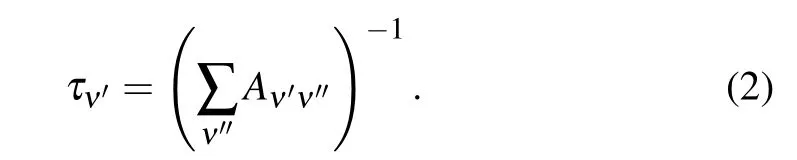
The calculated FCFs of A2Σ+-X2Π, F2Σ--X2Π and E2Σ+-X2Π transitions are listed in Table 4,and the FCFs change irregularly with the vibrational levelsν′andν′′.Due to the large difference between the equilibrium internuclear distances of E2Σ+and X2Π states,the FCF of E2Σ+-X2Π transition is obviously smaller than that of A2Σ+-X2Π and F2Σ--X2Π transitions. Compared with the experimental and theoretical results of the A2Σ+-X2Π transition in Table 4, it can be found that our calculation results are in good agreement with the experimental results of Johns,[44]and more accurate than the theoretical results of Bruna.[20]
According to the calculated transition energies,FCFs and TDMs, the radiative lifetimes can be calculated by Eq. (2).The radiative lifetimes of A2Σ+and F2Σ-at vibrational levelν′=0-3 are listed in Table 5. It could be found our calculated 748 ns(ν′=0)of A2Σ+is in reasonable agreement with the previous measurements of 820±240 ns.[45]The radiative lifetimes of A2Σ+increase with the number of vibration level;this trend is the same as the result of Resendeet al.[22]The order of magnitude of the radiative lifetime of A2Σ+is 1000 ns,and that of F2Σ-is 1 ns,which is caused by the larger TDMs of F2Σ--X2Π transition.
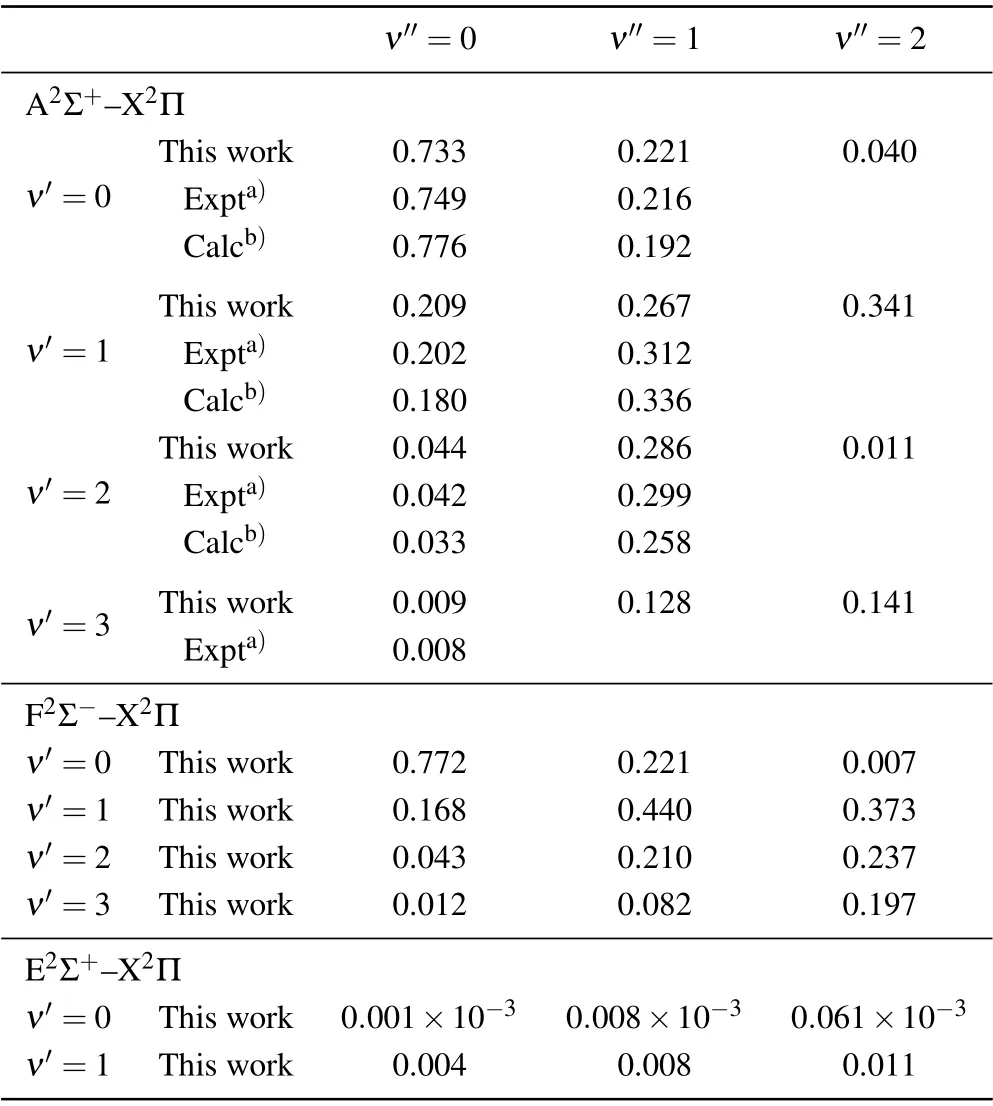
Table 4. The FCFs of A2Σ+-X2Π,F2Σ--X2Π and E2Σ+-X2Π transitions.
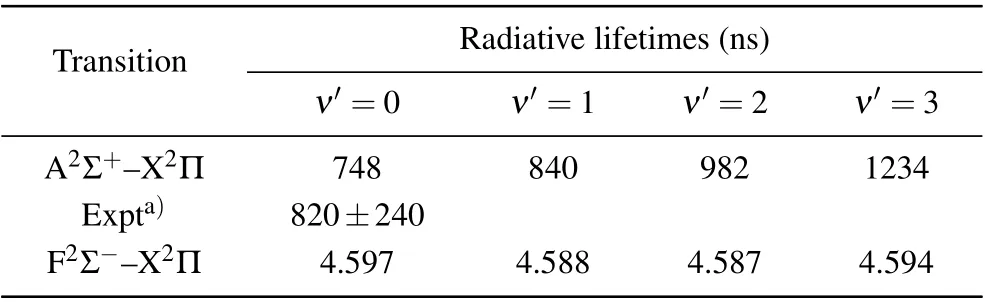
Table 5. Radiative lifetimes of A2Σ+ and F2Σ- states.
4. Conclusion
In this paper, the MRCI+Q method has been used to study the electronic states correlated with the five dissociation channels of the SH.In order to improve the calculation accuracy, the CV, Davidson correction, DK and SOC effects are considered in the calculation. Based on the PECs, the spectroscopic constants of the 10 Λ-S states are evaluated,and the influences of physical effects on spectroscopic properties are illuminated. At the intersection point, the absolute value of the SO matrix element between A2Σ+and b4Π is 130 cm-1,which can lead to strong predissociation. The possible predissociation channels c4Σ-and F2Σ-are also discussed. The Franck-Condon factors of spin-allowed transitions are determined, and the radiative lifetimes of A2Σ+and F2Σ-states are computed. Our investigations indicate that the SOC effect plays a vital role in the excited states of SH.
猜你喜欢
杂志排行
Chinese Physics B的其它文章
- Numerical simulations of partial elements excitation for hemispherical high-intensity focused ultrasound phased transducer*
- Magnetic-resonance image segmentation based on improved variable weight multi-resolution Markov random field in undecimated complex wavelet domain*
- Structure-based simulations complemented by conventional all-atom simulations to provide new insights into the folding dynamics of human telomeric G-quadruplex*
- Dual-wavelength ultraviolet photodetector based on vertical(Al,Ga)N nanowires and graphene*
- Phase-and spin-dependent manipulation of leakage of Majorana mode into double quantum dot*
- Deep-ultraviolet and visible dual-band photodetectors by integrating Chlorin e6 with Ga2O3
