Variability of Prorocentrum donghaiense response to allelopathic action from Alexandrium pacificum in laboratory culture*
2021-07-29HongjiaHUANGChuqiaoGANJiahuiHUANGChenZOUHongyeLIJieshengLIUWeidongYANG
Hongjia HUANG, Chuqiao GAN, Jiahui HUANG, Chen ZOU, Hongye LI, Jiesheng LIU,Weidong YANG
Key Laboratory of Aquatic Eutrophication and Control of Harmful Algal Blooms of Guangdong Higher Education Institute,College of Life Science and Technology, Jinan University, Guangzhou 510632, China
Abstract The dinoflagellates Alexandrium pacificum and Prorocentrum donghaiense are two wellknown harmful algal blooms (HABs)-forming species, both were usually found in the same sea areas in form of HABs in China. To date, there is no mechanistic model that can fully explain the occurrence of P. donghaiense blooms with A. pacificum. We found that diff erent strains of P. donghaiense had diff erent intrinsic growth rates of 0.107-0.215/d, and these strains exhibited diff erent responses to the allelopathic action from A. pacificum. Some strains of P. donghaiense could grow well despite some degrees ofinhibition in a short period, suggesting the two algal species P. donghaiense and A. pacificum could coexist, even if A.pacificum was allelopathic. Our findings may advance the understanding of phenotypes in P. donghaiense and provide a potential mechanism involved in the coexistence of P. donghaiense and A. pacificum in the same area.
Keyword: Prorocentrum donghaiense; allelopathy; Lotka-Volterra model; algal bloom; phenotype
1 INTRODUCTION
Harmful algal blooms (HABs), known as red tides,occurred in frequency and intensity, and spanned worldwide over the past two decades (Kremp et al.,2012; Cai et al., 2014). In China, eutrophication has become a serious environmental issue with the rapid development of economy, partly leading to the high frequent occurrence of HABs (Anderson et al., 2012;Kremp et al., 2012; Cai et al., 2014).
ProrocentrumdonghaienseandAlexandriumpacificumare two frequent HAB-forming dinoflagellate species in China, especially in the East China Sea. As a dominant species,P.donghaiensehas frequently formed large-scale blooms in the East China Sea since 1990s (Lu and Goebel, 2001; Lu et al., 2003).P.donghaienseblooms usually occur in area ranging from several thousand to more than ten thousand km2, lasting for several days to months,which results in great losses in economy and severe damages in marine environment. Diff erent fromP.donghaiense,A.pacificumis a notorious toxinproducing dinoflagellate species, which can produce paralytic shellfish poisoning (PSP) toxins and some extracellular substances (Tillmann and John, 2002)responsible for the PSP in humans.
It is reported thatA.pacificumhas an allelopathic eff ect on the growth of other algal species, includingP.donghaiense(Wang et al., 2006; Yang et al., 2010),which supports its ability to form blooms. However,the two algal species usually exist in the same sea areas, and in some cases form bi-phase HABs in China, especially in Zhejiang sea area recently. At present, no evidence is shown thatP.donghaienseproduces some toxins or has allelopathic eff ect on other microalgal species (Glibert et al., 2012; Lin et al., 2014), but several studies have demonstratedP.donghaiensehas high adaptability to a changing marine environment.P.donghaiensecan make good use of the metabolized dissolved organic phosphorus in the water (Ou et al., 2008), in addition to that,intraspecific genotypic and phenotypic variability might contribute the high adaptability ofP.donghaiense(Kremp et al., 2012). However, no mechanistic model yet is available that can fully explain the coexistence ofP.donghaienseandA.pacificum.

Table 1 Location of sampling stations and collection data
Several studies have investigated the algal competition among marine algal species, which demonstrated that inter-specific competition might play an important role in the succession of marine algal species (Wang et al., 2006, 2013; Yang et al.,2010; Qiu et al., 2011). Interaction between algae has a potential to construct the composition and structure of phytoplankton community in marine environment(Xu et al., 2020). Allelopathy, an important interspecific competition, can prevent competitive exclusion and promote phytoplankton diversity in aquatic ecosystems (Barreiro Felpeto et al., 2018).The allelopathic action may be complex and dynamic,and it seems to be determined by concrete group characteristics in the target and donor species (Fistarol et al., 2004). The co-existing competitors may diff er in their responses to allelopathy, and both the involved target and donor species might exhibit allelopathic properties (Legrand et al., 2003; Prince et al., 2008;Granéli and Salomon, 2010; Poulin et al., 2018).Moreover, the intra-specific trait diversity including allelochemical potency can promote the success of a species (Van de Waal et al., 2015; Brandenburg et al.,2018). Therefore, it is of paramount significance to learn the variability in alga-alga interaction betweenP.donghaienseandA.pacificumfor discovering underlying mechanism in their coexistence.
In this study, we focused on the responses of various strains ofP.donghaienseisolated from Zhejiang sea area to the allelopathy ofA.pacificumunder laboratory conditions. Therefore, a series of experiments, including bi-algal culture and cell-free filtrate culture were performed to determine whether the response to the allelopathic action varied between diff erent strains ofP.donghaiense. In addition, the competition betweenP.donghaienseandA.pacificumwas discussed.
2 MATERIAL AND METHOD
2.1 Culture condition
In total, 16 isolates ofP.donghaiensewere collected in May in 2014, respectively (Table 1), andA.pacificumstrain was collected from Daya Bay,Guangdong Province, the South China Sea. The collected algal samples were diluted up to the appropriate concentration, then algal cells were picked out by using a capillary tube. The isolated algal cells were cultivated using 6-well culture plates.All strains were grown as batch cultures in Erlenmeyer flasks containing f/2 medium, which was filtersterilized through 0.22-μm filters (Millipore). The pH of cultures was adjusted to 8.0 with 1-mol/L HCl. The cultures were grown at 20±1 °C in an artificial climate incubator (RXZ-430E). Cool-white fluorescent tubes provided an irradiance of 68 mmol photons/(m2·s)with a 12-h/12-h light/dark regime. All of the cultures were shaken manually at set times daily.
2.2 Co-culture of P. donghaiense and A. pacificum
The initial cell densities forP.donghaienseandA.pacificumin the co-culture were set in terms of their respective cellular volume (Wang et al., 2006).In the co-culture, the initial density ofA.pacificumwas 0.28×104cells/mL, and it was 1.0×104cells/mL or 1.0×105cells/mL forP.donghaiense.Correspondingly, the size/density ratio was 1:1 and 10:1, respectively. Experiments were carried out in a series of 150-mL Erlenmeyer flasks, where 100 mL of culture was present. Monocultures ofA.pacificumat 0.28×104cells/mL andP.donghaienseat 1.0×104cells/mL or 1.0×105cells/mL were used as related controls. The co-culture experiment lasted for 7 days. Cell densities of the microalgae were counted daily under a microscope (Olympus, CKX41) using triplicate subsamples of 0.1 mL. The intrinsic growth rate ofP. donghaiensestrains in monoculture was calculated. Inhibition ofA.pacificumagainstP.donghaiensein co-culture was expressed as the inhibitory rate (IR) as described by previous studies(Chen et al., 2015; Gao et al., 2015). Reciprocal interaction parameters between two species in the cocultures were evaluated by using the Lotka-Volterra model (Uchida et al., 1999; Wu and Wang, 2011), in which parameteraindicates the inhibition degree ofP.donghaiensebyA.pacificumwhen compared to its self-interference (Wang et al., 2006).
2.3 Exudate bioassay
In this experiment, we cultivated each strain ofP.donghaiense(1.0×105cells/mL) in filtrate fromA.pacificumculture. Filtrate was obtained from theA.pacificumculture at density of 1.12×104cells/mL when it was in the exponential growth phase by using 0.22-μm filters (Uchida et al., 1999). The filtrate pH was adjusted to 7.8-8.0 by 1-mol/L HCl, and the nutrient content of the filtrate was detected by using AutoAnalyzer3 (Brand Luebbe, Germany) and reenriched to f/2 level (Wang et al., 2013). In control,no filtrate was added inP.donghaiensecultures, but suffi cient nutrients were off ered as f/2 level. This experiment lasted for 7 days. The cell density ofP.donghaiensewas measured daily under a microscope (Olympus, CKX41).
2.4 Statistical analyses
Statistical analyses were performed using the software SPSS v.20.0, and all data were given as mean values±SD. To determine the significance in the growth between the experimental group and control,Student’st-test was carried out after testing for homogeneity of variance. The diff erences were considered significant at value ofP<0.05. To determine the changes in cell density over time,multiple comparison tests with a two-way analysis of variance (ANOVA) were performed with significance atP<0.05. The allelopathic potency ofA.pacificumtowardsP.donghaiensewas evaluated by Hierarchilcal cluster analysis (Average Linkage,between groups) using SPSS v.20.0 in terms of the parameterain bi-algal culture, and the inhibition rate on the first day both in bi-algal and in filtrate culture.Potential correlation between inhibition rate, intrinsic growth rate, and parameterawere evaluated by partial correlation analysis using SPSS v.20.0.
3 RESULT
3.1 Interaction between A. pacificum and P. donghaiense in bi-algal culture
As shown in Fig.1a, the growth ofP.donghaiensewere dramatically suppressed (P<0.05) on day 3 in bi-algal cultures with 1.0×104cells/mL ofP.donghaienseand 0.28×104cells/mL ofA.pacificum,while the growth ofA.pacificumremained almost unchanged compared to the control (P>0.05).However, when the initial cell density ofP.donghaiensewas increased to 1.0×105cells/mL,the growth of diff erentP.donghaiensestrains in the bi-algal cultures was significantly diff erent. Some strains including B5, B112, E21, F31, and G7 died after about 6 days, while B11, C2, C12, and F3 were significantly suppressed on day 8 compared to the control (P<0.05). Interestingly, the strains B111, C3,C3A, C13A, C13B, E2, and E23 showed obvious growth in spite of being inhibited in short-term period(Fig.1b). The cell densities of the strains C3, C3A,and C13A were increased over time after co-cultured for about 2 days (ANOVA,P<0.05).
Intrinsic growth rate in monoculture, inhibitory rates, and parameteraof diff erent strains ofP.donghaiensein bi-algal culture with initial cell density of 1.0×105cells/mL are presented in Table 2.Diff erent strains ofP.donghaienseexhibited diff erent responses toA.pacificumallelopathy. For example,the IR ofP.donghaienseat 6 d after co-culture withA.pacificumranged from 18.40%±4.90% to 100.00%±0.00%. Based on the IR ofA.pacificumon the growth ofP.donghaienseon the first day in bialgal culture, 16 strains ofP.donghaiensecan be divided into 3 hierarchical groups from weak to strong as follows: (1) weak (W): E23, C3A, C3, B111, B11,E21, B5; (2) moderate (M): F31, G7, E2, C2, C13A,C13B, B112; (3) strong (S): F3, C12 (Fig.2).
Similarly, diff erent strains ofP.donghaiensein bialgal culture showed diff erent parametera, which ranged from 13.29 to 145.00. In terms of the parametera, 16 strains ofP.donghaiensecan also be divided into 3 hierarchical groups follows (Fig.3): (1) weak(W): E2, F3, C12, E23; (2) moderate (M): C3, C3A,B111, C13A, E21; (3) strong (S): B5, G7, B112, B11,C2, C13B, F31.

Fig.1 Changes in cell density of A. pacificum and P. donghaiense in co-cultures with initial cell density of P. donghaiense at 1.0×10 4 cells/mL (a) or 1.0×10 5 cells/mL (b)
Interestingly, diff erent strains ofP.donghaiensealso displayed diff erent intrinsic growth rates in monocultures. However, partial correlation analysis showed that the inhibitory rate of diff erent strains ofP.donghaiensewas not correlated with parameteraand intrinsic growth rate.
3.2 Eff ect of filtrate from A. pacificum on the growth of P. donghaiense
To further study the inhibitory mechanism ofA.pacificumon the growth ofP.donghaiense, we conducted the exudate bioassay. As shown in Fig.4,similar to the results in bi-algal system, the growth ofB5, B112, C3, and F31 were inhibited significantly when cultured inA.pacificumfiltrate through all the experiment periods (P<0.05). However, the growth of strains B11, B111, C2, C3A, C12, C13A, C13B, E2,E21, F3, and G7 were inhibited on the first two days after culture (P<0.05), and cell densities of strains B11, C2, C12, C13B, E2, E21, and G7 began to increase since the third day (ANOVA,P<0.05). It is of note that the exudate bioassay results were not always consistent with that of bi-algal experiment. In bi-algal culture, strains E21 and G7 were inhibited
Data are expressed as mean±SD (n=3). Vertical lines show the standard deviation. P.d:P.donghaiense; P.d-filtrate:P.donghaiensein culture withA.pacificumfiltrate.severely, whereas they displayed an obvious growth trend despite some inhibition in filtrate ofA.pacificum.In contrast, the cell density of C3 was increased in bialgal culture, though it was lower than that in monoculture after co-cultured for 2 days (P<0.05).However, cell density of C3 inA.pacificumfiltrate was decreased to zero.
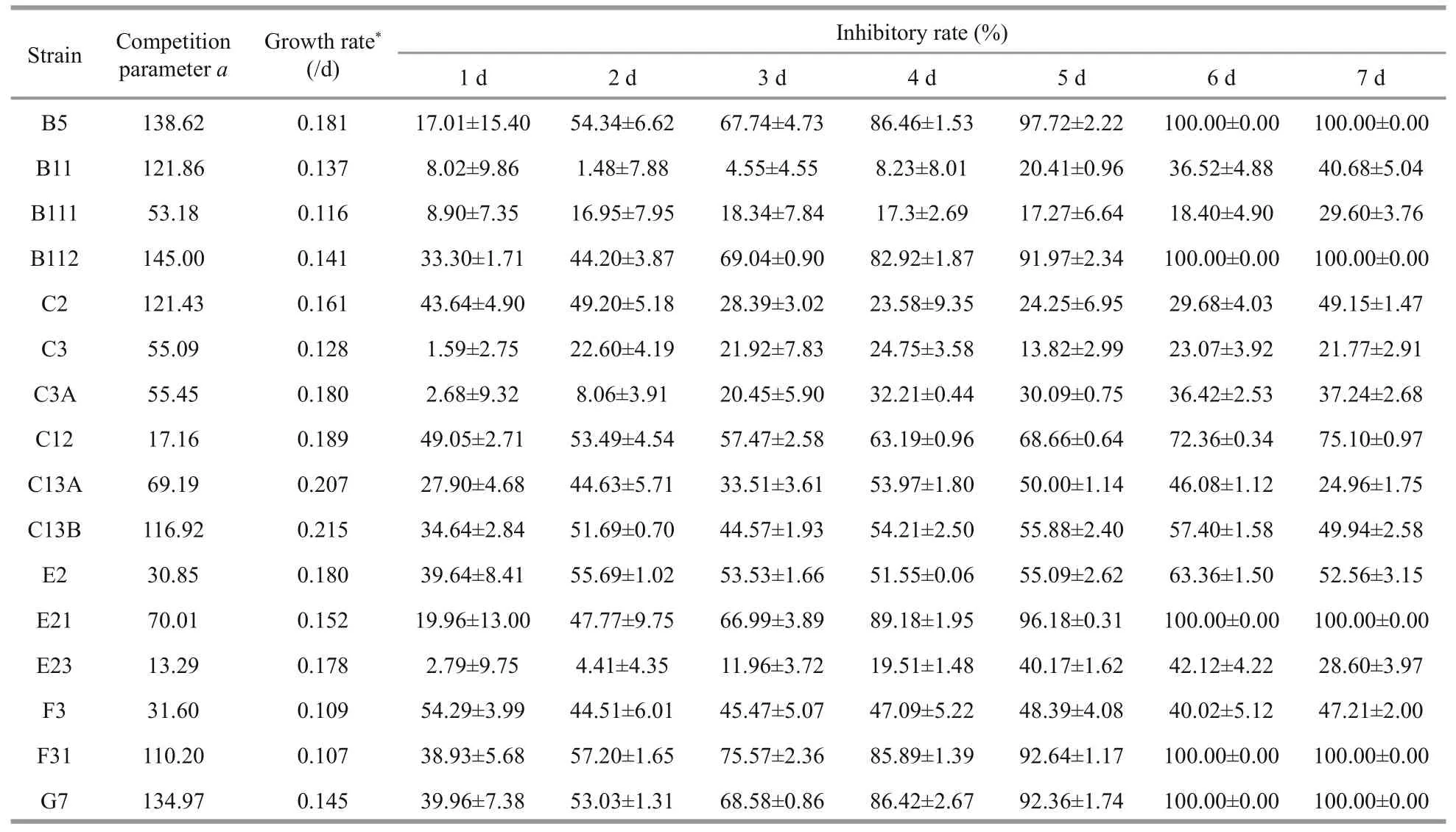
Table 2 Inhibition of A. pacif cium on P . donghaiense in bi-algal culture with initial density of 0.28×10 4 cells/mL for A. pacificum and 1.0×105 cells/mL for P. donghaiense
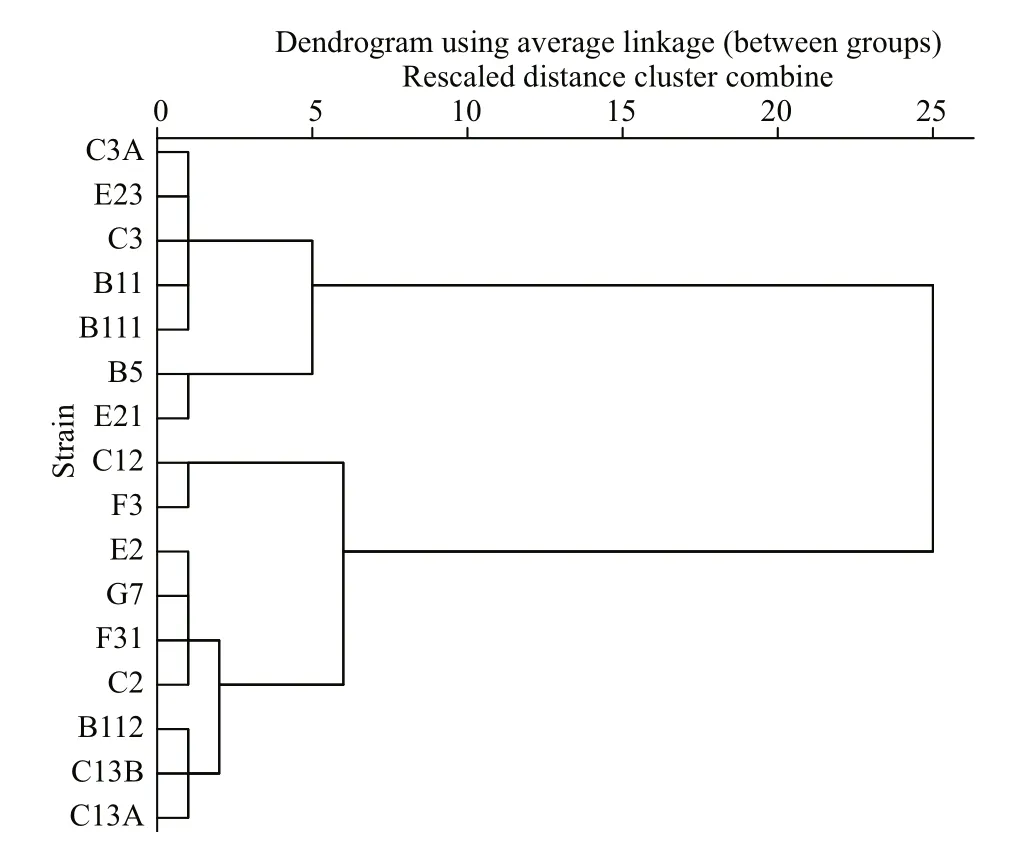
Fig.2 A hierarchical cluster of 16 strains of P. donghaiense in terms of the inhibitory eff ect of A. pacificum against P. donghaiense on the first day after coculture, where squared Euclidean distance was selected as the measurement
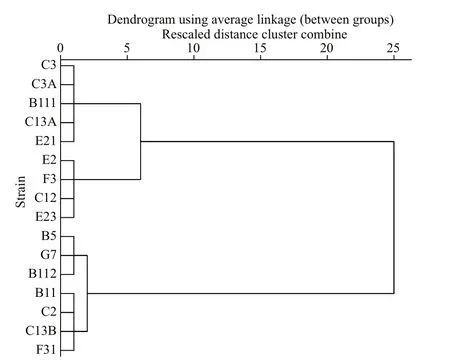
Fig.3 A hierarchical cluster of 16 strains of P. donghaiense based on the parameter a of P. donghaiense, where squared Euclidean distance was selected as the measurement
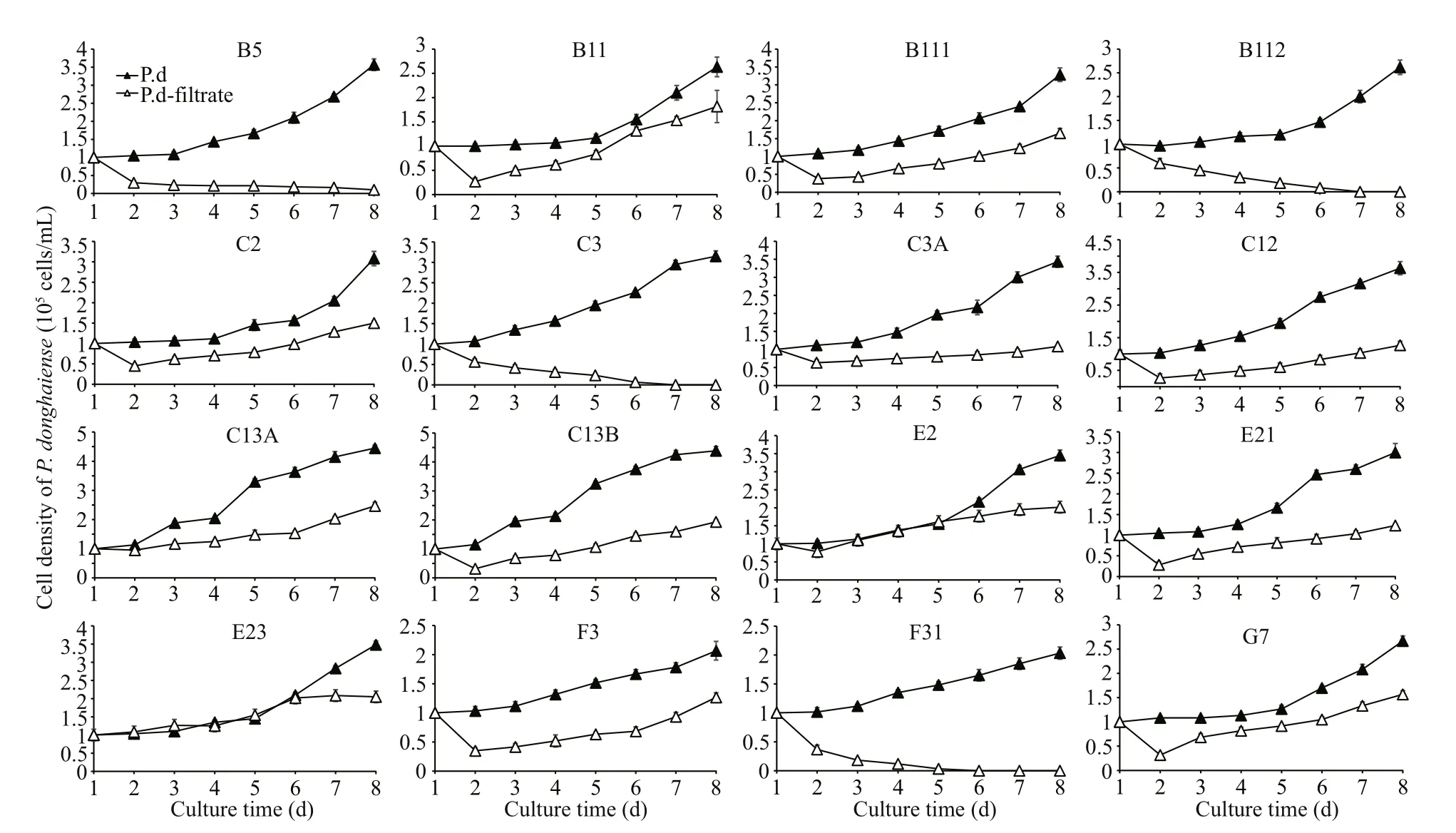
Fig.4 Eff ect of filtrate from A. pacificum on the growth of P. donghaiense
Inhibitory rates ofA.pacificumfiltrate onP.donghaienseare presented in Table 3. The IR ofP.donghaienseinA.pacificumfiltrate varied from-15.37%±9.72% to 100.00%±0.00%. According to the IR ofP.donghaienseon the first day in filtrate culture, the 16 strains ofP.donghaiensecan be divided into 3 hierarchical groups as follows (Fig.5):(1) weak (W): C13A, E2, E23; (2) moderate (M): C3,C3A, B112; (3) strong (S): C13B, E21, C12, B5, G7,B11, B111, F31, F3, C2.
4 DISCUSSION
Allelopathic eff ects are a natural phenomenon among higher plants and also between higher plants and microorganisms in ecosystems (Roy et al., 2006;Yang et al., 2010). This ecological phenomenon also exists among marine microalgae, and it is considered an important factor that promotes the HABs-forming species over other algal species. Many studies have dealt with the allelopathic action ofAlexandriumspecies (Alpermann et al., 2010). In the present study,we found that the growth ofP.donghaiensewas depressed deeply in the co-culture with initial cell density of 1.0×104cells/mL forP.donghaienseand 0.28×104cells/mL forA.pacificum, suggesting that allelopathy was one of the important phenotypes ofA.pacificum, which is in line with the results of previous studies (Wang et al., 2006; Alpermann et al.,2010; Yang et al., 2010; Yin et al., 2010).
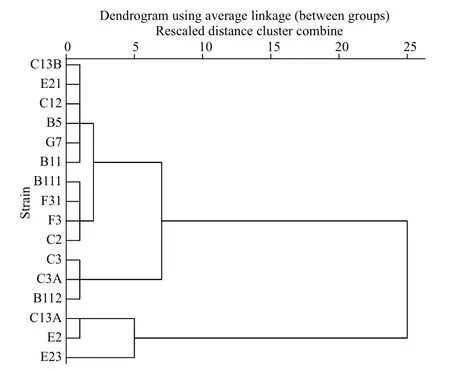
Fig.5 A hierarchical cluster of the 16 P. donghaiense strains in terms of the inhibitory eff ect of A. pacificum filtrate against P. donghaiense on the first day after culture,where squared Euclidean distance was selected as the measurement
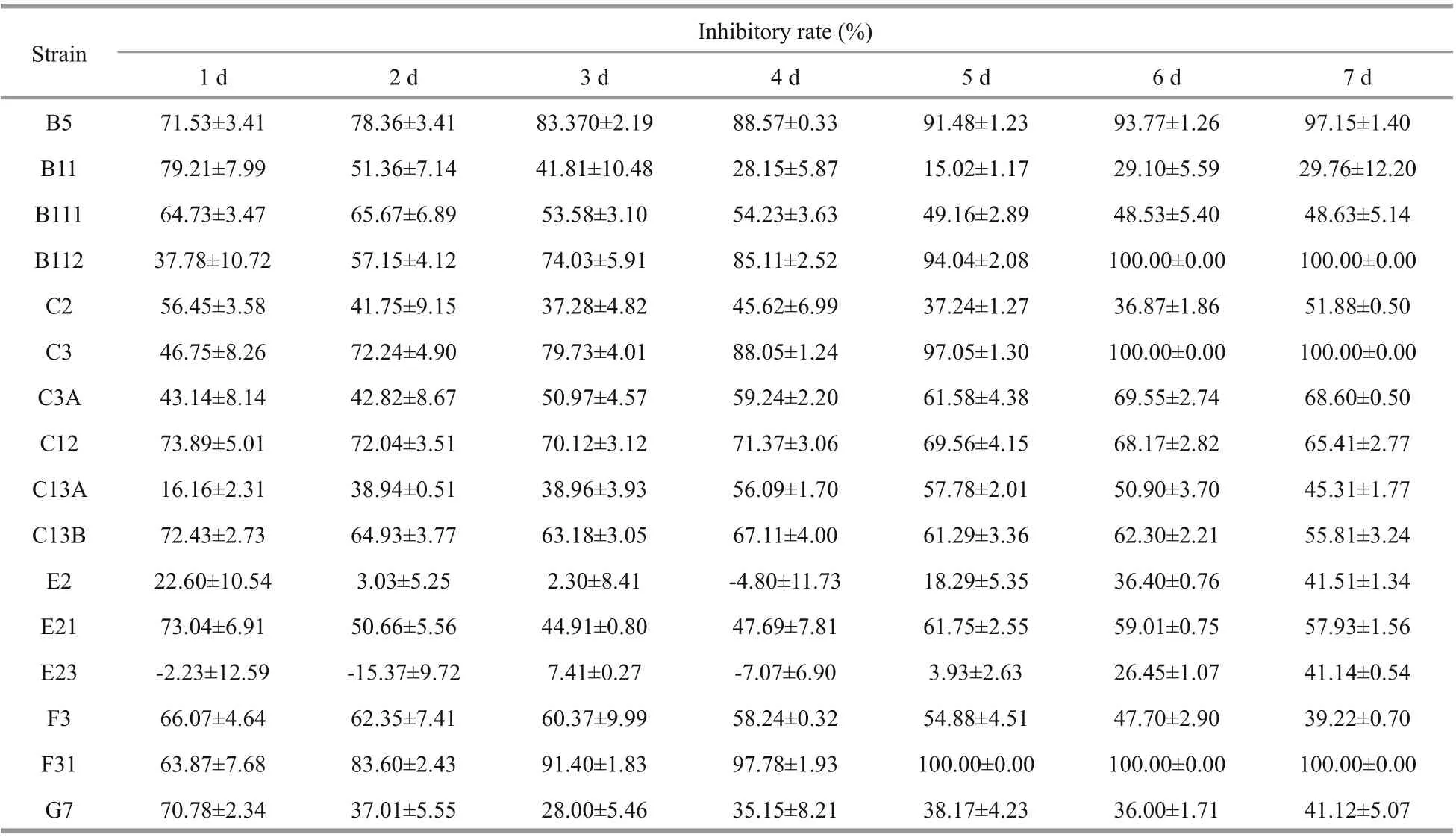
Table 3 Inhibition of A. pacificum filtrate on the growth of P. donghaiense
Allelopathic activity usually depends on the initial cell density of the associated target and donor species(Wang et al., 2006; Yamasaki et al., 2011). To further explore the response of variousP.donghaiensestrains toA.pacificum, we conducted the bi-algal culture with the 10:1 size/density ratio (1.0×105cells/mL forP.donghaiense, 0.28×104cells/mL forA.pacificum).In contrast with the results in the 1:1 bi-algal culture,diff erentP.donghaiensestrains showed diff erent responses to the co-culture withA.pacificum. The inhibition of strains B111, C3, C3A, C13A, and E23 was relatively weak, and these strains could maintain their growth, while the growth of strains B5, B112,E21, and G7 was depressed significantly, and the number of algal cells approached to zero. It is obvious that strains B111, B11, C2, C3, C3A, C13A, and E23 were able to maintain growth in the co-culture system though the growth of both algal species was inhibited to some extent. These results indicate that the two algal speciesP.donghaienseandA.pacificumcan coexist, even ifA.pacificumis allelopathic. Kaur et al. (2019) proposed that green algaScenedesmusabundanscould resist against allelochemicals fromMicrocystisaeruginosaby biochemical and proteomic reprogramming. However, whether and how theseP.donghaiensestrains resist allelopathic compounds fromA.pacificumremain to be elucidated in the future.
Cell-free filtrate cultivation is usually employed to determine the allelopathic eff ect of algae on target species (Zhang et al., 2015), so we observed the eff ect ofA.pacificumfiltrate on the growth of diff erent strains ofP.donghaiense. Similar to the results in bialgal culture, the growth of strains B5 and B112 was significantly inhibited when cultured in theA.pacificumfiltrate for 6 days, while the growth of strains B11, B111, C2, C3A, and C13A was inhibited on the first two days, and began to increase from the third day. These results suggest that theA.pacificumfiltrate really aff ect the growth ofP.donghaiense,possibly through extracellular substances produced byA.pacificum(Tillmann and John, 2002; Chen et al., 2015).
It is worth noting that the exudate bioassay results were not always consistent with that of bi-algal experiment. In bi-algal culture, strains E21 and G7 were severely inhibited, whereas they were inclined to grow despite some inhibition in the filtrate ofA.pacificum. Other forms of competition betweenA.pacificumandP.donghaiense, allelochemicals instability and physiological diversity might account for the discrepancy (Tillmann and Hansen, 2009).Unfortunately, the nature of allelochemicals ofA.pacificumis quite complex, and remains unclear to date, though several studies have been conducted (Ma et al., 2009, 2011; Chen et al., 2015). Surprisingly, cell density of strain C3 was increased despite the inhibition in bi-algal culture, whereasA.pacificumfiltrate could be lethal to it, which might be attributed to the potential physiological adaptation and high growth rate of strain C3 (Cullen and MacIntyre, 1999). However, the growth rate of strain C3 is only 0.128/d, lower than most of the strains ofP.donghaienseanalyzed. The intrinsic growth rate of microalgae plays an important role in the reciprocal interaction system. In general,high growth rate favors the donor species over other co-existing competitors. However, we did not observe a negative correlation between growth rate and inhibitory rate, suggesting that the diversity ofP.donghaiensein response to allelopathic action ofA.pacificummight be also related to other physiological characters. Heterotrophic bacteria may interfere with allelopathic action between phytoplankton species by degrading allelochemicals or producing some harmful compounds by themselves (Hulot and Huisman,2004). In our study, we performed all experiments in axenic and nutrient-enriched batch cultures. However,we cannot easily exclude the possible role of symbiotic bacteria in theA.pacificumallopathy againstP.donghaiense, especially when trying to explain any phenomena in field.
Based on the inhibition ofP.donghaienseon the first day in bi-algal culture and in filtrate culture, and parametera, the 16 strains ofP.donghaiensecan be divided into 3 hierarchical groups, but the three hierarchical groups do not completely match each other. For examples, strains E21 and B5 are clustered into W group according to the inhibition in co-culture,but they are attributed to group M and S, respectively,based on the parametera. In terms of the inhibition ofA.pacificumfiltrate, the two strains are ascribed to S group. These inconsistences suggest the complexity of response toA.pacificumin diff erent strains ofP.donghaiense. Remarkably,P.donghaienseis able to adapt to low irradiance, low temperature ranging from 10 to 20 °C and a broad range of salinities (Xu et al., 2010). High genetic diversity ofP.donghaiensepopulation might form the basis for variability in phenotypes such as the intrinsic growth rate,sensitivity to allelochemicals, suitable growth conditions, and empower it to display diff erent reaction to allelopathic action ofA.pacificum(Huang et al., 2020). Intraspecific variability has been regarded as a key driver for biodiversity sustenance in ecosystems (De Laender et al., 2014). Intraspecific variable phenotypic traits including nitrogen and carbon uptake rates, movement behaviors, and responses to changing environments have been reported in dinoflagellates (Van de Waal et al., 2015),diatoms (Godhe and Rynearson, 2017), and raphidophytes (Harvey et al., 2015). The variability in phenotype ofP.donghaienseremains an interesting topic for future study.
Blooms ofP.donghaiensehave been studied intensively in recent years. However, the reasons responsible for these blooms remain elusive.Allelopathy in phytoplankton is closely associated with competition for resources. Studies show thatP.donghaiensecan utilize a broad array of nutrients compound, especially nitrogen, which is an important regulator of the proliferation ofP.donghaiense(Hu et al., 2012). Zhang et al. (2019) demonstrated thatP.donghaienseevolved diverse dissolved organic phosphorus (DOP) utilization strategies to adapt to low phosphorus (P) environments, and that DOPs might play critical roles in theP.donghaiensebloom formation. Increasing studies have demonstrated the ability of dinoflagellates to respond to changing environments, which determine their success and proliferation, as well as bloom formations (Gobler et al., 2017). Many studies have dealt with the diversity of allelopathic activity among species and strains within the same species (Hakanen et al., 2014; Chen et al., 2015; Van de Waal et al., 2015). Meanwhile, a few studies have shown that allelopathic action is usually species dependent (Hakanen et al., 2014; Prasetiya et al., 2016). Here, maybe for the first time, we found the diversity of diff erent strains of target species to allelopathic action, and variability in growth rate ofP.donghaiense, suggesting that high adaptability ofP.donghaienseto the marine environment might be related to the phenotypic variability (Kremp et al.,2012). Alternatively, the immediate response ofP.donghaienseto environmental changes can involve both acclimation based on phenotypic plasticity, and adaptation based on selection (Barrett and Schluter,2008). As a result,P.donghaiensecould display a high level of phenotypic variability, which may stabilize a species response to environment changes,finally could co-exist withA.pacificum, and in some cases achieve dominance and form bloom withA.pacificumin Zhejiang sea area.
5 CONCLUSION
Diff erent strains ofP.donghaienseshowed diff erent intrinsic growth rate in monoculture. The toxic dinoflagellateA.pacificumhad a densitydependent allelopathic eff ect on the growth ofP.donghaiense, but diff erent strains ofP.donghaienseexhibited diff erent responses to the allelopathic action fromA.pacificum. Some strains ofP.donghaiensemight be resist to chemicals fromA.pacificum, and could grow well despite being inhibited in short-time period. This variability ofP.donghaiensein reaction to allelopathic action fromA.pacificummade it possible thatP.donghaienseandA.pacificumcoexist in the same sea area, and even form blooms in certain conditions. The variability in phenotype ofP.donghaiensedeserves further attention as an interesting topic for future studies. Our findings might advance the understanding to the phenotype ofP.donghaienseand suggest a potential mechanism involved in the coexistence ofP.donghaienseandA.pacificumin the same area.
6 DATA AVAILABILITY STATEMENT
The data generated in the current study were available from the corresponding author or the first author on reasonable request.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Numerical study of the seasonal salinity budget of the upper ocean in the Bay of Bengal in 2014*
- Study on evaluation standard of uncertainty of design wave height calculation model*
- A fast, edge-preserving, distance-regularized model with bilateral filtering for oil spill segmentation of SAR images*
- A Gaussian process regression-based sea surface temperature interpolation algorithm*
- Climatology and seasonal variability of satellite-derived chlorophyll a around the Shandong Peninsula*
- Sources of sediment in tidal flats off Zhejiang coast, southeast China*
