Potential therapeutic applications of mesenchymal stem cells for the treatment of eye diseases
2021-07-24GiulianaManninoCristinaRussoAnnaLongoCarmelinaDanielaAnfusoGabriellaLupoDeboraLoFurnoRosarioGiuffridaGiovanniGiurdanella
Giuliana Mannino, Cristina Russo, Anna Longo, Carmelina Daniela Anfuso, Gabriella Lupo, Debora Lo Furno,Rosario Giuffrida, Giovanni Giurdanella
Giuliana Mannino, Cristina Russo, Anna Longo, Carmelina Daniela Anfuso, Gabriella Lupo, Debora Lo Furno, Rosario Giuffrida, Giovanni Giurdanella, Department of Biomedical and Biotechnological Sciences, School of Medicine, University of Catania, Catania 95123, Italy
Abstract Stem cell-based treatments have been extensively explored in the last few decades to develop therapeutic strategies aimed at providing effective alternatives for those human pathologies in which surgical or pharmacological therapies produce limited effects.Among stem cells of different sources, mesenchymal stem cells (MSCs) offer several advantages, such as the absence of ethical concerns, easy harvesting, low immunogenicity and reduced tumorigenesis risks.Other than a multipotent differentiation ability, MSCs can release extracellular vesicles conveying proteins, mRNA and microRNA.Thanks to these properties, new therapeutic approaches have been designed for the treatment of various pathologies, including ocular diseases.In this review, the use of different MSCs and different administration strategies are described for the treatment of diabetic retinopathy, glaucoma, and retinitis pigmentosa.In a large number of investigations, positive results have been obtained by in vitro experiments and by MSC administration in animal models.Most authors agree that beneficial effects are likely related to MSC paracrine activity.Based on these considerations, many clinical trials have already been carried out.Overall, although some adverse effects have been described, promising outcomes are reported.It can be assumed that in the near future, safer and more effective protocols will be developed for more numerous clinical applications to improve the quality of life of patients affected by eye diseases.
Key Words: Mesenchymal stem cells; Eye diseases; Diabetic retinopathy; Glaucoma; Retinitis pigmentosa; Regenerative medicine
INTRODUCTION
Stem cell-based approaches have attracted considerable interest in the last few decades in view of their novel therapeutic applications.The regenerative potential of various types of stem cells has been widely explored, such as embryonic stem cells, induced pluripotent stem cells, and mesenchymal stem cells (MSCs).MSCs feature numerous advantages, such as the absence of ethical concerns, easy harvesting, low immunogenicity and tumorigenesis risks[1-3], and a marked ability to home to injury sites[4].
In the adults, MSCs are found in virtually all tissues where, by their self-renewal and multipotent differentiation capability, they ensure tissue growth, remodeling and repair[5].When culturedin vitro, MSCs show a fibroblast-like morphology and, as indicated by the International Society for Cellular Therapy, they are positive for typical MSC markers[6] and are able to differentiate into adipocytes, osteoblasts, and chondrocytes.When administeredin vivo, they induce beneficial effects, mostly thanks to the production of a variety of cytokines and growth factors (GF), such as basic fibroblast GF (bFGF) vascular endothelial GF (VEGF), transforming GF-β (TGF-β), hepatocyte GF, insulin-like GF-1 (IGF-1), ciliary neurotrophic factor (CNTF), brainderived neurotrophic factor (BDNF), and glial cell-derived neurotrophic factor (GDNF)[2,7,8].Moreover, they can release extracellular vesicles (EVs) conveying proteins, mRNA and microRNA (miRNA).In addition, like other cell types, MSCs feature immunomodulatory actions[9-11].Because of these properties, new therapeutic approaches are being developed for the treatment of various pathologies, including ocular diseases[12].Eye diseases may be due to different causes, such as photoreceptor loss, retinal ganglion cell (RGC) degeneration, and intraocular pressure (IOP) elevation.In this review, the use of different MSCs and different administration strategies are described for the treatment of diabetic retinopathy, glaucoma, and retinitis pigmentosa (RP).Most studies used MSCs derived from bone marrow (BMSCs) or adipose tissue (ASCs), but also those derived from the umbilical cord (UCMSCs) or conjunctiva (CJMSCs) were tested.
DIABETIC RETINOPATHY
Diabetic retinopathy (DR) is a vascular complication that occurs in diabetic patients.DR pathogenesis is associated with inflammation processes leading to microcirculation damage.As a result, a disruption of the blood-retina barrier (BRB) develops, mainly due to pericyte loss, which represents one of the earliest hallmarks of DR[13,14].To date, only limited beneficial effects have been obtained with available therapeutic strategies.In fact, pericyte loss is virtually irreversible since in the adult retina they are not able to replicate[15,16].Medical options trying to mitigate DR-induced damage include laser surgery, vitrectomy, and pharmacotherapy.However, these treatments are generally carried out when the disease is already in an advanced state.In addition, DR also affects retinal neuronal and glial cells.In fact, the visual loss occurring in the early stages of DR is related to retinal sensory dysfunction, mainly due to RGC loss.For these reasons, many studies are focusing on developing therapies that can mitigate or arrest disease progression before irreversible damage occurs[17,18].
MSC-based treatments
Several studiesin vitroor in animal models suggest that MSC-based strategies may represent a valuable tool for DR treatment.Main results are summarized in Table 1.A successful pericyte-like differentiation of ASCs was achieved by using specific culture media, as demonstrated by the expression of typical pericyte markers [α-smooth muscle actin, nerve/glial-antigen 2, platelet-derived growth factor receptor (PDGFR)] as well as by their perivascular localization in three-dimensional cultures[19,20].In anin vitromodel of the BRB, pericyte-like ASCs were also able to reinforce endothelial junctions, as indicated by the increased expression of VE-Cadherin, zone occludens-1 and occludin.In these experiments, also trans-endothelial electrical resistance values were increased[20].It is also suggested that a high glucose (HG) concentration does not affect the pericyte-like functions of ASCs[21,22].However, a conditioned medium (CM) from ASCs chronically cultured in HG conditions was able to normalize the HGchallenged retinal endothelial cells to normal glucose levels[23].HG exposure of retinal endothelial cells activated NF-κB and increased expression of downstream proinflammatory genes, such as interleukin (IL)-1B, VEGF and tumor necrosis factor (TNF).In bovine retinal endothelial cells, ASC-CM suppressed proinflammatory genes such as prostaglandin-endoperoxide synthase 2, and the proangiogenic gene VEGF.On the contrary, ASC-CM obtained using a shorter time of HG exposure did not induce beneficial effects.In vitroexperiments indicated that, acting through Notch signaling, ASCs induce a stabilization of DR dysfunctional microvascular networks; in fact, genetic knockdown of Notch2 in ASCs disturbed the vascular network formation of human umbilical cord vein endothelial cells[24].However,in vivoangiogenesis did not seem significantly influenced by Notch signaling, whose downregulation impairs ASC migratory ability, likely decreasing PDGFR expression.
Promising results were reported in experiments carried out in a murine model of DR[19].A retinal capillary dropout was prevented and the revascularization of the central retina was achieved by an intravitreal injection of native or pretreated ASCs.Following intravitreal injection, it was also reported that BMSCs can repair retinal damage by their engrafting as photoreceptors[25].In a mouse model of DR induced by streptozotocin, intravitreal administration of ASCs prevented RGC loss.In these studies, a retinal neuroprotection was obtained by reducing oxidative damage and increasing the intraocular levels of several neurotrophic factors[26].In particular, mRNA and protein levels of nerve growth factor (NGF), bFGF, and GDNF were increased in treated eyes compared to controls.In addition, mRNA and protein levels of trombospondin-1, a potent anti-angiogenic factor, also increased.In a similar rat model, intravitreal administration of ASCs was carried out two months after diabetes onset.Compared to controls, retinal histopathological evaluation revealed a significant decrease of vascular leakage and apoptotic cells around the retinal vessels.Moreover, a significantly improved ‘‘b’’ wave amplitude was observed in the electroretinogram.The authors suggest that ASCs may counteract retinal inflammation by blocking the up-regulation of several pro-inflammatory cytokines and DR-related genes[27].
Retinal stabilization against vascular insults was also reported in hyperglycemic Akimba mouse, by intravitreal ASC administration after retinal capillary dropout[28].To compare their therapeutic efficacy, ASCs from either healthy or diabetic mice were injected.It was found that ASCs from healthy mice were more effective than diabetic ones for retinal protection.Moreover, engrafted ASCs were found associated with the retinal vasculature.ASC-mediated vascular stability is likely due to the secretion of FGF, IGF binding protein-3, monocyte chemoattractant protein-1, and stromal cellderived factor (SDF-1).However, CM administration produced no significant effects, both when obtained from diabetic and healthy ASCs.However, small differences in the CM composition were noted: CM obtained from healthy mice was richer in some growth factors, such as IGF binding protein-3 and SDF-1.Dynamic effects were observed after intravitreal injection of both ASCs and their CM in mouse DR models.
Three weeks post-injection, mice treated with ASCs showed less vascular leakage and improved electroretinography tests, whereas visual acuity was not enhanced[17].However, all visual deficits were ameliorated following CM administration.The better CM performance in this case was likely due to a cytokine ASC pretreatment before CM collection.Despite some discrepancies, there is a general agreement that many beneficial effects are exerted by MSC-derived EVs, which contain a considerable amount of bioactive molecules, such as miRNAs, cytokines, and immunomodulatory factors[29].
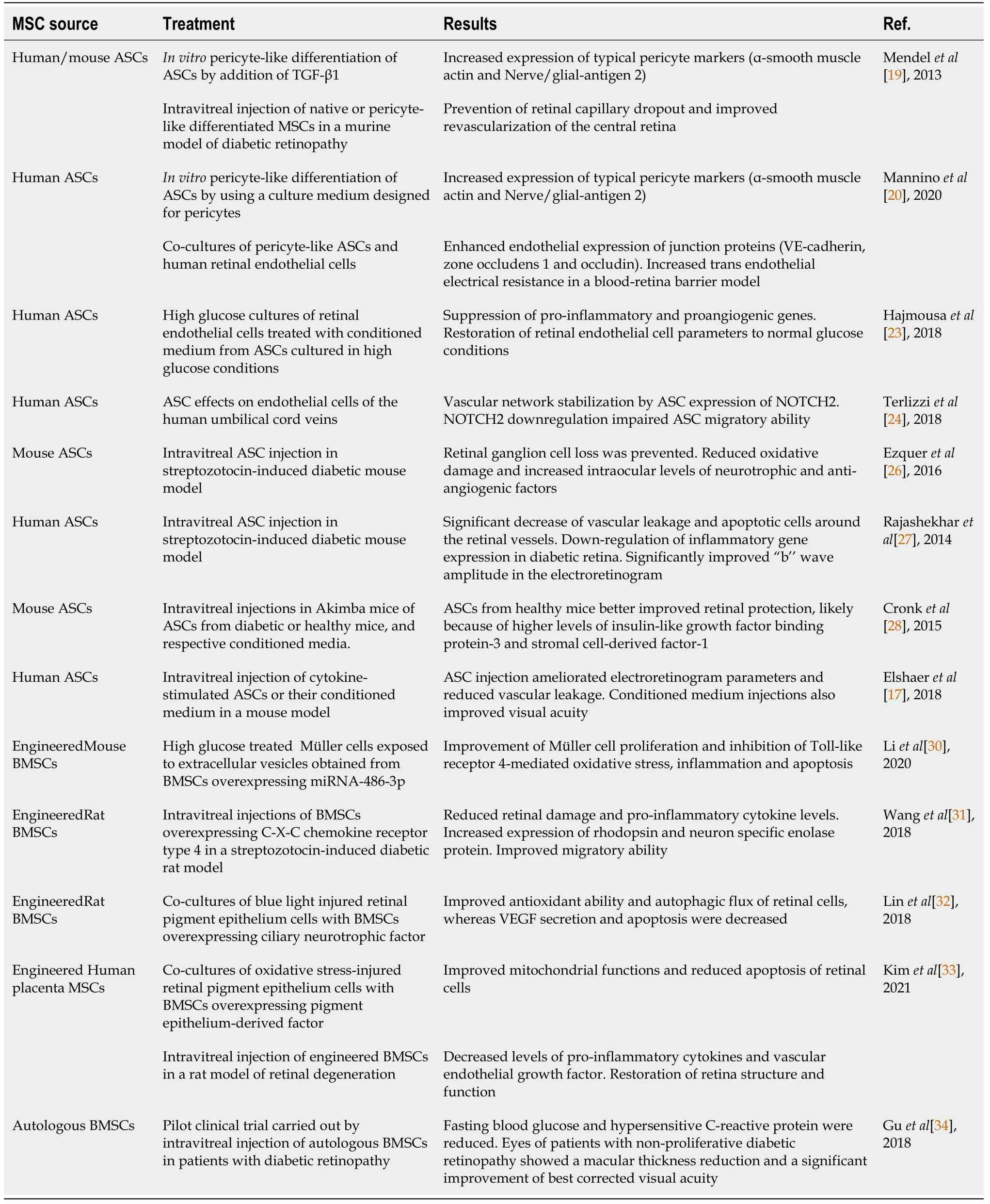
Table 1 Summary of the main results obtained in mesenchymal stem cell-based experiments for diabetic retinopathy
Genetically engineered MSCs
In anin vitrostudy, beneficial effects were observed in HG-treated Müller cells when exposed to EVs released by genetically engineered BMSCs.In particular, compared to control conditions, EVs from BMSCs overexpressing miRNA-486-3p were able to inhibit Toll-like receptor 4-mediated oxidative stress, inflammation and apoptosis, as well as promoting Müller cell proliferation[30].BMSC ability to home to the injury site is guided by SDF-1 through its C-X-C chemokine receptor type 4 (CXCR4)[31].To improve this ability, MSCs were infected by a lentivirus constructed with CXCR4.In vitroobservations revealed that transduced MSCs had high expression levels of the CXCR4 gene and protein, showing improved migration activities.When intravitreally injected, they reduced retinal injury in a DR animal model.In particular they overexpressed rhodopsin and neuron specific enolase, while decreasing the inflammatory cytokines IL-6 and TNF-α.The authors conclude that this strategy may be useful in DR treatment.The neuroprotective ability of CNTF-overexpressing BMSCs was tested in co-cultures with blue light-injured retinal pigment epithelium (RPE) cells[32].In fact, it was demonstrated that CNTF protected RPE cells and modulated the secretion of neurotrophic factors and cytokines.Results showed an improved antioxidant ability and autophagic flux of RPE cells, whereas VEGF secretion and apoptosis were decreased.In another investigation, placenta-derived MSCs were genetically modified to overexpress pigment epithelium-derived factor (PEDF).They were then able to maintain stemness and showed an increased mRNA expression of biogenesis regulators and antioxidant enzymes[33].When co-cultured with RPE cells injured by oxidative stress, engineered MSCs improved mitochondrial functions and reduced apoptosis by activating the cell survival JNK/ERK signaling pathway.Beneficial effects were also observed after their intravitreal administration in a rat model with H2O2-induced retinal degeneration.In fact, proinflammatory cytokines and VEGF expression decreased, whereas an increased PEDF expression promoted a restoration of retina structure and function.
Clinical trials
In a pilot clinical trial, the safety and efficacy of intravenous administration of autologous BMSCs were evaluated for the treatment of patients with proliferative or non-proliferative DR.Results show that fasting blood glucose and hypersensitive Creactive protein were reduced in both groups of patients, whereas no significant differences were observed concerning hemoglobin A1Cor IL-6 levels.Only eyes of patients with non-proliferative DR showed a macular thickness reduction and a significant improvement of best corrected visual acuity (BCVA)[34].
GLAUCOMA
More than 70 million people suffer irreversible blindness due to glaucoma.Whereas primary glaucoma is of unknown origin, secondary glaucoma is associated with identifiable causes leading to increased IOP, optic nerve injury, and vision loss[29].Moreover, two major types of glaucoma are genetically distinct: closed-angle and open-angle glaucoma.Overall, glaucoma is an age-related multifactorial and slow progressive eye disease, in which IOP elevation may result from an increased production and/or decreased outflow of aqueous humor, generally consequent to the degeneration of the trabecular meshwork (TM), the ocular tissue regulating aqueous humor outflow[35].The main consequences comprise degeneration of RGCs and their axons, optic nerve shrinkage, and changes in brain visual areas[36].The slow progression of the disease requires long-term ophthalmic medications, and 15%-25% of patients progressively lose sight, even if IOP is kept under control.Other mechanisms are likely involved in disease progression[29,37-39].Lamina cribrosachanges may reduce RGC axonal transport and favor apoptotic mechanisms[40].In fact, RGC axons normally support the retina with various neurotrophic factors, such as NGF, BDNF, CNTF, and GDNF.Since RGC regeneration is problematic[29], their loss or dysfunction severely impairs vision and quality of life.By targeting TM, the main therapeutic approaches for glaucoma are currently aimed at reducing IOP, and avoiding further RGC loss, but these interventions only delay disease progression.Indeed, some studies report the presence of MSC-like cells within the TM and between the TM and the corneal endothelial periphery, but they are not able to efficiently restore cell damage[41].
MSC-based treatments
Results obtained following MSC-based treatments are summarized in Table 2.Most of them indicate an improved RGC survival, a production of functional RGC-like cells, and an expansion and differentiation of resident retinal stem cells in mature RGCs[42,43].Although intravitreal administration often produces an exiguous number of MSC engraftments and retinal differentiation, beneficial effects are also attributable to MSC release of neurotrophic factors[36].Co-culturing BMSCs with pigmented cells from the ciliary margin, the photoreceptor specific marker, cone-rod homeobox (Crx), was found increased at both mRNA and protein expression levels[43].In addition, MSC retinal differentiation was supported by increased percentages of cells that were immunopositive for typical retinal markers such as rhodopsin, visual system homeobox 2 and heparin sulphate.In fact, these markers are specific for rods, bipolar neurons and Müller glia, respectively.Many studies have been aimed at promoting TM regeneration; for example, Manuguerra-Gagnéet al[44] reported that restored IOP levels were observed in a rat model after BMSC administration into the anterior chamber, this was likely due to paracrine mechanisms.Neuroprotective effectsviaTM protection were also reported in another study by BMSC injection in a rat model of glaucoma-like ocular hypertension[45].Rapid and long-lasting BMSC-induced effects were associated with MSC engrafting to the ciliary processes and the TM.The same authors also showed that increased TM cellin vitrosurvival was obtained using MSCCM, likely activating the anti-apoptotic pathway Akt.In fact, MSC anti-apoptotic effects had been previously reported[46].At the same time, TM cell relaxation was induced by decreasing myosin phosphorylation and the TGF-β2-dependent profibrotic phenotype was inhibited.In a laser-induced rat model of glaucoma, it was reported that only local BMSC administration, but not systemic administration, induced neuroprotective effects by improving RGC axon survival[47].Most BMSCs were preferentially located to a perivascular position, and some of them migrated within the nerve fibers and the retinal ganglion cells.In co-cultures of retinal explants with rat or human BMSCs, a reduction of apoptosis and an RGC survival increase was observed.In fact, compared to cultures of the retina alone, a high density of cells expressing Islet-1 and NeuN was observed in the RGC layer.On the contrary, the addition of fibroblasts in analogous conditions was not able to reproduce these effects.Although inducing lesser neuroprotective effects, also the use of BMSC-CM has been proven effective, compared with fibroblast-CM.The differential analysis between the two conditioned media revealed that a significant difference was related to the presence of PDGF, which was contained only in BMSC-CM.It was then concluded that PDGF is responsible for these neuroprotective effects[48].According to Meadet al[38], most MSC positive effects are due to their paracrine release of neurotrophic factors and EVs.Intravitreal BMSC- or fibroblast-EV administration was carried out on two glaucoma rat models obtained by intracameral injection of microbeads or laser photocoagulation of the TM and limbal vessels.Data reported that decreased RGC apoptosis was obtained following BMSC-EV treatment, rather than fibroblast-EV injection.BMSCmiRNA would play an important role in RGC regeneration mechanisms by modulating the expression of the phosphatase and tensin homolog, a suppressor of RGC axonal growth and survival.Overall, more evident improvements were found in aged rats, characterized by a faster RGC loss when compared with young animals[37].

Table 2 Summary of the main results obtained in mesenchymal stem cell-based experiments for glaucoma
Genetically engineered MSCs
In a work by Suet al[49], BMSCs were co-cultured with RGCs in a transwell system mimickingin vivoconditions.Whereas RGCs cultured alone showed significantly decreased cell viability if subjected to oxygen-glucose deprivation and reperfusion, cocultures with MSCs significantly increased RGC viability in a dose-dependent manner.Improved RGC survival was likely due to MSC-induced production of stanniocalcin 1, thus inhibiting caspase-8-mediated apoptosis.MSC transfection with miRNA-21, essential for stanniocalcin 1 production, further ameliorated RGC survival.
Clinical trials
No significant effects have been described to date in clinical trials.In patients with advanced glaucoma, intravitreal injection of autologous BMSCs did not induce appreciable improvements on visual acuity or the visual field[50].
RETINITIS PIGMENTOSA
Retinitis pigmentosa (RP) is a genetic disease affecting 1.5 million people worldwide.It leads to night blindness, tunnel vision, progressive central vision loss and total blindness.Modified molecular processes involved in the turnover of rhodopsin progressively induce the total loss of rod cells[51-53].Subsequently, an increase of reactive oxygen species (ROS) levels occurring in RPE induces cone loss at later stages[54-57].In fact, ROS oxidize fatty acids triggering inflammation, synaptic injury, apoptosis, and cell death[58].Since there are no satisfactory therapeutic approaches for this disease, new strategies are being developing to replace or repair damaged or dead cells by using stem cell therapy[59,60].
MSC-based treatments
MSC intraocular administration would improve visual function[3,61-63] through different mechanisms: cell replacement following MSC trans-differentiation, paracrine effects, or inflammation modulation.In addition, also electroretinographic parameters could be improved[61,62,64].Main results are summarized in Table 3.
In vitrostudies have reported the ability of MSCs to differentiate into retinal progenitor cells, photoreceptors, and retinal neuron-like cells[65,66].It has been shown that either in the presence of retinal cells, conditioned media from retinal cell cultures, or retinal cell extracts, MSCs differentiate into retinal-like cells, expressing typical genes and markers[67].By using a taurine containing induction medium, CJMSCs were differentiated into photoreceptor-like cells in fibrin gel, as indicated by the expression of typical markers such as Nestin, rhodopsin, and RPE65.The authors claim that this 3D scaffold, fibrin gel-based strategy may lead to increased availability of photoreceptor progenitor cells for retina repair and regeneration[68].
When BMSCs were intravitreally injected in a rat model of retina degeneration, they expressed rhodopsin and pancytokeratin (PCK) markers.Rhodopsin indicates a photoreceptor-like differentiation, while PCK expression is indicative of an RPE-like phenotype[65].Injected in the subretinal space of rhodopsin knockout mouse, engrafted BMSCs were found not only in the RPE, but also in the neuroretina, acquiring neuronal and glial morphologies, and prolonging photoreceptor survival[69].In a rat model of RP, intravenous BMSC administration before photoreceptor loss was able to preserve both rod and cone cells.By multiunit activity recording from the superior colliculus, it was concluded that also visual functions, such as BCVA and luminance threshold, were significantly preserved[70].
Intravitreal injection of mouse ASCs or human UCMSCs, and their derived EVs, promoted repair and protective actions in rodent models of laser-induced retinal damage[71].It was found that both MSCs and their exosomes induced comparable beneficial effects.They were able to reduce retinal damage, inhibit apoptosis, and suppress inflammatory responses, thus obtaining an improved visual function.
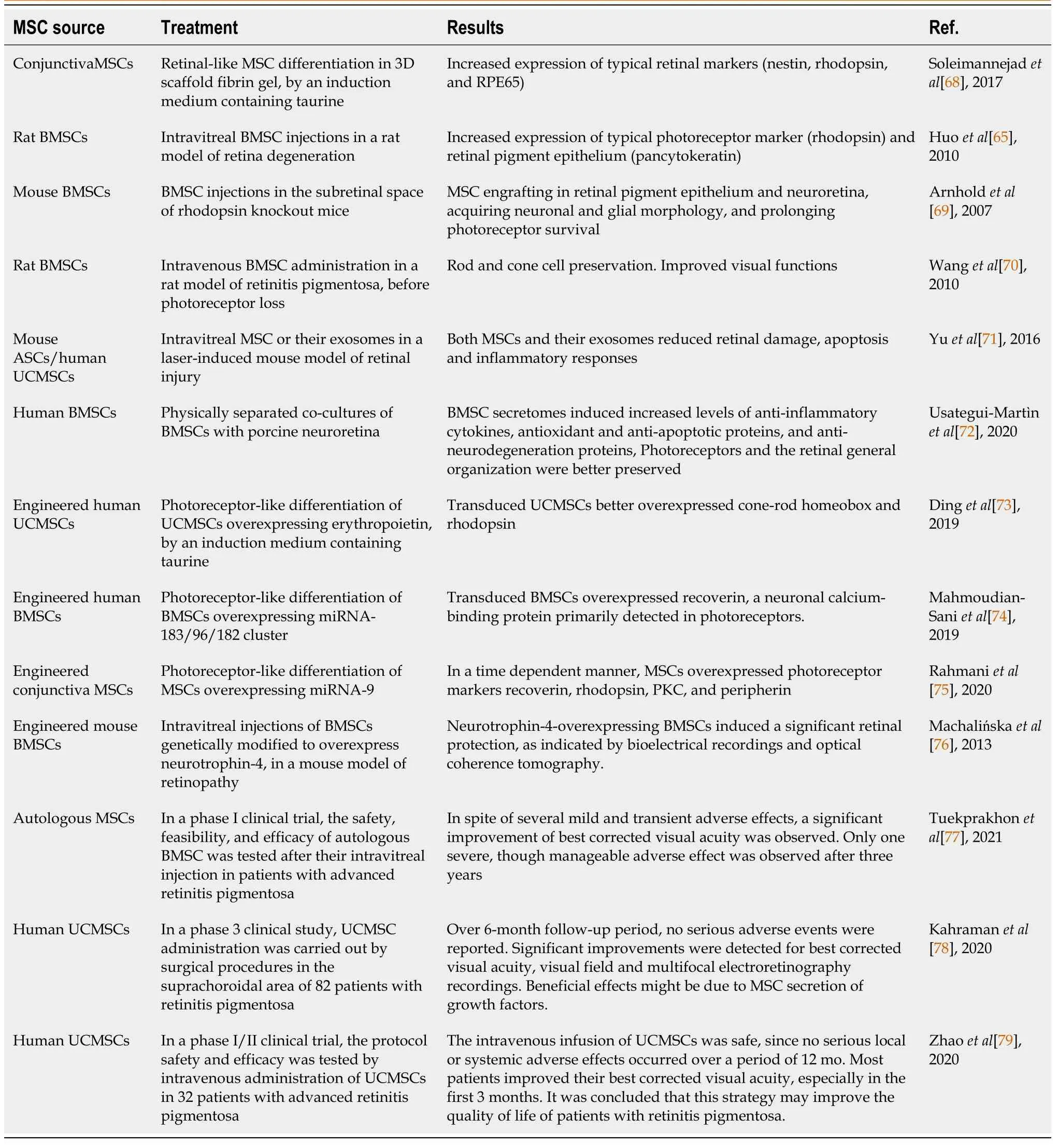
Table 3 Summary of the main results obtained in mesenchymal stem cell-based experiments for retinitis pigmentosa
In physically separated co-cultures of BMSCs with neuroretinas, it was found that neuroprotective effects were induced by BMSC secretomes.In particular, anti-inflammatory cytokines, antioxidant and anti-apoptotic proteins, as well as anti-neurodegeneration proteins, were increased in these co-cultures.Probably by secreting these neuroprotective molecules, photoreceptors and the retinal general organization were better preserved[72].
Genetically engineered MSCs
In anin vitrostudy, UCMSCs were tested for photoreceptor differentiation by using a specific culture medium containing taurine[73].Indeed, compared to control cultures, taurine-treated cells showed an increased expression of Crx and rhodopsin.However, an enhanced expression of these photoreceptor markers was obtained when UCMSCs were previously transduced with lentivirus particles encoding erythropoietin (EPO).In fact, EPO was proven to play a role in neuro-regeneration and protection, cell differentiation and rescue potential on dying photoreceptor cells.Since the fluorescence intensity was higher in rhodopsin-positive cells, it was suggested that a rod photoreceptor differentiation was favored.
In anin vitrowork by Mahmoudian-Saniet al[74], human BMSCs were induced to differentiate into photoreceptor cellsviatransfection with the miR-183/96/182 cluster.Results showed that recoverin, a neuronal calcium-binding protein primarily detected in photoreceptors, was upregulated.Therefore, the authors concluded that overexpression of miR-183 can induce a successful reprogramming of BMSCs into photoreceptor cells.
A new approach was introduced by Rahmaniet al[75] to guide the differentiation of CJMSCs into photoreceptor-like cells by miRNA-9 transduction.By using neither chemical nor biological growth factors, transduced cells were able to express, in a time-dependent manner, photoreceptor markers such as recoverin, rhodopsin, PKC, and peripherin.In particular, rhodopsin was the most enhanced marker after miRNA-9 overexpression.
BMSCs genetically modified to overexpress neurotrophin-4 (NT-4) were intravitreally injected in a mouse model of retinopathy[76].NT-4-overexpressing BMSCs migrated into the injured retina, actively producing NT-4 and inducing a significant retinal protection.In particular, the preservation of retinal bioelectrical activity was indicated by electroretinography, and a complete restoration of the retina organization was shown by optical coherence tomography.Overall, the authors claim that this treatment induced a positive impact on neural cell survival, growth, and differentiation.
Clinical trials
In a phase I clinical trial, Tuekprakhonet al[77] investigated the safety, feasibility, and efficacy of autologous BMSC intravitreal injection in patients with advanced RP.In three groups of patients, BCVA, visual field (VF), central subfield thickness (CST), and subjective experiences were evaluated over 12 mo.In spite of several mild and transient adverse effects, a statistically significant improvement of BCVA was observed, whereas VF and CST remained stable, indicating no further progression of the disease.Only one severe, though manageable, adverse effect was observed after three years.
In a phase 3 clinical study, UCMSC administration was carried out in the suprachoroidal area of 82 RP patients by a surgical procedure[78].Over a 6-month follow-up period, no serious adverse events or ocular complications were reported.Overall, statistically significant improvements in BCVA and VF were observed, as well as improvements in multifocal electroretinography recordings.The authors conclude that beneficial effects may possibly be due to MSC secretion of growth factors.
In a phase I/II clinical trial, intravenous administration of UCMSCs was performed in 32 advanced RP patients to evaluate the safety and efficacy protocol[79].After a single dose injection, all subjects were followed-up over a period of 12 mo.Results showed that the intravenous infusion of UCMSCs was safe, since no serious local or systemic adverse effects occurred.Most patients improved their BCVA, especially in the first 3 mo.It was concluded that the intravenous infusion of UCMSCs may improve the quality of life of advanced RP patients.
CONCLUSION
In the last few decades, stem cell-based strategies have been extensively explored to develop therapeutic protocols aimed at counteracting human pathologies when other remedies fail.Although a variety of stem cells feature formidable potential therapeutic applications, in many cases they cannot be currently used because of ethical issues and/or safety problems.MSCs probably represent an acceptable compromise, being easily available even for autologous administration.Many experimental data obtained both inin vitrostudies and in animal models report encouraging results, although after transplantation, only a limited fraction of injected cells really engrafted and differentiated into functional cells.Because of the wide MSC paracrine activity, also cell-free treatments have been explored with positive effects.Genetic manipulations of MSCs have also been performed to improve their ability to home to damaged sites and to overexpress cytokine/growth factors helping retinal restoration.Many clinical trials have already been carried out and, despite some adverse effects, promising outcomes are reported.It is not difficult to assume that in the near future, safer and more effective protocols will be designed for clinical applications, to improve the quality of life of patients affected by eye disease.
杂志排行
World Journal of Stem Cells的其它文章
- Genome engineering and disease modeling via programmable nucleases for insulin gene therapy; promises of CRISPR/Cas9 technology
- Immunotherapy in the treatment of lymphoma
- Recent trends in stem cell-based therapies and applications of artificial intelligence in regenerative medicine
- Epigenetic regulation of autophagy: A key modification in cancer cells and cancer stem cells
- Review of the potential of mesenchymal stem cells for the treatment of infectious diseases
- Growing and aging of hematopoietic stem cells
