A Preliminary Investigation of Pathogenic Fungi from Lotus (Nelumbo nucifera Gaertn) in Nanchang City
2021-07-24HUANGJingyueTANGHongGUBoTUJuanjuanHUANGJiawenZHONGChengHUDianming
HUANG Jingyue, TANG Hong, GU Bo, TU Juanjuan, HUANG Jiawen, ZHONG Cheng, HU Dianming
A Preliminary Investigation of Pathogenic Fungi from Lotus (Gaertn) in Nanchang City
HUANG Jingyue1,2, TANG Hong3, GU Bo1, TU Juanjuan1, HUANG Jiawen1, ZHONG Cheng1, HU Dianming1,2*
(1. School of Biological Sciences and Engineering, Jiangxi Agricultural University, Nanchang 330045, China; 2. Bioengineering and Technological Research Centre for Edible and Medicinal Fungi, Jiangxi Agricultural University, Nanchang 330045, China; 3. Jintan Town Government Service Center of Jishui County, Ji’an 331600, China)
Lotus (Gaertn) is an economically important aquatic plant in China. Fungal disease is a serious problem in lotus cultivation. In this study, the pathogenic fungi on lotus in Nanchang City were investigated to lay the foundation for the disease control.Lotus leaves and stems in ponds of Nanchang City were collected, the fungi on leave/stem spots were isolated and purified. Colonies morphological characters and ITS sequences were used to identify the strains.49 strains were isolated and identified to 20 species, belonging to 12 genera.15 species may firstly be reported on lotus in this study, i.e.,,,,,,,,,,,,,,, and
taxonomy; lotus leaf; pathogenic fungi; fungal diversity
Introduction
Lotus (Gaertn.) is an economically important aquatic plant in China. It is a well known and popular vegetable for its nutritious rhizomes and seeds[1]. The planting area of lotus in China is about 100 000 hm2, mainly distributed in the middle and lower reaches of the Yangtze River Basin[2].
Lotus rhizome is a nutritionally balanced food—low in fat but high in protein, minerals and vitamins, and is commonly found in the dishes in China. Studies have shown that the whole plant of lotus has various pharmacological effects. Flavonoid-enriched extracts from lotus leaves inhibits proliferation of breast cancer in vitro and in vivo[3]. Natural antioxidant components with antiviral and immunoregulatory activities, which could be potentially important for anti HIV-1 drug development and application to HIV-1 therapy, were reported from lotus rhizome[4].
Fungal disease is a serious problem in lotus cultivation. For example, rhizome rot, a prevalent disease caused byor, can cause yield loss of up to 80%, or even total loss[1]. Leaf spot of lotus () caused bywas repoeted in China[5].andplant parasitic fungi on lotus have been recorded in Taiwan for the first time[6]. And the powdery mildew(Erysiphales, Ascomycota) has been recorded on lotus based on a collection from the Botanical Garden in Frankfurt am Main, Germany[7].
About 23 300-26 700 hm2of lotus are planted in Jiangxi Province[8], however, no systematic investigation into pathogenic fungi on lotus in this area was reported. In this study, a preliminary investigation into pathogenic fungi from lotus was conducted in Nanchang City to lay the foundation for the disease control of lotus.
1 Materials and Methods
1.1 Samples collection
Lotus leaves or stems with obvious lesions or spots were collected in ponds of Nanchang City. Samples were individually placed into zip lock plastic bags, returned to the laboratory and kept at ca. 4 °C until further processing.
1.2 Isolation and cultivation of fungal strains
Small tissue blocks (ca. 2 cm×2 cm) from the junction of the spots/lesions of leaves or stems were cuted for further processing. The tissue blocks were surface sterilized (75% alcohol 30 seconds, sterilized water 60 seconds), then put in a peri dish with potato dextrose agar (PDA) medium, and incubated at 27 ℃. Mycelia growing on the margin of tissue blocks were isolated and cultivated on PDA medium at 27 ℃. All the fungal strains were deposited in the culture collection of Jiangxi Agriculture University (JAUCC).
1.3 Morphological studies
Colonies of fungal strains on PDA were observed, described and photographed.
1.4 DNA analysis
The whole genomic DNA were exacted from pure culture mycelium with CTAB method[9]. The internal transcribed spacer (ITS) DNA fragments were amplified with primers ITS1 and ITS4 according to the method described by Hu et al.[10], then sequenced at Tsingke Biological Technology Company, Beijing. Phylogenetic trees of each genera in this study were constructed based on ITS sequences by the methods described by Hu et al.[10].
2 Results
2.1 Isolation and purification of fungal strains
A total of 49 strains were isolated in this study (Table 1). Combining morphology and ITS rDNA phylogenetic analysis, the isolates were classified into 12 genera and 20 species.,,,, andare common genera in this study.

Table 1 Strains isolated in this study

Table 1 Strains isolated in this study

JAUCC 3245F. solaniNectriaceaeHypocrealesSordariomycetes JAUCC 3248Neofusicoccum parvumBotryosphaeriaceaeBotryosphaerialesDothideomycetes JAUCC 3249Nigrospora sphaericaBotryosphaeriaceaeBotryosphaerialesDothideomycetes JAUCC 3250Phomopsis eucommiiDiaporthaceaeDiaporthalesSordariomycetes JAUCC 3251Sclerotium hydrophilumIncertae sedisIncertae sedisIncertae sedis
2.2 DNA analysis
The Maximum likelihood (ML) phylogenetic trees were costructed in this study. The Phylogenetic tree of the genus(Figure 1) showed that the 16 strains (JAUCC 3203, JAUCC 3204, JAUCC 3244, JAUCC 3246, JAUCC 3206, JAUCC 3207, JAUCC 3209, JAUCC 3205, JAUCC 3214, JAUCC 3208, JAUCC 3210, JAUCC 3211, JAUCC 3212, JAUCC 3213, JAUCC 3215, JAUCC 3216) culstered in theclade. In Figure 2, three strains (JAUCC 3217, JAUCC 3219, JAUCC 3218) clustered in theclade. In Figure 3, two strains (JAUCC 3220, JAUCC 3221) clustered in theclade. In Figure 4, six strains (JAUCC 3222, JAUCC 3223, JAUCC 3224, JAUCC 3225, JAUCC 3226, JAUCC 3227) clustered in theclade. In Figure 5, the strain JAUCC 3229 clusterd in theclade. In Figure 6, 12 strains (JAUCC 3230, JAUCC 3231, JAUCC 3232, JAUCC 3233, JAUCC 3234, JAUCC 3235, JAUCC 3236, JAUCC 3237, JAUCC 3238, JAUCC 3239, JAUCC 3240, JAUCC 3241) clusterd in theclade. In Figure 7, the strain JAUCC 3242 clusterd inclade. In Figure 8, the strain JAUCC 3243 and JAUCC 3247 clusterd in theclade. In Figure 9, the strain JAUCC 3248 clusterd in theclade. In Figure 10, the strain JAUCC 3249 clusterd inclade. In Figure 11, the strain JAUCC 3250 clusterd inclade. In Figure 12, the strain JAUCC 3251 clusterd inclade.

Figure 1 Phylogenetic tree of the genus
Figure 2 Phylogenetic tree of genus

Figure 3 Phylogenetic tree of the genus
Figure 4 Phylogenetic tree of the genus

Figure 5 Phylogenetic tree of the genus
Figure 6 Phylogenetic tree of the genus

Figure 7 Phylogenetic tree of the genus
Figure 8 Phylogenetic tree of the genus

Figure 9 Phylogenetic tree of the genus
Figure 10 Phylogenetic tree of the genus

Figure 11 Phylogenetic tree of the genus
Figure 12 Phylogenetic tree of the genus
2.3 Taxonomy
2.3.11)(Fr.) Keissl., Beih. bot. Zbl., Abt. 2 29: 434 (1912)

a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 13
Colonies grew fast on the PDA, light gray, being covered with off-white cotton-like hyphae; the reverse side was brown.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 5, NCLL 15, NCLL 17, NCLL 31, NCLL 34).
2)E.G. Simmons, Mycotaxon 25(1): 198 (1986)

a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 14
Colonies grew fast on PDA, white, being covered with white fluffy hyphae; the reverse side was white.
Specimen: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 32, NCLL 33).
3)(Cooke) McClellan, Phytopathology 34: 229 (1944)

a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 15
Colonies grew fast on the PDA, gray-black, being covered with white gray-white plexiform hyphae; the reverse side was brown-black.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 37).
4)(Ellis & G. Martin) L.R. Jones & Grout, Annual Report of the Vermont Agricultural Experimental Station 9: 86 (1896)

a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 16
Colonies grew fast on the PDA, light brown, with small amount of white hyphae; the reverse side was brown to white.
Specimen: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 6 , NCLL 16).
5)(Kunze) Wiltshire, Trans. Br. mycol. Soc. 18(2): 157 (1933)

a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 17
Colonies grew fast on the PDA, gray-black, with gray hairy hyphae; the reverse side black green.
Specimen: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 4, NCLL 7, NCLL 16, NCLL 24, NCLL 35).
2.3.2 Arthrinium(Corda) Dyko & B. Sutton, Mycotaxon 8 (1): 119 (1979)

a: The reverse side of a colony on PDA, b-c: The obverse side of a colony on PDA, d: Substate
Figure 18
Colonies grew fast on the PDA, light gray, with gray hairy hyphae, being covered with white fluffy hyphae; the reverse side was white.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 22, NCLL 37, NCLL 38 ).
2.3.3 Botryosphaeria(Moug.) Ces. & De Not., Comm. Soc. crittog. Ital. 1(fasc. 4): 212 (1863)

a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 19
Colonies grew fast on the PDA, black, being covered with gray hairy hyphae; the reverse side was black.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 20, NCLL 28).
2.3.41) Colletotrichum(Penz.) Penz. & Sacc., Atti Inst. Veneto Sci. lett., ed Arti, Sér. 6 2: 670 (1884)

a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 20colony morphology
Colonies grew fast on the PDA, gray, being covered with downy hyphae; the reverse side was dark brown.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 3, NCLL 4, NCLL 5, NCLL 8, NCLL 26).
2)Prihast., L. Cai & K.D. Hyde, in Prihastuti, Cai, Chen, McKenzie & Hyde, Fungal Diversity 39: 98 (2009)

a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 21colony morphology
Colonies grew fast on the PDA, gray, being covered with gray-white plexiform hyphae; the reverse side was dark blue to gray.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 26).
2.3.5(Bainier) Boedijn, Bull. Jard. bot. Buitenz, 3 Sér. 13(1): 127 (1933)
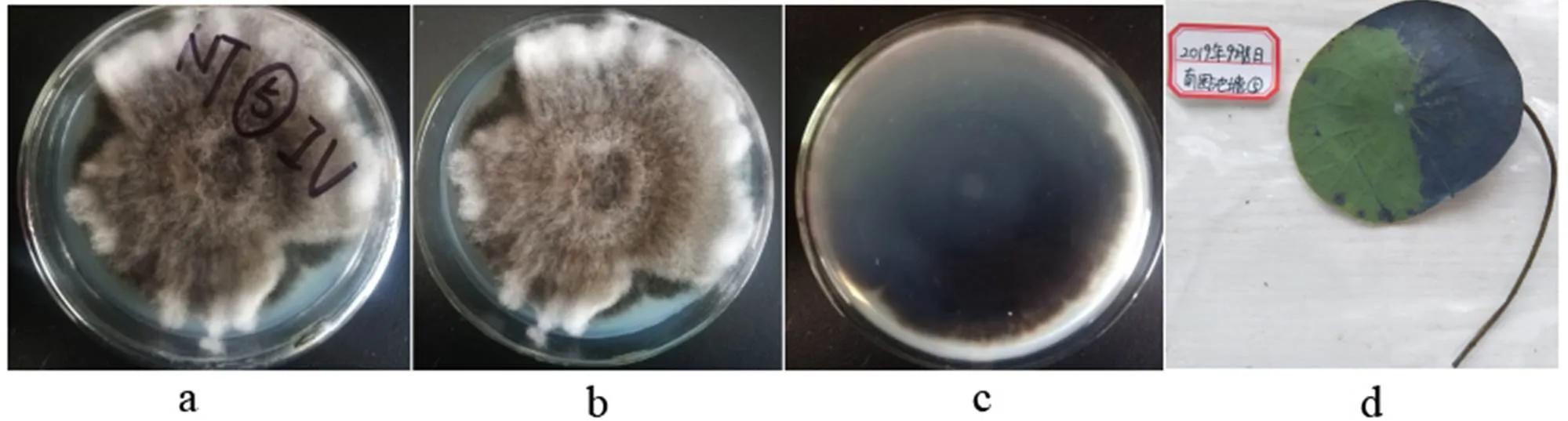
a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 22colony morphology
Colonies grew fast on the PDA, gray-black, being covered with hairy hyphae; the reverse side was black brown.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 8, 2019, Huang Jingyue (NCLL 5).
2.3.61)(Cooke & Ellis) Sacc., Syll. fung. (Abellini) 1: 692 (1882)

a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 23
Colonies grew fast on the PDA, white, being covered with white hyphae; the reverse side was brown.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 20).
2)Sacc. & Speg., Michelia 1 (no. 4): 386 (1878)

a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 24
Colonies grew fast on the PDA, light gray, being covered with white flocculent hyphae; the reverse side was white grey.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 16, NCLL 24).
3)Doilom, Dissan. & K.D. Hyde, in Doilom et al., Fungal Diversity: 10.1007/s13225-016-0368-7 (2016)
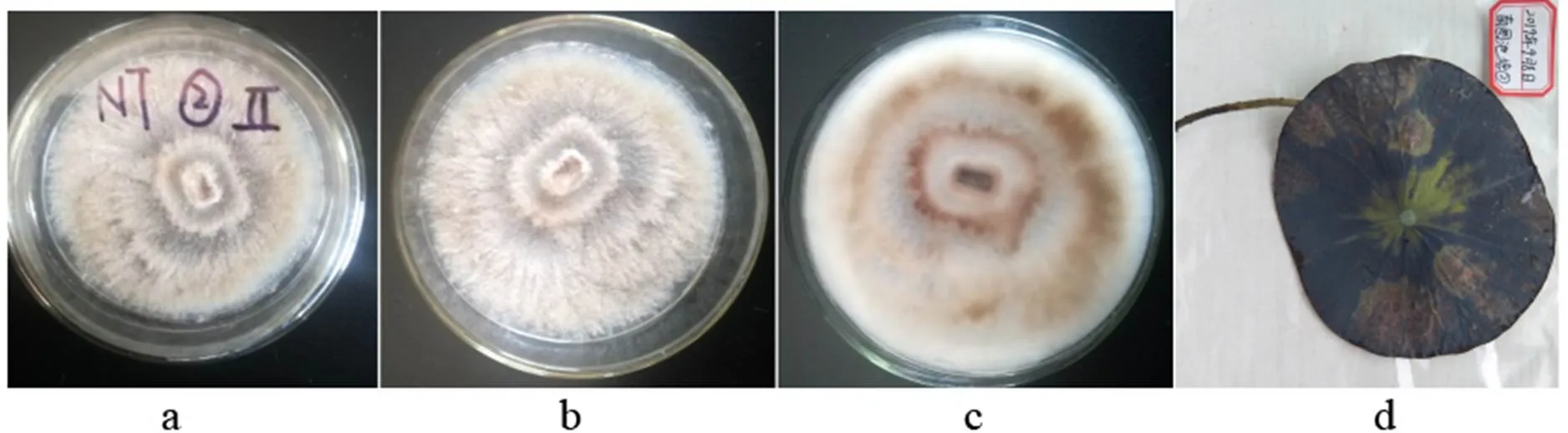
a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 25
Colonies grew fast on the PDA, light gray, being covered with creeping light brown hyphae; the reverse side was yellow-brown to white.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 8, 2019, Huang Jingyue (NCLL 2, NCLL 4, NCLL 5, NCLL 9).
2.3.7Link, Mag. Gesell. naturf. Freunde, Berlin 7: 32 (1816) [1815]

a: Front side, b: Front side (open the petri dish), c: Back side, d. Substate
Figure 26
Colonies grew fast on the PDA, yellow, being covered with white cotton-like hyphae; the reverse side was yellow-brown.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 33).
2.3.81)(Matsush.) Nirenberg, Mitt. biol. BundAnst. Ld- u. Forstw. 169: 38 (1976)

a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 27
Colonies grew fast on the PDA, gray-black, being covered with a small amounts of hairy hyphae; the reverse side was gray-white.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 8, 2019, Huang Jingyue (NCLL 3, NCLL4).
2)(Mart.) Sacc., Michelia 2(no. 7): 296 (1881)
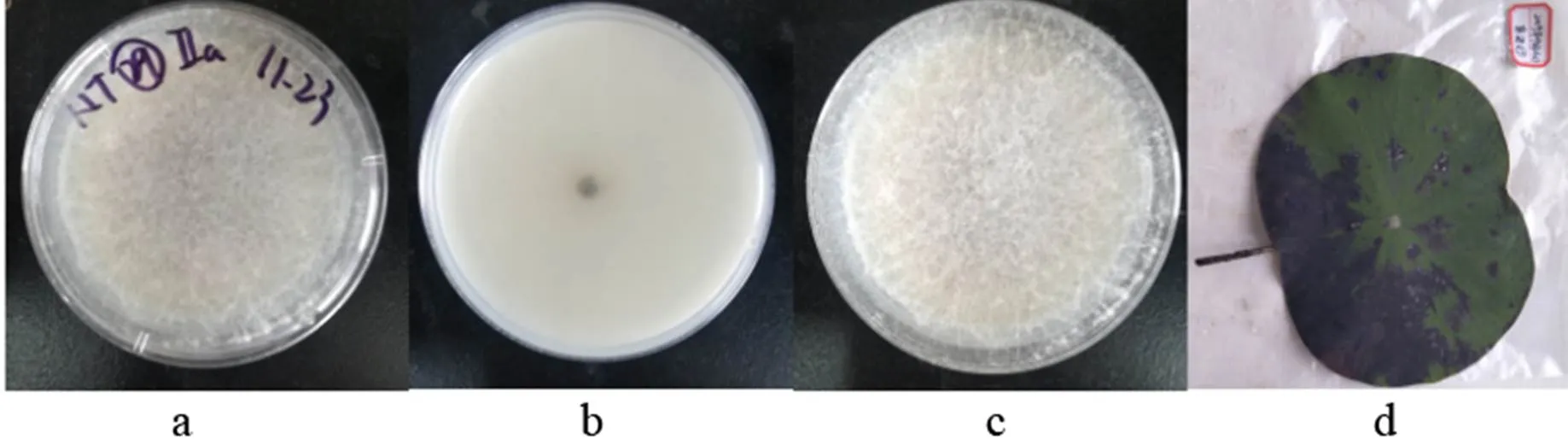
a,c: The obverse side of a colony on PDA, b: The reverse side of a colony on PDA, d: Substate
Figure 28
Colonies grew fast on the PDA, gray-black, being covered with gray-white fluffy hyphae; the reverse side was white.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 29).
2.3.9(Pennycook & Samuels) Crous, Slippers & A.J.L. Phillips, in Crous, Slippers, Wingfield, Rheeder, Marasas, Phillips, Alves, Burgess, Barber & Groenewald, Stud. Mycol. 55: 248 (2006)

a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 29
Colonies grew fast on the PDA, black, being covered with white clump hyphae; the reverse side was black and white.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 27).
2.3.10(Sacc.) E.W. Mason, Trans. Br. mycol. Soc. 12 (2-3): 158 (1927)

a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 30
Colonies grew fast on the PDA, light yellow, being covered with white flocculent hyphae; the reverse side was yellow.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 28).
2.3.11C.Q. Chang, Z.D. Jiang & P.K. Chi, Mycosystema 24 (2): 147 (2005)

a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 31colony morphology
Colonies grew fast on the PDA, white; the reverse side was light brown.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 20).
2.3.12Sacc. & P. Syd., Syll. fung. (Abellini) 14 (2): 1141 (1899)
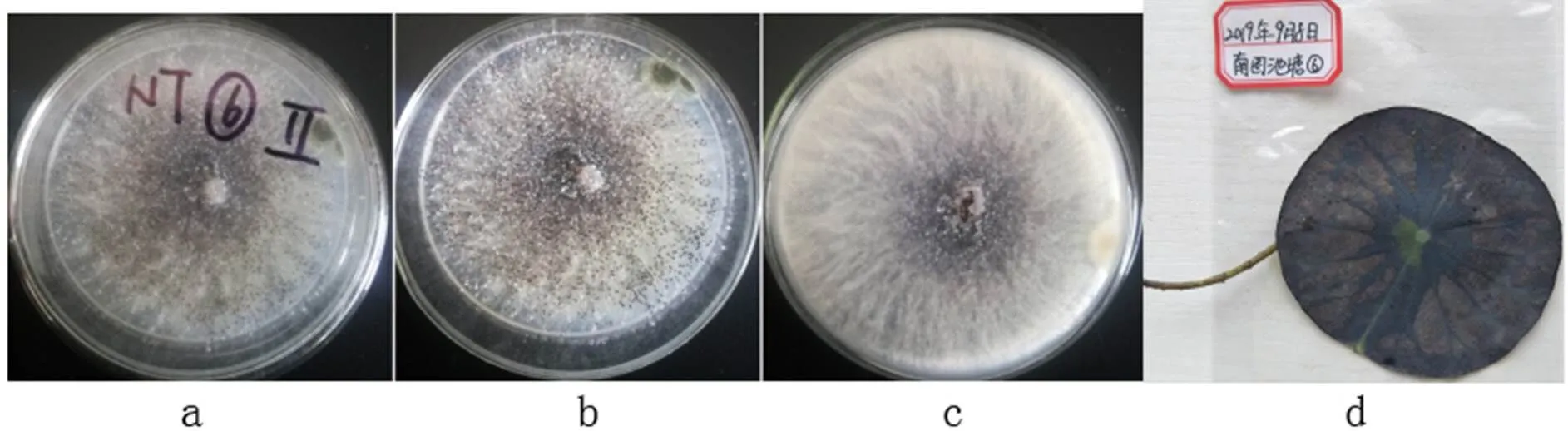
a-b: The obverse side of a colony on PDA, c: The reverse side of a colony on PDA, d: Substate
Figure 32 Sclerotium hydrophilum
Colonies grew fast on the PDA, light gray, being covered with white flocculent hyphae; the reverse side was white.
Specimens: Nanchang City, Jiangxi Province, China, near 29°11' N and 116°35' E, on lotus leave lesions in a pond, September 23, 2019, Huang Jingyue (NCLL 6).
3 Discussion
In this study, 20 species were isolated from the leave spots of lotus, namely,a,,,,,,,,,,,,,,,,,,,. It is the first investigation into the lotus pathogenic fungi in Nanchang City.
Zheng et al.[11]listed 18 taxa of pathogenic fungi, i.e.(Ell.et Ev.) Enlows et Rand.,sp.,Pers. Ex Fr.,Sakaguchi.,sp.,Penz.,(Bert. Et Curt.),Jain.,,(Zinss meister) Scholten.,Nomura,,sp.,Cooke et Massee.,Speg.,sp.,(Cke.et Ell.) Deighton.,Sacc. Among the 18 taxa, onlywas isolated in our study.
Chen and Kirschner listed 42 fungal species on lotus, among which,,,andwere isolated in this study.
To our knowledge, 15 species may firstly be reported on lotus in this study, i.e.,,,,,,,,,,,,,,Unfortunately, the microscopic characters of these species were not obtained. Morphological characters of colonies combined with ITS sequences data are not sufficient to precisely identify these species. And the pathogenicity of these species to lotus need to be confirmed by cultivation experiment.
[1] Yin X, Li X Z, Yin J J, et al. First report ofcausing rhizome rot of Asian lotus in China[J]. Plant disease, 2016, 100(2): 532.
[2] He J, Chen X, Fan C, et al. Suggestions on development of seed lotus industry in China [J]. Journal of Changjiang vegetables, 2018(24): 36-39.
[3] Yang M Y, Chang Y C, Chan K C, et al. Flavonoid-enriched extracts fromleaves inhibits proliferation of breast cancer in vitro and in vivo[J]. European journal of integrative medicine, 2011, 3(3): 153-163.
[4] Jiang Y, Ng T B, Liu Z, et al. Immunoregulatory and anti-HIV-1 enzyme activities of antioxidant components from lotus (Gaertn.) rhizome[J]. Bioscience reports, 2011, 31(5): 381-390.
[5] Zhang Q H, Huang L L, Liu Y J, et al. First report of leaf spot of lotus () caused byin China[J]. Plant disease, 2018, 102(5): 1038-1038.
[6] Chen K L, Kirschner R. Fungi from leaves of lotus ()[J]. Mycological progress, 2018, 17(1): 275-293.
[7] Kirschner R. First record ofon lotus, a host outside the Magnoliales[J]. Mycological progress, 2010, 9(3): 417-424.
[8] Huang G. Characteristics and value of traditional lotus culture system in Guangchang, Jiangxi Province[J]. Chinese agricultural science bulletin, 2021, 37(10): 48-53.
[9] Hu D M, Wang M, Cai L. Phylogenetic assessment and taxonomic revision of[J]. Mycological progress, 2017, 16(4): 271-283.
[10] Song H Y, El Sheikha A F, Zhong P A, et al.sp. nov., producing phytase[J]. Mycotaxon, 2020, 135(2): 281-292.
[11] Zheng X, Jie Z, Zu J, et al. The list of lotus pest insects, diseases and natural enemies[J]. Biological disaster science, 2016, 39(2): 116-120.
HUANG J Y, TANG H, GU B, et al. A preliminary investigation of pathogenic fungi from Lotus (Gaertn) in Nanchang City[J]. Biological disaster science, 2021, 44(2): 123-135.
2021-05-02
2021-05-30
Key projects of the Natural Science Foundation of Jiangxi Provincial Department of Education (GJJ190168), Advantages of Technological Innovation Teambuilding Program of Nanchang City, Innovation and Entrepreneurship Training Program of Jiangxi Agricultural University in 2020 (No. 147)
HUANG Jingyue (1999—), Undergraduate, engaged in Bioengineering, 2698398097@qq.com; *Correspon0000ding author: Hu Dianming, associate professor, engaged in microbiology, hudianming1@163.com.
S435.672
A
2095-3704(2021)02-0123-13
