Prevention and management of scarring after thermal injury
2021-07-20JoshuaWongWeberLinJieDingEdwardTredget
Joshua Wong, Weber Lin, Jie Ding, Edward E. Tredget,
1Wound Healing Research Group, Department of Surgery, University of Alberta, Edmonton, Alberta T6G 2S2, Canada.
2Division of Plastic and Reconstructive Surgery and Critical Care, Department of Surgery, University of Alberta, Edmonton, Alberta T6G 2S2, Canada.
Abstract Survival from burn injury has improved considerably over the past two decades such that the quality of life of the victim of thermal injuries has become a major concern. Severe proliferative scarring or hypertrophic scarring (HTS) is an all too frequent complication of burn wound healing that severely compromises quality of life for surviving burn victims. Prevention of such scarring in burn patients involves better understanding of the pathophysiology of scar formation, development of newer methods for determining depth of burn injury and earlier and advanced surgical interventions. Many established and evolving novel treatments for HTS in patients after thermal injury exist and include antifibrotic pharmaceuticals and cellular-based therapies as reviewed herein.
Keywords: Thermal injury, hypertrophic scarring, transforming growth factor-β, fibrocytes, macrophages, scar prevention
INTRODUCTION
The World Health Organization estimates that over 180,000 deaths result annually from fire-related burns[1], with low- and middle-income countries disproportionately affected. In North America, more than 400,000 cases of thermal injury occur each year, where 40,000 require hospitalization for long durations and require multiple reconstructive procedures[2]. In a majority of cases of thermal injury, functionally debilitating hypertrophic scarring (HTS) will develop and lead to long-term complications such as chronic pain, stiffness and aesthetic deformity. The psychologic consequences of scars in recovering burn patients can be severe and disabling, particularly where cosmetically sensitive regions are injured, making any efforts to prevent scars and deformity after burn injury very important considerations in their clinical care[3].
EPIDEMIOLOGY OF SCARRING AFTER BURN INJURY
Despite advances in burn wound depth assessment and critical care management of the burn patients, a recent systematic review shows that the prevalence of HTS after burn injury is still strikingly high at 32%-72%[4].Surprisingly, the incidence of HTS after surgical intervention in burn injury can be as high as 70%[5]. A study of prospective burn patients requiring treatment, whether operative or non-operative, involving anatomic joints showed that non-operative approaches restored normal range of motion by 9 months. However, 20% of those who received operative management, most commonly skin grafting over the joint involved, still had persistent joint contracture at 12 months post-injury[6].
When looking at the risk factors that lead to pathologic scarring after burn injury, the exhaustive multivariate review of 703 patients by Gangemiet al.[7]reveals that female sex, younger age, burn sites on the neck or upper limbs, multiple surgical procedures, and meshed skin grafts were independent risk factors that contributed to increased incidence. In pediatric burn injury, Rotatoriet al.[8]reviewed 237 patients and found an astounding 64% with HTS. Specific risk factors for HTS included Hispanic ethnicity, increased total burn area, increased depth of injury, and increased percentage of burn requiring skin autografting. Risk factors involved in developing donor site HTS were increased time of epithelialization, increased depth of skin harvest, and thigh as the anatomical donor[8].
It is important to realize that the spectrum of scarring can range from pathologic to hypertrophic with an associated contracture. Also, HTS can be associated with other comorbidities such as chronic pain and pruritus, especially in patients who receive skin autografting procedures[9].
Traditionally, post-burn scarring has been assessed and measured with objective means based on clinician assessment. Recent studies suggest that a substantial portion of the morbidity from post-burn HTS requires patient self-assessment and pain scoring. Govermanet al.[10]suggests that even 2 years after initial injury, burn patients report as high as 80% incidence of raised or thick scars and an increase in symptoms related to HTS, such as dry or fragile skin, scars restricting range of motion and scar pain or itch. A review of longterm outcomes using the Burn Specific Health Scale Brief shows that even ten years post-injury, patients are still struggling with heat sensitivity and scar stiffness affecting work and that a significantly higher incidence of these issues occurs in females[11].
PATHOPHYSIOLOGY OF HTS AFTER BURN INJURY
It is now well recognized that burn injury into the deep or reticular dermis is most commonly associated with the development of HTS. Fibroblasts isolated from the deep dermis possess many of the features typical of similar cells explanted from HTS[12][Table 1].
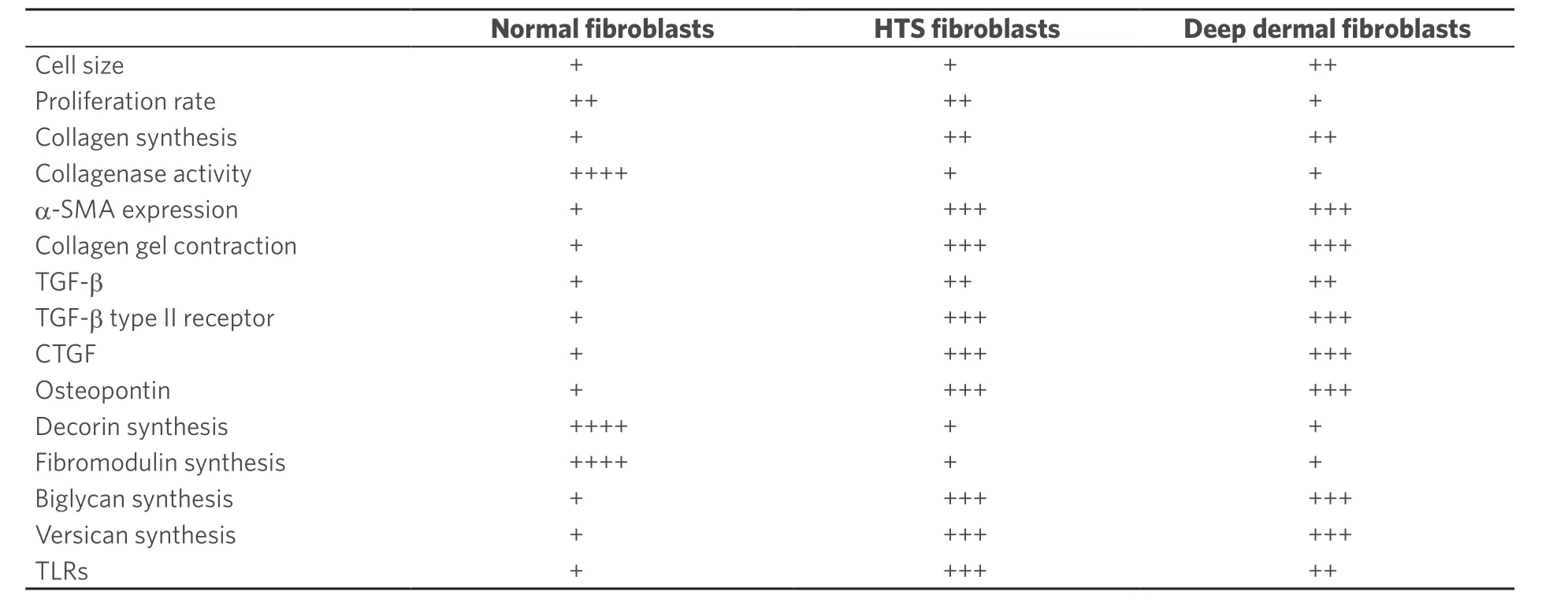
Table 1. Comparison of the characteristics of human dermal fibroblasts derived from normal skin, hypertrophic scars and deep dermis
In addition, single cell isolation has recovered unique populations of fibroblasts that contribute to scar formation in the perivasculature of the skin and deeper structures[13]. A clinical study in human skin with a progressively deeper incision injury demonstrates the development of HTS in lateral hip skin once the injury exceeds 0.56 mm or more than one-third of the thickness of the human skin[14]. Activation of deep dermal fibroblasts through toll-like receptors (TLRs)[15]leads to prolonged release of inflammatory cytokines including interleukin (IL)-1, tumor necrosis factor (TNF)-α and interferon (IFN)-γ, which chemoattracts inflammatory monocytes into slowly healing wounds.
The systemic immunologic response to major burn injury leading to severe HTS includes a polarized T-helper cell 2 environment[16,17], which also promotes the differentiation of blood-borne fibrocytes[18], secreting extracellular matrix (ECM) proteins, proteases, and fibrotic cytokines, including transforming growth factor-β (TGF)-β. This response to burn injury persists for up to 1 year after burn injury, such that reconstruction in patients with large burns and limited skin donor sites is best delayed where possible, until resolution of the systemic inflammatory response. Major burn injury contributes circulating monocytes to the healing wound in response to chemokines, including chemokine ligand 12 (CXCL-12; also termed stromal cell-derived factor 1) released by activated fibroblasts, where binding to chemokine receptor CXCR-4 leads to continued inflammation[19]. Chronic release of TGF-β in the wound tissues results in fibroblast proliferation, collagen and proteoglycan synthesis and excessive matrix synthesis. The morphology of the healing matrix is distorted by increased collagen I and III but also abnormal proteoglycans including versican, aggrecan and biglycan, which causes a fibrocartilaginous transformation of the healing skin and reduces remodeling[20]. Typically, hypertrophic scars are deficient in decorin, a small leucine-rich proteoglycan that binds collagen leading to tightly packed fibers and fiber bundles seen in normal skin, where it is produced in abundance by normal fibroblasts[21]. However, both HTS fibroblasts and deep dermal fibroblasts produce little decorin partly because of small inhibitory RNAs that downregulate decorin gene transcription[22].
Ultimately, limiting inflammation in the skin and extension of the depth of injury after thermal injury becomes critical in preventing a superficial wound, which heals with minimal scar formation such as the non-scarred region of the scratch injury, from converting into a deeper dermal injury and the inherent scar formation as a result[14].
PREVENTION OF SCARRING AFTER THERMAL INJURY
Clinical assessment of the depth of burn wounds is well recognized to be difficult and accurate in only 65%-70% of cases even when performed by an experienced burn surgeon, partly because of the evolving inflammatory response to the injured tissue, which may lead to deepening of the wound over the first several days after injury[23]. A number of clinical management features become important in preventing extension of the original burn injury into the deep dermal region of the skin[24]. Prevention of burn scarring involves the understanding that, beyond a critical depth, activated deep dermal fibroblasts of specific lineage with fibrogenic potential will lead to HTS. Therefore, accurate determination of burn depth is paramount and can be achieved with serial examination aided by objective instruments[25]to avoid unnecessary surgery. Despite the creation of a new wound and possible scar at the donor site, skin graft resurfacing is indicated for deep dermal burns to avoid HTS, particularly in critical cosmetic regions such as the face[26]. Decreased time to epithelialization is proportionately related to the depth of injury and also the incidence of developing HTS. Factors involved in ensuring minimal time to epithelialization are preventing wound conversion by avoiding over-resuscitation, preventing secondary wound infection, avoiding chemical toxicity from topical wound agents, and accurate surgical decision-making for deep wounds only[27-29]. The major challenge in burn depth assessment is in partial-thickness wounds where clinical evaluation by experienced clinicians is often inaccurate, in part because of the evolving inflammation that progresses in deep dermal wounds in the zone of stasis[30,31]. Thus, new instruments to aid as adjuncts to evaluate burn depth are becoming useful tools. One such tool includes the laser Doppler imaging (LDI) system, which evaluates microvascular dermal perfusion[32,33]. LDI is performed between 48 hours and 5 days after burn injury and has an accuracy ranging from 90% to 97%, compared with 52.5% to 71.4% with clinical evaluation[34-36]. LDI has a positive predictive value for burns that will not heal within 14 to 21 days of 85.1% to 98% and is accurate and noninvasive; however, sedation is often required for burns in young children, where LDI likely has its greatest applicability. Commercial videos illustrating LDI application are available online[37]with newer iterations of the technology that have quicker response time, decreased lag time and higher utility in specialized populations such as pediatrics and the elderly. In a recent meta-analysis of 321 publications related to burn depth assessment with LDI, Shin and Yi concluded that LDI is an accurate measurement tool when combined with careful clinical assessment of deep burn wounds[38].
Other modalities to distinguish burn depth at early time points have been investigated, including thermography[39], ultrasonography[40], nuclear magnetic resonance[41], near-infrared spectroscopy imaging, and confocal microscopy[42]. An emerging technology in microvascular surgery that overlaps with indeterminate burn depth assessment is in indocyanine green (ICG) angiography. In a prospective, multicenter triple-blinded study, ICG was shown to have higher accuracy in indeterminate burn wound assessment compared to clinical assessment and 100% sensitivity and specificity with tissue biopsy as a gold standard[43]. In the age of smartphone use in medicine and surgery, some centers have applied infrared thermography from a handheld device for assessing burn wounds and shown more than 90% overlap of estimation of salvageable tissue margins when compared to ICG angiography[44]. To date, however, these other techniques have gained only modest application in clinical practice because they may require expensive equipment, standardized training, and controlled environmental conditions during assessment.
Operating early on deep and full-thickness burns to prevent HTS is an approach useful for clearly deeper burn injuries identified by serial clinical examination of wound depth, scanning laser Doppler measurement and the time to epithelialization. In deep burns of the face, a better cosmetic result is achieved by surgical intervention and planned skin grafting using carefully selected donor tissues. Improved cosmetic outcome is achieved in these cases and is illustrated by less HTS in regions of skin-grafted tissues as compared to wounds that healed over prolonged intervals in the same patient[45]. Accurate wound depth estimation is paramount for serial assessment in particular areas that may be left longer to heal such as the face and glabrous surfaces. As such, serial assessment of epithelialization can be fraught with error when burn units have changes of attending physicians every 1 to 2 weeks. A recent study showed that sequential highresolution photo assessment had higher intra-class correlation to digital image analysis than serial clinical wound assessments[46]. As more centers transition to electronic medical records, the use of daily or regular photo documentation will assist in accurate assessment of the progression of healing.
NON-OPERATIVE MANAGEMENT OF BURN SCARS
Prior to complete burn HTS maturation, several therapeutic options are currently implemented to halt the progression of HTS. These options include scar massage, pressure garment therapy and topical silicone gel sheeting or silicone spray. The most common and least resource-intensive therapy is scar massage, and preliminary evidence suggests that scar massage upwards of 20-30 min per day can decrease scar height, vascularity, pain and pruritis and improve scar pliability in burn HTS. However, evidence-based guidelines are not well defined as larger controlled clinical trials are required[47]. Most compiled highquality evidence suggests that compression garment therapy at normal compression (20 mmHg or greater) can improve scar thickness and probably decreases scar redness. Silicone therapy shows positive results in improving scar pliability and redness[48]. Unfortunately, the studies are small in number and do not use accurate or standardized objective measurements of scar for outcome assessment and ultimately do not delineate “indications, duration and efficacy” of treatment[49]. There is evidence to suggest that no significant difference exists between compression and silicone therapy alone or in combination[50,51]. In fact, a similarity in efficacy can be seen between topical silicone spray and silicone gel sheeting but with less side effects in the silicone spray group compared to gel sheeting, which more commonly leads to skin maceration and contact dermatitis. This could have a large impact on treatment as compliance with silicone spray is much better than gel sheeting, especially in cosmetic areas such as the face, or when 23 h/day of more uncomfortable compression garment therapy is required.
Conventional treatments for HTS after thermal injury include corticosteroid injections, laser therapy, or surgery including scar release and skin resurfacing[52].
Pulsed-dye laser and fractional carbon dioxide laser have shown promise as an adjunct to established treatments for burn scar treatment. Pulsed-dye laser therapy selectively targets hemoglobin at the 585-nm wavelength, making it effective in hypervascular immature burn scars to reduce erythema. Using pulseddye and fractional carbon dioxide lasers, Hultmanet al.[53]demonstrated significant improvements in before-and-after burn scar scale scores and patient-reported outcomes. Ablative lasers such as the neodymium:yttrium-aluminum-garnet laser have been effective in contact mode, where 102 scar patients treated every 3 to 4 weeks for 1 year demonstrated significant improvements overall[54]. Unfortunately, scar recurrence developed in the upper chest, arm, and back areas, particularly if residual erythema and induration persisted following therapy. Thus, although laser treatment of post-burn HTS offers a new, potentially transformative approach to difficult scar challenges, further objective controlled trials are required[55].
OPERATIVE REVISION OF BURN SCARS: NEW APPROACHES
Burn reconstruction may be accomplished with the following: contracture release; scar excision and resurfacing; local transposition, rotation, and advancement flaps; tissue expansion; or distant axial flap, as well as with the use of skin substitutes such as IntegraTM[56,57]. Despite the requirements for greater operative times and specialized training and equipment, surgeons can also offer significant reconstruction advantages for burn patients through the use of reconstructive microsurgery. Acutely, free flaps may be used for limb salvage or defect coverage, permitting preservation of exposed vital structures such as nerves, tendons, vessels, or bone, often in high-voltage electrical burns, to avoid limb amputation[58].
Microvascular free flaps are also used in reconstruction of joint contractures and HTS when injured or deficient regional tissue precludes local flaps, skin grafts, or tissue expansion, where success rates for free flap transfer in burn reconstruction range from 78% to 96%[59]. Excessive free flap bulk is averted by the use of thinner fasciocutaneous flaps such as the anterolateral thigh[60]or parascapular[61]flaps in the head and neck region[62]and thin fascial flaps such as the temporoparietal fascial or serratus fascial flaps in the dorsum of the hand, which offer better color, thickness, and texture match[63].
POTENTIAL NOVEL TREATMENTS OF HTS AFTER BURN INJURY IN THE FUTURE
Splinting, pressure garments and silicone gel physical therapies are conventional treatments of HTS after burn injury but are time-consuming and not always suitable for HTS in specific regions such as the hands, feet and facial areas. Topically applied pharmacological agents such as antimicrobial creams have been traditionally used to prevent infection, and new enzymatic debridement agents are available for removal of necrotic wound eschar. However, high level scientific support for topical therapies in the form of controlled multicenter trials is difficult and expensive to achieve. Despite these complexities, ongoing research into the basic pathophysiology of fibroproliferative scars is yielding newer, novel strategies directed against specific molecules.
Strategies to manipulate TGF-β in the healing wound
TGF-β expression is involved in many wound healing processes including inflammation, angiogenesis, reepithelialization, ECM synthesis, and wound remodeling. TGF-β promotes myofibroblast proliferation, differentiation and wound contraction in many fibrotic diseases[64,65]. Fetal wound healing studies seeking to exploit the specific features of regenerative healing of the fetus in utero, have identified the specific TGF-β isoform TGF-β3 as an important cytokine present in the regenerating scarless fetal wound environment as opposed to TGF-β1 and TGF-β2 expressed in adults, where scarring is the inevitable consequence of adult wounds[64,65]. Unfortunately, despite encouraging preclinical evidence, current clinical trials on antagonizing the effects of TGF-β have been disappointing. For example, Juvista (Renovo, UK), a commercially developed recombinant form of TGF-β3 product, which demonstrated positive results in animal models and early phase human trials, was unsuccessful in significantly improving scar outcomes in a phase III trial[66,67]. Similarly, an inhibitor of the mannose-6 phosphate receptor for TGF-β1/TGF-β2, Juvidex (Renovo, UK) also was unsuccessful in a phase II trial[64]. TGF-β has important roles in burn wound healing and during HTS development. However, substantial blockage of TGF-β receptors to prevent fibrosis using recombinant human antibodies, can delay or prevent wound healing leading to chronic, non-healing wounds[65]. Similarly, treatment of patients with systemic sclerosis with recombinant human antibodies to neutralize TGF-β1 did not improve efficacy over controls during phase I/II trials[64]. Thus, although strategies to manipulate TGF-β continues to be an important potential therapeutic opportunity in fibrotic diseases, newer approaches to modulating TGF-β expression appear to be required.
Potential role of interleukins in burn wound healing
After burn injury, neutrophils and macrophages release inflammatory growth factors including IL-1, -2, -6, -8 and others as well as IFN-γ and TNF-α[68]. IL-10 has been shown to reduce inflammation by sequestering IL-6 or IL-8 and by reducing inflammatory T-cell cytokine production. Administering Prevascar (Renovo, UK), a recombinant human IL-10 product, intradermally during early wound healing[68]improved scar healing in human patients during phase I/II trials[64]. Other clinical trials with recombinant IL-10 to potentially combat various inflammatory diseases[68]are ongoing. However, a phase II clinical trial[69]conducted with IL-10 demonstrated no effectiveness in reducing scar formation in humans of continental African ancestral origin[68,70]. IL-2 may also contribute to the resolution of inflammation and improve the strength of the healed wound. Unfortunately, IL-2 clinically causes systemic inflammation and thus has a narrow therapeutic window[71].
Agents that modulate mechanical stress in wounds
Mechanical stress during wound healing facilitates fibrosis via cellular activation that stimulates cytokine release and promotes HTS[72,73]. New polymer-stress shielding devices to reduce mechanical stress to modulate local biomechanics, have been used to minimize scar development by off-loading mechanical forces, reducing mechanical stress imposed upon healing incisions[72,74]in high-tension body locations which are susceptible to developing HTS such as the central chest, shoulders, knees, ankles, and/or the back[52]. However, physical devices that reduce wound tension cannot be used easily on excisional wounds, burn injuries, and wounds that formed in convex surfaces such as the facial area. However, noninvasive drugs that target key mechanical signal transduction pathways involved in converting mechanical stress to intracellular biological signals have been developed[75,76]and are at the early stage of pre-clinical development.
Stem cells and other cellular therapies
Stem cells and cultured epithelial cells have potential benefit in severe burn injuries when used as wound closure techniques or anti-scarring biological agents in addition to standard skin grafting techniques. The efficacy and biosafety of many of these approaches are incompletely understood and as a result the technology is tightly regulated[77].
Although allogenic epithelial cells stimulate an immunologic rejection response, allogeneic cells as well as autologous approaches have been developed using cells derived from skin and other tissues for burn wound management[77]. Cultured epithelial allografts have been used for temporary coverage of acute burns as a bridge to eventual cultured epithelial autografting. Cost-utility analysis suggested that they facilitate healing in partial-thickness burns[78]. Cultured epithelial autografts may speed wound closure when combined with meshed skin grafts[78]. Fibroblasts added to dermal scaffolds may produce ECM proteins and growth factors that improve healing[77,79]. Keratinocyte stem cells regulate epithelial stratification and regeneration of skin appendages and hair follicles[77,80]and are beginning to be tested in burn wound management[81], as along with undifferentiated stem cells and progenitor cells[77]. Mesenchymal stem cells (MSC) administered systemically and locally have been shown to speed wound healing and regeneration and minimize scarring[77,82,83]. Induced pluripotent stem cells from human embryonic stem cells may also be useful as temporary skin substitutes for burn patients with large surface area burns. However, human embryonic cell research is associated with ethical issues and safety concerns yet to be resolved. MSC may be useful to correct defective granulation tissue formation and to heal chronic wounds[84]. Macrophage differentiation in granulation tissue formation initiates regenerative M2 polarization, fibroblast proliferation and differentiation with wound contraction, blood vessel formation, and matrix deposition. After burn injury, impairment of granulation tissue formation can result in delayed wound healing and HTS. The primary effect of MSC appears to be due to the release of growth factors into the wound and their paracrine effects on nearby resident cells, rather than engrafting and transdifferentiating into host tissue, thereby accelerating wound healing and reducing scarring. MSC sense and re-establish a reparative and regenerative local environment in response to local conditions in the wound. These environmental molecules include pathogen-associated molecular patterns (PAMPs) and/or damage-associated molecular patterns (DAMPs), which activate TLRs, or growth factors and/or inflammatory mediators and their respective receptors. Because MSC possess the unique capacity to sense and restore regenerative healing in wounds, they may be ideal therapeutic cells that adapt to changes in the local environment of disordered wounds.
Perivascular dermal MSC comprise about 0.3%-2.5% of total mesenchymal cells in the skin[85,86]. Quantitatively, MSC are not able to correct the disordered wound environment, but when delivered in higher therapeutic numbers, they can restore wound repair and regeneration in preclinical studies to date. Current data demonstrate the unique responses of therapeutically administered MSC in adapting to specific healing wounds; however, the inability of endogenous MSC in these settings to heal the wounds is poorly understood in part because of the lack of specific markers for MSCin vivo.
Challenges to the evaluation of new antifibrotic treatments
Experimental treatment for hypertrophic scarring after burn injury includes IFN-α2b, an anti-fibrotic T-helper cell cytokine that significantly improves scar remodeling and normalizes TGF-β[18,87,88]. New promising pharmacologic agents include topical imiquimod, calcium channel blockers, tacrolimus, 5-fluorouracil, pirfenidone, and bleomycin, as well as more experimental biological agents including IL-10, inhibitory microRNA against TGF-β, and peptide inhibitors of CXCR4 as potential future therapies[12,19,89].
Development of new scarring models and outcome measures
Unfortunately, designing clinical trials of antifibrotic agents is difficult due to the inter-individual characteristics, variable severity and depth of injury between and within subjects and sensitivity and objectivity of outcome measurements. As a result, new approaches have been developed by standardized scratch wounds where progressively increasing wound depth from one end to the other leads to wounds that heal with HTS at the deep end and regenerative non-proliferative scars at the superficial end[14,90,91]. This approach controls for variation between individuals when two scratches are created allowing for double-blinded, placebo-controlled studies. When coupled with patient- and observer-rated scar rating systems and more objective and sensitive outcome assessments including colorimetry and scar volume measurements with ultrasound and scar pliability instruments, more accurate assessment of the benefits of new modalities to prevent and treat scars will be achieved.
CONCLUSIONS
HTS following burn injury remain a major cause of morbidity for thermally injured victims. Advances in clinical care coupled with improved understanding of the pathophysiology of fibroproliferative scarring as outlined herein will improve the management of burn patients in the future.
DECLARATIONS
Authors’ contributions
Wrote the the manuscript: Wong J, Tredget EE Edited the manuscript: Lin W
Provided original data: Ding J
Availability of data and materials
Not applicable.
Financial support and sponsorship
The authors were supported by the Firefighter’s Burn Trust Fund of the University of Alberta.
Conflicts of interest
All authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2020.
杂志排行
Plastic and Aesthetic Research的其它文章
- More than meets the eye: a comprehensive review of blepharoptosis
- Skin collagen through the lifestages: importance for skin health and beauty
- Current and future trends in periodontal tissue engineering and bone regeneration
- Sternohyoid muscles plication and sternocleidomastoid muscles rejuvenation in neck lift: a retrospective study of 1,019 consecutive patients
- Contemporary techniques for nerve transfer in facial reanimation
- Abdominal wall reconstruction with component separation at the time of incisional hernia among survivors of emergency laparotomy after traumatic injuries: a population-based analysis of complications and healthcare utilization
