Earthworm contributions to soil nitrogen supply in corn-soybean agroecosystems in Quebec,Canada
2021-07-16ZhorABAILandJoannWHALEN
Zhor ABAIL and Joann K.WHALEN*
1 National Institute of Agricultural Research(INRA),Kenitra B.P.257(Morocco)
2 Depar tment of Natural Resource Sciences,Macdonald Campus of McGill University,Ste-Anne-de-Bellevue,Quebec H9X 3V9(Canada)
(Received September 16,2019;revised December 9,2019)
ABSTRACT Accurately quantifying the soil nitrogen(N)supply in crop fields is essential for enabling environmentally sustainable and economically profitable crop production.It requires using field-based methods to account for the contribution of soil biota,including earthworms,to N mineralization in temperate agroecosystems.The direct contribution of earthworms to the soil N cycle is the N they release throughout their life and after death,and it can be estimated using the secondary production method.This study was conducted in 2014 and 2015 in two adjacent fields with annual corn-soybean rotation in Ste-Anne-de-Bellevue,Quebec,Canada.The cumulative biomass of Aporrectodea spp.in two no-till corn-soybean agroecosystems was determined,and the direct N flux from these earthworms was estimated during the corn and soybean phases of the rotation.Secondary production was estimated by sampling earthworms biweekly during April—June and September—November and inferring the change in earthworm biomass between sampling dates using a size frequency calculation.The N flux was calculated as the sum of the N released through excretion,during periods when earthworms were active,and from mortality.The secondary production of the Aporrectodea population was estimated to be 8—43 g ash-free dry weight m-2 year-1,and the N flux was 22—105 kg N ha-1 year-1.The N flux was higher at the early vegetative growth stage,which is a period of high N demand for corn.These findings suggest that refining the N fertilization recommendation by accounting for soil N supplied by earthworms could potentially reduce fertilizer costs and environmental N losses.
Key Words: nitrogen excretion,nitrogen flux,nitrogen release,secondary production,size frequency method
INTRODUCTION
Nitrogen(N)is often the most limiting nutrient for crop production,and N fertilizers are essential to support high yields of non-leguminous crops.Worldwide,the average N recovery from fertilizers and other N sources is 47%—59%(Liuet al.,2010;Lassalettaet al.,2014),meaning that a large proportion of N inputs to agroecosystems are not retained in crop biomass.This mismatch between N supply to crops and N uptake by crops results in N losses from agroecosystems that impair air and water quality.For instance,16%of the Canadian nitrous oxide(N2O)emission from agriculture in 2015 was attributed solely to the application of inorganic N fertilizers(Environment and Climate Change Canada,2018).Nitrate nitrogen(NO-3-N)leached from agricultural soils is the primary source of contamination in aquatic ecosystems and drinkable water(Rasouliet al.,2014).Determining the fraction of crop N requirements that should be met with N fertilizers is essential for achieving environmentally sound and economically profitable crop production.Reaching this goal requires more accurate quantification of the soil N supply,which is the mineral N that is released by the biological decomposition of crop residues,animal manure,and soil organic matter.In particular,earthworms are known to stimulate decomposition and subsequent mineralization of organic N compounds,leading to a significant increase in mineral N concentrations.A meta-analysis by van Groenigenet al.(2014)reported a 25%increase in crop yield because of the enhancement of Nmineralization by earthwormactivities.Furthermore,Xiaoet al.(2017)showed that the presence of earthworms improves plant growth by 20%while increasing their N content by 11%.
Earthworm contributions to the soil mineral N pool can be partitioned into their indirect and direct impacts on microbially mediated N cycling.The indirect contributions of earthworms to N mineralization are estimated to range from 11 to 267 kg N ha-1year-1and are attributed to their ecological engineering activities associated with changes in soil physical and chemical properties,improvement of plant root growth,stimulation of microbial activity,and other processes(Marinissen and De Ruiter,1993).In addition,the direct effects of earthworms produce between 3 and 74 kg N ha-1year-1,which is released through their metabolic products,such as mucus and urine,and the turnover of their biomass(Boström,1988;Parmelee and Crossley,1988;Curryet al.,1995;Whalen and Parmelee,2000).Although earthwormderived N includes organic and mineral N compounds,most of the excretion products and tissues of dead earthworms are a source of mineral N for crop uptake.Research has found that 36%—84%of N excreted by earthworms in urine and mucus is recovered in mineral N and dissolved organic N(DON)pools after two days(Whalenet al.,2000;Abail and Whalen,2019),and 30%—70%of the N released from dead earthworm tissues is incorporated into plant shoots in 8—16 d(Whalenet al.,1999).Furthermore,field-based estimates suggest that N flux through earthworm populations is equivalent to 11%—38%of the total N uptake by sorghum and corn plants(Parmelee and Crossley,1988;Whalen and Parmelee,2000).However,the contributions of earthworms to the soil N supply are seldom accounted for,since most estimates of potentially mineralizable N focus on the N transformation by microorganisms and microfauna.However,Rashidet al.(2014)reported that earthworm-mediated N mineralization together with potentially mineralizable N determined from aerobic soil incubation in the laboratory is a good predictor of N uptake in temperate grasslands.
The direct N flux through earthworm populations is generally estimated by summing N released from earthworms through secondary production and excretion of mucus and urine.Secondary production is the accumulation of biomass through growth and reproduction,which is calculated using the size frequency method.This involves sampling earthworms at regular time intervals and determining the changes in earthworm biomass and abundance between two consecutive sampling dates(Benke,1979,1984;Whalen and Parmelee,2000).On the basis that annual earthworm production and mortality are approximately equivalent(Andersen,1983;Boström,1988;Christensen,1988),we assume that all N accumulated in the earthworm population through secondary production is returned to the soil annually because of earthworm mortality.Nitrogen excretion is estimated by multiplying the mean earthworm biomass by the average N excretion rate.The direct contribution of earthworms to the soil N supply is expected to be proportional to the earthworm population size.The objectives of this study were to determine the secondary production ofAporrectodeaspp.in no-till corn-soybean rotation agroecosystems and to estimate the direct N flux from this earthworm population during the corn and soybean phases of the rotation.
MATERIALS AND METHODS
Study sites
The study was conducted in 2014 and 2015 in two adjacent agricultural fields,which were 50 m apart,at the Macdonald Research Farm of McGill University in Ste-Anne-de-Bellevue,Quebec,Canada(45°25′N,73°56′W).The climate in this region is humid temperate with mean monthly temperatures ranging from-10.8°C in January to 20.9°C in July and a mean annual precipitation of 885 mm(Government of Canada,2017).The soil in these fields was a sandy-loam,mixed,frigid Typic Endoaquent(US Soil Taxonomy).
The agricultural fields were under a no-till corn(Zea maysL.)-soybean(Glycine maxL.)rotation,and both phases were grown each year.Corn was produced for grain in one field and silage in the other field,which affected the amount of crop residue remaining after harvest.Before 2013,both fields were under alfalfa(Medicago sativaL.)production and managed similarly,so recent differences in residue inputs and their effects on earthworm populations could be compared between the corn grain-soybean and corn silagesoybean agroecosystems.Based on corn and soybean yields in 2013,the year before the experiment,and during the study period in 2014—2015,the high residue-producing corn grain-soybean agroecosystem had an extra 3—5 Mg ha-1year-1of aboveground residues in the field after harvest,compared to the low residue-producing corn silage-soybean agroecosystem.Further details on the soil and agronomic management of these fields are provided in Abail and Whalen(2018).
Experimental design
Earthworm populations were studied in each agroecosystem in a single plot,which was 50 m wide by 25 m long,split into five blocks that were 10 m wide×25 m long.Each block was further divided into subunits of 5 m×2.5 m.Samples were taken from a unique,randomly selected subunit in each block at approximately biweekly intervals,in 2014 and 2015 at the following times:April(4th week),May(2nd and 4th weeks),June(2nd and 4th weeks),September(2nd and 4th weeks),October(2nd and 4th weeks)and November(2nd week).In total,100 different subunits from each agroecosystem were selected for sample collection during the two-year study.Earthworms were collected,as described below,from each selected subunit,and soil temperature and moisture were measured,at each sampling date,from September 2014 to November 2015.Soil temperature in the 0—10 cm depth soil layer was recorded using a hand-held thermometer,and a soil sample(0—10 cm)was taken to measure the gravimetric moisture content.
Earthworm sampling and identification
One soil block,which measured 40 cm×40 cm×25 cm,was removed from the center of each randomly selected subunit and hand sorted to collect surface-dwelling earthworms.Deep-dwelling earthworms,at depths of more than 25 cm,were collected after pouring dilute 0.5%formaldehyde solution into the bottom of the 25-cm-deep pit.The preserved earthworms were categorized as adults if they had fully developed clitella,pre-clitellates if they had undeveloped clitella,juveniles,or fragments which were incomplete earthworms.Sexually mature specimens were identified to the species level using the taxonomic keys of Reynolds(1977)and Schwert(1990).Juveniles were assigned to the generaAporrectodea,Allolobophora,orLumbricus,depending on their pigmentation and other morphological characteristics,such as the shape of the prostomium.All earthworms and fragments were cleaned in water,the body length(in cm)of every intact individual was recorded,and the oven-dry(60°C for 48 h)and the ash-free dry weights(AFDW,500°C for 4 h)were determined for the four earthworm categories:juveniles,pre-clitellates,adults,and fragments.Earthworm communities in both agroecosystems were dominated byAporrectodeaspecies,namelyAporrectodea turgidaandAporrectodea tuberculata.Therefore,all adults identified asA.turgidaandA.tuberculataand juveniles that could not be identified to the species level were designated as members of theAporrectodeapopulation,which might include a negligible number(2%—6%of the total number of juveniles)of unpigmentedAllolobophorajuveniles.
Size frequency method
The aim of the size frequency method is to organize the distribution of specific traits of individuals into cohorts,which is more challenging for earthworms because they do not show synchronous development like arthropods.Therefore,earthworm cohorts were identified according to measurements of their body length(Whalen and Parmelee,2000),which were between 0.5 and 12.0 cm for individuals.Only one individual reached 12 cm.Earthworms were separated into six cohort classes(0—1.9,2—3.9,4—5.9,6—7.9,8—9.9,and 10—12.0 cm),and the mean biomass of individuals in each cohort class was determined following Whalen and Parmelee(2000),based on the relationship between the body length and biomass ofAporrectodeaspp.,using Eq.1:
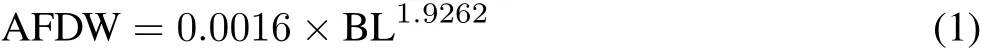
where AFDWis the ash-free dry weight(g)and BL is the body length(cm).
Secondar y production of Aporrectodea spp.
Annual production was calculated by multiplying the cohort production by the cohort production interval(CPI).Cohort production equals the sum of production losses between two consecutive length classes multiplied by the total number of length classes.Cohort production forAporrectodeaspp.was calculated using six length classes,and secondary production ofAporrectodeaspp.was then calculated on an annual basis for the populations in each agroecosystem(Benke,1979,1984).
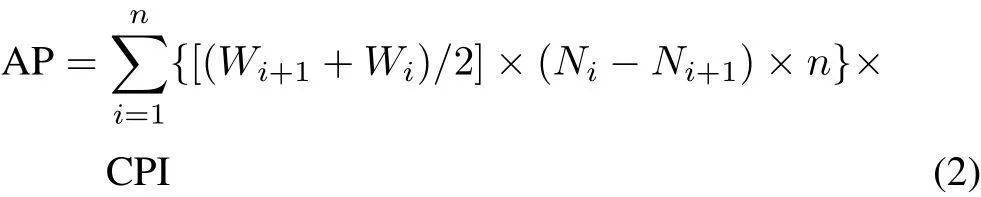
where AP is the annual production(g AFDWm-2year-1)for all length classes(classes 1-i),Wi+Wi+1represents the mean mass of individuals from two consecutive length classes(g AFDW),Ni-Ni+1represents the decrease in the number of individuals from two consecutive length classes,nis the number of length classes,and CPI is the time needed to develop from hatching to the largest length class and was assumed to be equal to 1 year for theAporrectodeaspp.(Andersen,1987;Whalen and Parmelee,2000).
Nitrogen flux through Aporrectodea spp.in the corn and soybean phases of rotation
The direct flux of N through earthworm populations(NDF,g N m-2year-1)is the N released from earthworm tissues through mortality and excretion of metabolic products,such as mucus and urine.It is calculated using Eq.3:

where AP×Newrepresents the amount of N released from dead earthworm tissues which is estimated using secondary production,Newis the N content of earthworm tissues(g N g-1AFDW),andEis the N released in earthworm excretion products(g N m-2year-1),calculated using Eq.4:

where ABmeanis the mean annual biomass of earthworms(g AFDWm-2),Nexis the N excretion rate ofA.turgida(g N g-1AFDW d-1,609±33μg N g-1fresh weight(FW)d-1)obtained from a laboratory experiment(Abail and Whalen,2019),andAdis the number of days(d)when earthworms are active during the frost-free period.The Newvalue used was that estimated from a random sample(n=40)of juveniles and adults ofA.turgidacollected in another part of the agricultural field(<10 m from the study site).These individuals were allowed to clear their guts for 24 h,they were then euthanized by spraying with 70%ethanol,and their tissues were oven dried(60°C for 48 h)and ground to determine N content using a Thermo Finnigan Flash 1112 EA CN analyzer(Carlo Erba,Milan,Italy).The N content determined was 145.6±3.2 g N kg-1AFDW.
Considering that the AFDWis 14%of FW(Whalen and Parmelee,2000),the N excretion rate used was 4.35 mg N g-1AFDW d-1.Earthworms are assumed to be active when the soil temperature is between 4 and 20°C and moisture content is above 15%(Whalen and Parmelee,1999;Holmstrup,2001;Eriksen-Hamel and Whalen,2006).At our field sites,soil moisture ranged from 16%to 28%during the spring(April—June)and fall(September—November).Since soil moisture was generally above 15%,theAdvalues were calculated to be 142 d in 2014 and 141 d in 2015,based on the daily soil temperature estimated from daily air temperature obtained from the Environment Canada weather station in Ste-Anne-de-Bellevue,about 1.5 km from the agricultural fields.The ranges of values in literature for parameters used in the calculation of N flux are summarized in Table SI(see Supplementary Material for Table SI).
Statistical analysis
The data were tested for normality using a Shapiro-Wilk test;they were not normally distributed,so we analyzed the data using non-parametric statistics.Earthworm abundance and biomass were compared between treatments using a Friedman test,and at sampling dates when treatments were significant(P<0.05),mean abundance and biomass values were compared using apost-hocWilcoxon signed-rank test.Statistical analyses were conducted using SPSS software(IBMSPSS Statistics 20.0).
RESULTS
Size frequency distribution of Aporrectodea spp.in corn and soybean phases of rotation
The mean number ofAporrectodeaspp.was similar(P=0.521)in the corn and soybean phases of the corn grain-soybean agroecosystem,but fewerAporrectodeaspp.(P<0.01)were collected from the soybean phase than the corn phase in the corn silage-soybean agroecosystem(Table I).Individuals in all length classes were found at almost every sampling date(Figs.1 and 2)in both agricultural fields.Small individuals(<4 cm)made up 27%of theAporrectodeaspp.in the corn silage-soybean agroecosystem(Fig.1)and 30%of theAporrectodeaspp.in the corn grainsoybean agroecosystem(Fig.2).The highest proportions of small individuals(<4 cm)were retrieved in May-Juneand October-November.Individuals having a body length between 4 and 8 cm were predominant,constituting more than 50%of all individuals ofAporrectodeaspp.collected during the study period(Figs.1 and 2).Large individuals(>8 cm)represented,on average,between 9%and 17%of the total number of individuals and were more abundant in June-September(Figs.1 and 2).
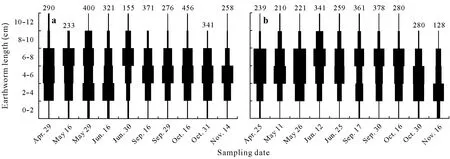
Fig.2 Size frequency distribution of Aporrectodea spp.in no-till corn grain-soybean agroecosystem during the soybean phase in 2014(a)and corn phase in 2015(b).The width of horizontal bar indicates the proportion of individuals in each length class in the total number of individuals collected at each date.The number above histogram indicates the mean number of individuals(n=5)of Aporrectodea spp.measured at each date.
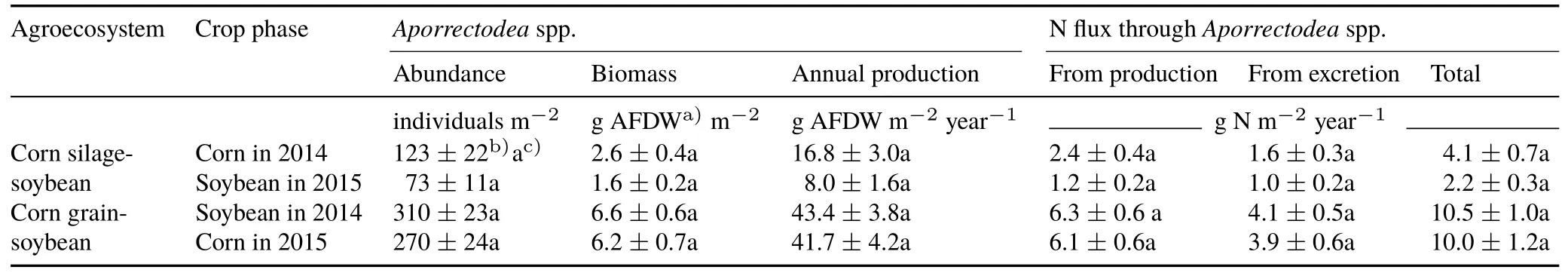
TABLE IAbundance,biomass,annual production,and N flux through Aporrectodea spp.in no-till corn-soybean agroecosystems(based on 142 and 141 active days in 2014 and 2015,respectively)
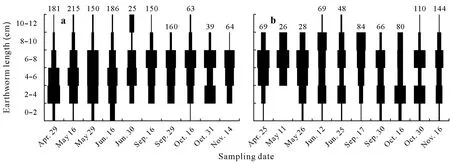
Fig.1 Size frequency distribution of Aporrectodea spp.in no-till corn silage-soybean agroecosystem during the corn phase in 2014(a)and soybean phase in 2015(b).The width of horizontal bar indicates the proportion of individuals in each length class in the total number of individuals collected at each date.The number above histogram indicates the mean number of individuals(n=5)of Aporrectodea spp.measured at each date.
Secondar y production through Aporrectodea spp.in the corn and soybean phases of rotation
Production ofAporrectodeaspp.was 8—43 g AFDWm-2year-1,with lower production in the corn silage-soybean agroecosystem than the corn grain-soybean agroecosystem(Table I).Production ofAporrectodeaspp.did not differ significantly between the corn and soybean phases in either agroecosystem(P>0.05),but the production was two times greater,on average,during the corn phase than during the soybean phase of the corn silage-soybean agroecosystem.
Annual N flux through Aporrectodea spp.in the corn and soybean phases of rotation
If we assume that direct N flux from theAporrectodeaspp.(Table I)can be extrapolated to an agroecosystemrelevant scale,by multiplying it by 10 to get kg N ha-1year-1,then earthworms were responsible for a N flux of 22—105 kg N ha-1year-1.A lower N flux,with more variation among crop phases,occurred in the corn silagesoybean agroecosystem,whereas the higher N flux in the corn-grain agroecosystem was similar between the crop phases(Table I).Biomass turnover and N derived from excretion products represented 54%—61%and 39%—46%,respectively,of the total N flux from theAporrectodeaspp.in these agroecosystems.The maximum N flux occurred in June and September,with the cumulative N flux reaching 49%—59%of the total N flux from theAporrectodeaspp.in the early part of the corn growing season(Fig.3).
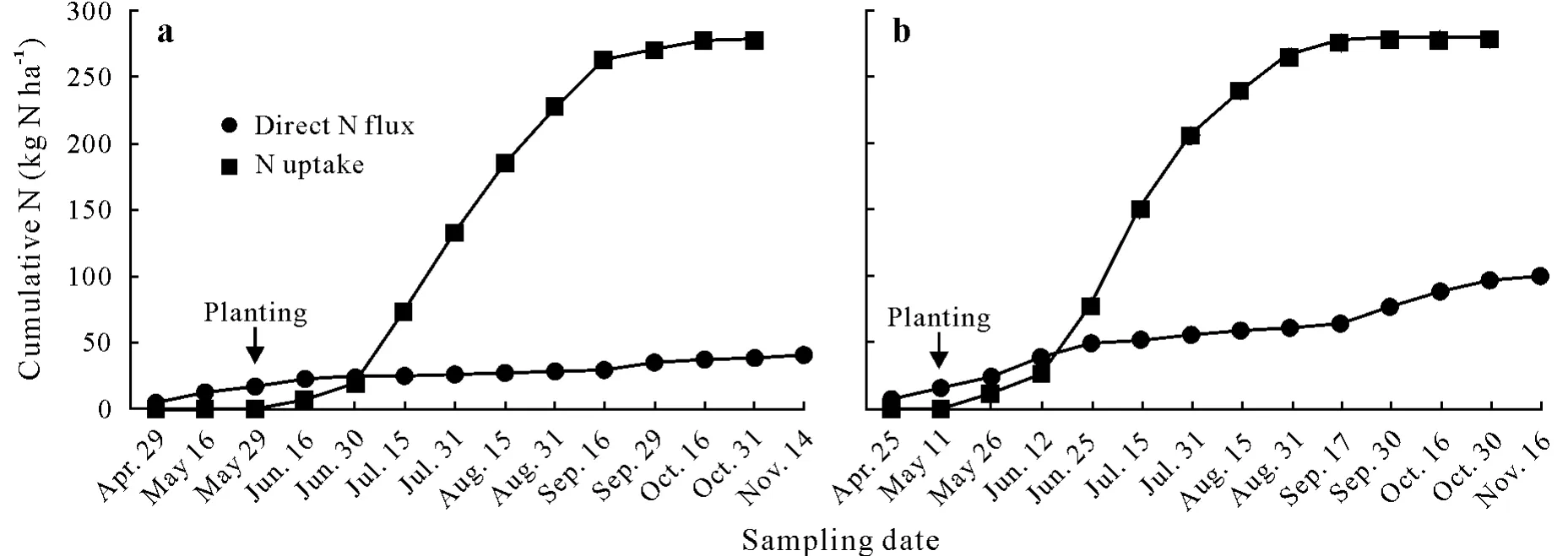
Fig.3 Cumulative direct N flux through Aporrectodea spp.and the hypothetical total cumulative N uptake by corn that produces,on average,21 Mg ha-1 of total biomass with 11 Mg ha-1 of grain adapted from Jones(2003)in no-till corn silage-soybean agroecosystem(a)and corn grain-soybean agroecosystem(b).
DISCUSSION
As expected,the annual production ofAporrectodeaspp.was higher in agroecosystems with a larger earthworm population.In general,agroecosystems receiving high residue inputs and amended with organic fertilizers support two to five times larger earthworm populations and thus higher annual production than those receiving inorganic fertilizers and low inputs of organic residues(Edwards and Lofty,1982;Andersen,1983;Whalenet al.,1998;Whalen and Parmelee,2000).There was less temporal variation in the earthworm population of the corn grain-soybean agroecosystem than the corn silage-soybean agroecosystem,presumably because earthworms had access to organic substrates from the corn residues(11 Mg ha-1)that remained after corn grains were harvested in 2013 and there was 5 Mg ha-1of soybean residues remaining after harvest in 2014(Abail and Whalen,2018).The corn silage-soybean agroecosystem had less crop residues from nonharvested biomass during the same period,with 5 Mg ha-1of corn residues and 6 Mg ha-1of soybean residues,and thus there were fewerAporrectodeaspp.in this agroecosystem(Abail and Whalen,2018).Production of theAporrectodeaspp.population was between 8 and 43 g AFDWm-2year-1in this study,which is consistent with other reports of earthworm production that varied from 3 to 47 g AFDW m-2year-1(Boström,1988;Parmelee and Crossley,1988;Senapatiet al.,1991;Curryet al.,1995;Whalen and Parmelee,2000;Eriksen-Hamelet al.,2009).Some of the differences between these production estimates may have been due to differences in the size of the earthworm population,the number of active days,and the sampling frequency.Production will be higher for a larger earthworm population and when there is a longer period of favorable conditions for earthworm activity.We recommend biweekly sampling of earthworm populations for a better estimate of the magnitude of biomass gain or loss,which is interpolated between sampling dates.
Direct N flux through theAporrectodeapopulation followed the same trend as earthworm production and was substantial,ranging from 22 to 105 kg Nha-1year-1.These estimates are higher than N fluxes of 3—74 kg N ha-1year-1reported in the literature for earthworm populations in annually cropped agroecosystems and hayfields(Andersen,1983;Boström,1988;Parmelee and Crossley,1988;Curryet al.,1995;Whalen and Parmelee,2000).There are two reasons for this outcome:i)theAporrectodeapopulation in this study,reaching up to 300 individuals m-2,was larger than earthworm populations in other N flux studies and ii)we used a higher excretion rate(609μg N g-1FWd-1)than that used in previous studies(30—576μg N g-1FWd-1).Our excretion rate was obtained in the laboratory using a noninvasive15N tracing procedure withAporrectodeaspp.(Abail and Whalen,2019),which are representative of the investigated earthworms.However,our measurements were performed on earthworms incubated at 16°C,whereas in our estimates of N flux,earthworms were assumed to be active in the field at temperature ranging from 4 to 20°C.Nitrogen excretion is expected to increase with temperature(Christensen,1987;Bouchéet al.,1997).Bouchéet al.(1997)reported that N turnover through the body of the anecic speciesLumbricus terrestrisandAporrectodea longawas dependent on temperature between 8 and 16.5°C,with a temperature coefficient(Q10)value of 1.65(Ferrière and Bouché,1985;Hameedet al.,1994).Furthermore,Whalenet al.(2000)showed that N excretion rates ofLumbricus rubellus,L.terrestris,and A.tuberculatawere not affected by soil temperature between 10 and 18°C,suggesting this temperature range to be optimal for their metabolic processes.In the absence of information about the low-temperature effect(<10°C)on N excretion rates,we assumed that the N excretion rate was temperature-independent in our calculation of N flux.We acknowledge that this assumption could lead to an over-estimation of the N fluxviaexcretion,particularly for the active days when the field temperature was below 10°C,which occurred for 20%—26%of the total number of active days.Nevertheless,excretion products contributed 10—39 kg N ha-1year-1to the total N flux in this study,which is consistent with the estimates of 21—36 kg N ha-1year-1reported in previous studies(Parmelee and Crossley,1988;Curryet al.,1995;Whalen and Parmelee,2000).Yet,our estimate of excreted N may have been underestimated,since the N excretion rate was measured on adult species while 72%of theAporrectodeaindividuals were juveniles.One would expect the N excretion rate of adult earthworms to be lower than juveniles,which are metabolically more active,but no quantitative evidence currently exists to support this assumption.
Furthermore,our estimates of total N flux,although higher than those reported in previous studies,may have underestimated the potential N flux through earthworm populations in the agroecosystems studied here for two main reasons.First,we calculated secondary production of theAporrectodeapopulation only because of the dominance of the speciesA.turgidaandA.tuberculataat the field sites studied.However,the earthworm community also includedL.terrestris,which represented as many as 27%of the individuals and up to 47%of the earthworm biomass in the studied agroecosystems,and a negligible quantity ofL.rubellus(Abail and Whalen,2018).The Nflux would be significantly higher if theLumbricuspopulations in these fields were included.Second,we did not account for the indirect N flux that could be derived from other earthworm trophic(i.e.,casting)and non-trophic(i.e.,their ecosystem engineering functions)activities stimulating N mineralization by microbial communities.Previous studies reported that the total N flux through earthworm populations may reach up to 363 kg N ha-1year-1when considering N generated from direct and indirect earthworm activities(Syers and Springett,1984;Scheu 1987;Marinissen and De Ruiter,1993;Curryet al.,1995;Rashidet al.,2014).Earthworm-mediated N cycling can make considerable contributions to N input in agroecosystems,which may provide a substantial N supply to crops,reducing our reliance on exogenous N inputs to sustain crop production.
Considering the growing season of corn,which is a crop with high N requirements and is widely cropped in Quebec,our results indicate that the annual Nflux through theAporrectodeapopulation represents a considerable amount of N,which is equivalent to 24%—88%of the recommended N fertilizer rate(120—170 kg N ha-1)for corn in Quebec(CRAAQ,2003).However,we do not know if any of the N released from earthworms was absorbed by plant roots since the earthworm N supply must be synchronized with the crop N uptake to be counted as a source of fertility.The temporal patterns of cumulative earthworm N flux and corn N uptake showed that a large proportion of the N flux from theAporrectodeapopulation was available during the period when corn has a high Ndemand,which is when plants are at the V6—V8 growth stage,30—40 d after planting(Ma and Biswas,2016).However,before planting or in the early and end periods of the growing season,N released from earthworms is unlikely to be absorbed by a crop and could be transformed into unavailable N forms or lost from the agroecosystem.For instance,approximately 16%—64%of N excreted by earthworms in laboratory incubation is not recovered in mineral Nand DONpools and is suggested to be likely retained in microbial biomass or lostviadenitrification or leaching(Whalenet al.,2000;Abail and Whalen,2019).The current methods for evaluating the soil N supply in crop fields remain based on laboratory incubations that exclude soil macrofauna like earthworms.Neglecting to consider the contribution of earthworms may lead to an underestimation of the actual soil N supply(Rashidet al.,2014)and result in the application of excess N fertilizer to the crop.A combination of ecological model estimates of N flux through earthworm populations with laboratory-based estimates of potentially mineralizable N may reduce the gap between the actual and predicted crop N requirement.Refining the current N fertilization recommendation by accounting for N supply from earthworms has the potential to reduce costs and environmental losses of N.
CONCLUSIONS
This study was the first to estimate the direct N flux through earthworms of naturally occurring field populations in agroecosystems of Quebec,Canada.Our research suggests that in corn-soybean agroecosystems of Quebec,receiving an annual crop residue input averaging 10 Mg ha-1year-1and supporting a high density ofAporrectodeaspp.(≥300 individuals m-2),the direct flux of N through the population ofAporrectodeaspp.was substantial,being equivalent to as much as 88%of the recommended N fertilizer requirements of corn in Quebec.These estimates are based on assumptions and laboratory data and will be improved by generating temperature-dependent excretion rates for field-dwelling populations ofAporrectodeaspp.The substantial amount of Nflux from earthworm populations has the potential to contribute to the soil N supply.Furthermore,we note that the direct N flux from earthworms must be synchronous with crop N demands to be counted as a source of fertility.Additional studies are required to document what proportion of the direct N flux from earthworm populations reaches the crop,which could be done experimentally with15N stable isotopes to track N transformations or by integrating the earthworm-mediated N flux into a soil N supply model.Such studies would be pertinent in agroecosystems where earthworms are abundant and active throughout the crop growth period.
ACKNOWLEDGEMENTS
Financial support for this study was provided by the Natural Sciences and Engineering Research Council of Canada(NSERC)through the Discovery Grant(No.RGPIN-2017-05391).Zhor ABAIL was supported by a postgraduate scholarship from the IDB Merit Scholarship Program for High Technology.The authors kindly thank Dr.Kevin Butt,University of Central Lancashire,UK,for his thorough and helpful review of the manuscript.We also thank Hicham Benslim,Hélène Lalande,Khosro Mousavi and Marc Samoisette for their technical assistance.
SUPPLEMENTARY MATERIAL
Supplementary material can be found in the online version.
杂志排行
Pedosphere的其它文章
- Soil properties,grassland management,and landscape diversity drive the assembly of earthworm communities in temperate grasslands
- Earthworm community development in soils of a reclaimed steelworks
- Community structure of Lumbricidae in beech woodland of the Bieszczady National Park,Southeast Poland
- Earthworm populations are stable in temperate agricultural soils receiving wood-based biochar
- Earthworm functional groups are related to denitrifier activity in riparian soils
- Impacts of wetting-drying cycles on short-term carbon and nitrogen dynamics in Amynthas earthworm casts
