Application of Liquid Chromatography-High Resolution Time-of-flight Mass Spectrometry in the Detection of Raw Milk and Dairy Products
2021-07-08YiLILumanHUOLixueDONGXuesongWANGLitianZHANGRuihuanDUAijunLILeiWANG
Yi LI Luman HUO Lixue DONG Xuesong WANG Litian ZHANG Ruihuan DU Aijun LI Lei WANG
Abstract Milk and dairy products are more and more popular with consumers due to their various nutrients, and their quality and safety issues have always been concerned. Therefore, the development of rapid, accurate and simple screening techniques is of great significance. Liquid chromatography-high resolution time-of-flight mass spectrometry has high-resolution and high-throughput detection functions, and has gradually begun to be applied in the detection of milk and dairy products. This paper summarized the application of milk and dairy products in liquid chromatography-high resolution time-of-flight mass spectrometry, laying a foundation for the development of new methods.
Key words Milk and dairy products; High resolution time-of-flight mass spectrometry; Pesticide and veterinary drug residues
Milk contains a variety of nutrients such as amino acids, proteins, vitamins, minerals and phospholipids, and its nutrients can be completely absorbed by the human body. It is known as the natural and healthy food closest to perfect. With the improvement of people's consumption level and the change of consumption concept, milk is becoming more and more popular among consumers. According to statistics, China's dairy product output reached 27.194 million tons in 2019, with an increase of 323 000 tons over 2018; and as of the fourth quarter of 2019, the cumulative dairy product sales reached 27.106 million tons, with an increase of 0.2% over the same period in 2018.
There are many methods for detecting veterinary drug residues in raw milk and dairy products. The common ones are liquid chromatography, gas chromatography-mass spectrometry, liquid chromatography-tandem mass spectrometry, enzyme-linked immunoassay, etc. According to the characteristics that raw milk is not easy to preserve, there are few reports that can accurately determine the residues of multiple veterinary drugs in raw milk in a short time at one time with high throughput, and there are still certain technical defects. Therefore, paying attention to the quality and safety of raw milk and dairy products and developing accurate, simple, and rapid screening methods for raw milk and dairy products by liquid chromatography-high resolution time-of-flight mass spectrometry is the current focus of research.
Concept of Time-of-flight Mass Spectrometry (TOF-MS)
The concept of TOF-MS was put forward by Stephens in 1946. Due to the backward electronic technology and instrument design, the resolution of the early TOF-MS was less than 100. With the use of dual-gate structure, delayed extraction technology, pulsed field focusing, ion mirror and other technologies, the resolution of TOF-MS has been greatly improved. In the 1960s, the ionization technology of TOF-MS continued to develop. During this period, a series of optical ionization sources were applied to TOF-MS. In the mid-1970s, the laser ionization technology of TOF-MS made great progress. In recent years, the ESI technology proposed by Fenn and the MALDI technology proposed by Tanaka and Hillenkamp have enabled the application of TOF-MS to increase exponentially. As of today, TOF-MS technology has been applied in many fields such as food, chemistry, life sciences, proteomics, and is attracting more and more attention due to its fast, efficient, and simple pre-treatment methods, as well as the advantages of high sensitivity and high resolution.
Application in Testing of Raw Milk and Dairy Products
Application in raw milk testing
In the process of dairy cattle breeding, the large-scale use of veterinary drugs, non-strict implementation of the withdrawal period, and illegal use of feed additives, etc., may cause serious veterinary drug residues in the finished milk[1]. Residues of veterinary drugs in dairy products can cause the human body to develop drug resistance, endangering human health, and even endangering life in severe cases.
Gong et al.[2]established a rapid screening method for 14 sulfonamides in fresh milk using ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Raw milk was treated with 0.1% acidified acetonitrile and then purified by QuEChERS method. Gradient elution was carried out using acetonitrile-0.1% formic acid aqueous solution as the mobile phase, and the ESI source was used. Data was collected in the positive ion mode. The 14 sulfonamide drugs were well separated within 8 min. The average recoveries under the 3 spiked levels of 10, 20, and 50 μg/kg were in the range of 72.5%-117.1%, and the relative standard deviations ranged from 1.3% to 10.9%.
Application in dairy product testing
Zhang et al.[3]developed a method for the simultaneous detection of 19 antibiotics in dairy products using ultra performance liquid chromatography-high resolution time-of-flight mass spectrometry. After the sample was treated with acidified acetonitrile, it was subjected to freezing, centrifugation and concentration, and in the positive ion scanning mode, 19 antibiotics were separated and detected within 10 min. The detection limit was 3-5 μg/L, and the average recoveries were in the range of 68.4%-96.7%. The isotope peak shape matching degree was not less than 87.4%. All 19 spiked antibiotics were detected, and most of the antibiotics had high identification scores.
Peng et al.[4]established a screening method for 55 drugs in milk using ultra-high pressure liquid chromatography-quadrupole time-of-flight mass spectrometry. Using acetonitrile as the extraction solvent, the extract was purified and concentrated by HLB solid phase extraction, and then loaded on the machine. The detection limits of the 55 drugs were in the range of 2-10 μg/kg, and the linearity was good in the range of 5-100 ng/ml. Adding 20-100 μg/kg drugs to milk, the recoveries ranged from 22.9% to 114.3%.
Yu et al.[5]established a rapid screening method for 28 prohibited veterinary drug residues in milk using ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry. The milk was extracted with 0.2% acidified acetonitrile and purified with an Oasis PRi ME HLB solid phase extraction cartridge. In the positive and negative ion mode, a full scanning was carried out with an electrospray ion source, and 28 veterinary drugs were well separated within 20 min. In the range of 1-10 μg/kg, the average recoveries were in the range of 68.9%-102.0%.
Comparison of methods
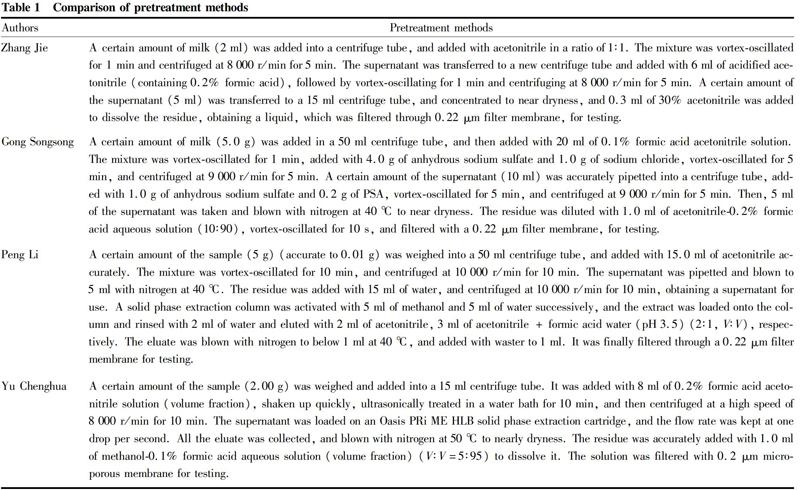
As shown in Table 1, acidified acetonitrile is used in the four pretreatment methods to extract pesticide residues in milk. Experiments have shown that acidified acetonitrile can better extract target compounds and precipitate proteins. The centrifugation speed is
Zhang JieA certain amount of milk (2 ml) was added into a centrifuge tube, and added with acetonitrile in a ratio of 1∶1. The mixture was vortex-oscillated for 1 min and centrifuged at 8 000 r/min for 5 min. The supernatant was transferred to a new centrifuge tube and added with 6 ml of acidified acetonitrile (containing 0.2% formic acid), followed by vortex-oscillating for 1 min and centrifuging at 8 000 r/min for 5 min. A certain amount of the supernatant (5 ml) was transferred to a 15 ml centrifuge tube, and concentrated to near dryness, and 0.3 ml of 30% acetonitrile was added to dissolve the residue, obtaining a liquid, which was filtered through 0.22 μm filter membrane, for testing.
Gong SongsongA certain amount of milk (5.0 g) was added in a 50 ml centrifuge tube, and then added with 20 ml of 0.1% formic acid acetonitrile solution. The mixture was vortex-oscillated for 1 min, added with 4.0 g of anhydrous sodium sulfate and 1.0 g of sodium chloride, vortex-oscillated for 5 min, and centrifuged at 9 000 r/min for 5 min. A certain amount of the supernatant (10 ml) was accurately pipetted into a centrifuge tube, added with 1.0 g of anhydrous sodium sulfate and 0.2 g of PSA, vortex-oscillated for 5 min, and centrifuged at 9 000 r/min for 5 min. Then, 5 ml of the supernatant was taken and blown with nitrogen at 40 ℃ to near dryness. The residue was diluted with 1.0 ml of acetonitrile-0.2% formic acid aqueous solution (10∶90), vortex-oscillated for 10 s, and filtered with a 0.22 μm filter membrane, for testing.
Peng LiA certain amount of the sample (5 g) (accurate to 0.01 g) was weighed into a 50 ml centrifuge tube, and added with 15.0 ml of acetonitrile accurately. The mixture was vortex-oscillated for 10 min, and centrifuged at 10 000 r/min for 10 min. The supernatant was pipetted and blown to 5 ml with nitrogen at 40 ℃. The residue was added with 15 ml of water, and centrifuged at 10 000 r/min for 10 min, obtaining a supernatant for use. A solid phase extraction column was activated with 5 ml of methanol and 5 ml of water successively, and the extract was loaded onto the column and rinsed with 2 ml of water and eluted with 2 ml of acetonitrile, 3 ml of acetonitrile + formic acid water (pH 3.5) (2∶1, V∶V), respectively. The eluate was blown with nitrogen to below 1 ml at 40 ℃, and added with waster to 1 ml. It was finally filtered through a 0.22 μm filter membrane for testing.
Yu ChenghuaA certain amount of the sample (2.00 g) was weighed and added into a 15 ml centrifuge tube. It was added with 8 ml of 0.2% formic acid acetonitrile solution (volume fraction), shaken up quickly, ultrasonically treated in a water bath for 10 min, and then centrifuged at a high speed of 8 000 r/min for 10 min. The supernatant was loaded on an Oasis PRi ME HLB solid phase extraction cartridge, and the flow rate was kept at one drop per second. All the eluate was collected, and blown with nitrogen at 50 ℃ to nearly dryness. The residue was accurately added with 1.0 ml of methanol-0.1% formic acid aqueous solution (volume fraction) (V∶V=5∶95) to dissolve it. The solution was filtered with 0.2 μm microporous membrane for testing.
between 8 000 r/min and 10 000 r/min. The higher the centrifugation speed is, the better precipitation can be obtained. The dissolving solution can be selected from 30% acetonitrile, acetonitrile-0.2% formic acid aqueous solution, water, methanol-0.1% formic acid aqueous solution, and the like.
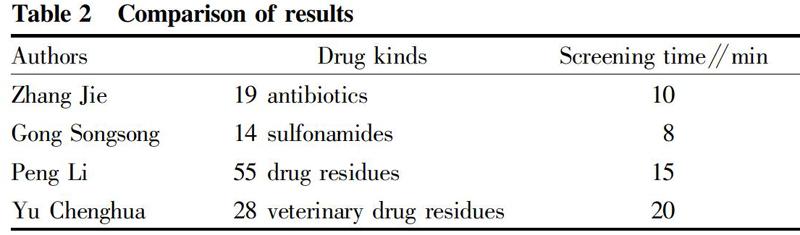
As shown in Table 2, high resolution time-of-flight mass spectrometry has the ability to screen multiple drug residues in a short period of time, with short screening time, fast analysis speed, high sensitivity, and a wide variety of screening drugs.
Application in Identifying Adulterated Substances in Milk
The problem of milk quality and safety is a major food safety problem facing China. At present, the methods for identifying adulterated substances in milk mainly include specific gravity method and color rendering method. The method of using liquid chromatography-high resolution time-of-flight mass spectrometry to identify adulterants in milk is not yet mature. How to use liquid chromatography-high resolution time-of-flight mass spectrometry to quickly and accurately identify adulterants in milk is an important issue for our research. How to use liquid chromatography-high resolution time-of-flight mass spectrometry to quickly and accurately identify adulterants in milk is an important issue for our research. Li et al.[6]compared the peak differences of the BPC spectra of three samples including milk, soy milk, and milk mixed with soy milk. The obvious separation of the three samples was completed, and the results showed that this method can be used to identify adulteration of complex mixtures of soybean milk.
Conclusions
At present, in general, TOF-MS technology has been widely used in food, chemistry, life sciences, etc. the TOF-MS pre-treatment technology is becoming simpler, more convenient, and faster, and the mass spectrometry resolution is getting higher and higher. With the advancement and development of science and technology, TOF-MS technology will be more and more integrated into our various aspects of food, clothing, housing and transportation testing, which will be more beneficial to protect people's safety.
With regard to the application of TOF-MS technology for rapid screening of veterinary drug residues in milk, the technology has become mature, and the types of screening are gradually increasing. However, there are relatively few studies on the rapid screening of pesticide residues and adulterated substances in milk. We can focus our research on rapid screening of pesticide residues and identification of adulterants.
References
[1]LI H, DING JJ, LI JF. Source, harm and prevention and control of antibiotic residues in milk[J]. China Dairy, 2011, 2011(008): 47-48. (in Chinese)
[2]GONG SS, DU X, CAO H, et al. Rapid screening of 14 sulfonamides in raw milk by ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry[J]. Journal of Instrumental Analysis, 2014, 33(012): 1342-1348. (in Chinese)
[3]ZHANG J, YAN LQ, PAN CS, et al. Simultaneous analysis of 19 antibiotics in dairy products using ultra-performance liquid chromatography coupled with high resolution time-of-flight mass spectrometry[J]. Chinese Journal of Chromatography, 2012(10): 1031-1036. (in Chinese)
[4]PENG L, WU NP, ZHANG CW, et al. Screening method for 55 kinds of drugs in milk by ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry[J]. Chinese Journal of Veterinary Drug, 2014(9): 45-52. (in Chinese)
[5]YU CH, LIN L, YANG JQ, et al. Rapid screening of 28 banned veterinary drugs in milk by ultra performance liquid chromatography coupled with quadrupole-time of flight mass spectrometry[J]. Journal of Food Science and Biotechnology, 2017(10): 62-68.
[6]LI G, QI L, JIN BM, et al. Identification of adulteration and quality control of milk by UPLC/Q-TOF MS/MS[J]. Modern Preventive Medicine, 2016(23). (in Chinese)
杂志排行
农业生物技术(英文版)的其它文章
- Review on Effects of Sunlight on the Internal Quality of Peach Fruit
- Research Progress on Genetic Breeding of Sweet Sorghum Related to Sugar Traits
- Screening of Red-flesh Small Watermelon Varieties for Substrate Cultivation in Spring Greenhouses
- Planting Techniques of Pennisetum giganteum in Huanghuai Area
- Bibliometric Analysis of Status Quo and Trend of the Research on Duck Based on the Web of Science Database
- Preparation and Insecticidal Activity of Sea Anemone Peptide AP-GI from Aiptasia pallida
