Evaluation of the Acute Toxicity of Prochloraz-manganese Chloride Complex to Environmental Species
2021-07-08XuyeZHANGJiMAXuanqingKONGKuaiYUXiaojunDENGWensenOUYANGYuJIANGXiaomingOUChenzhongJIN
Xuye ZHANG Ji MA Xuanqing KONG Kuai YU Xiaojun DENG Wensen OUYANG Yu JIANG Xiaoming OU Chenzhong JIN
Abstract [Objectives]This study was conducted to evaluate the toxicity risk of prochloraz-manganese chloride complex to environmental organisms. [Methods]The acute toxicity tests of prochloraz-manganese chloride complex on 8 common environmental organisms, such as Coturnix coturnix japonica, Apis mellifera L., Eisenia foetida, Bombyx mori, Trichogramma japonicum, Brachydanio rerio, Daphnia magna Straus and Scenedesmus obliquus were carried out. [Results]Prochloraz-manganese chloride complex to D. magna EC50 (48 h): 1.11 mg a.i./L, with a 95% confidence limit of 1.00-1.26 mg a.i./L; B. rerio LC50 (96 h): 2.84 mg a.i./L, with a 95 % Confidence limit of 2.43-3.48 mg a.i./L; S. obliquus ErC50 (72 h): 1.88×10-2 mg a.i./L, with a 95% confidence limit of 1.70×10-2-2.16×10-2 mg a.i./L; C. coturnix LD50 (168 h): 1.01×103 mg a.i./kg body weight, with a 95% confidence limit of 7.87×102-1.27×103 mg a.i./kg body weight; T. japonicum LR50 (24 h): 2.53×10-3 mg a.i./cm2, with a 95% confidence limit of 2.14×10-3-2.99×10-3 mg a.i./cm2; B. mori LC50 (96 h): 2.03×102 mg a.i./L, with a 95% confidence limit of 1.79×102-2.30×102 mg a.i./L; A. mellifera (peroral) LD50 (48 h)>100 μg a.i./bee; A. mellifera (contact) LD50 (48 h)>100 μg a.i./bee; E. foetida LC50 (14 d)>1.00×102 mg a.i./kg dry soil. According to the Guidance for Evaluating and Calculating Degradation Kinetics in Environmental Media for Pesticide Registration, prochloraz-manganese chloride complex had low toxicity to A. mellifera, C. coturnix, B. mori and E. foetida, medium toxicity to D. magna and B. rerio, and high toxicity and low risk to S. obliquus and T. japonicum. [Conclusions]This study provides a data basis for the toxicity and environmental safety evaluation of the agent.
Key words Prochloraz-manganese chloride complex; Environmental organisms; Acute toxicity
Prochloraz-manganese chloride complex is compounded from prochloraz and manganese chloride, with the chemical name of N-propyl-N-[2 (2,4,6-trichlorophenoxy) ethyl]-1 H- imidazole-1-methanamide-manganese chloride. It is an imidazole broad-spectrum microbicide the action mode of which is to effectively inhibit the biosynthesis of sterols of plant pathogens, having special effects on a variety of plant diseases caused by ascomycetes[1]. It can be used for disease control of rice, cotton, rape, pear, walnut, citrus, apple tree, tomato and other plants with good control effects[2-7], and can also be used for fruit preservation[8]. Prochloraz-manganese chloride complex can be quickly digested in water and soil of paddy fields, and belongs to easily degradable pesticides[9]. It not only maintains the original excellent characteristics of prochloraz agents, but also enhances the safety of prochloraz on plants, fruits and vegetables. It is more widely used[10-11].
At present, there are very few reports on the toxicity of prochloraz-manganese chloride complex to environmental organisms in China, and there are large gaps. The existing studies have proved that the LC50 of prochloraz-manganese chloride complex on the tadpoles of Bufo gargarizans at 24, 48 and 72 h is respectively 7.43, 3.75, and 3.22 mg/L[12]. However, there are few studies on other environmental organisms (birds, earthworms, bees, algae, etc.). Therefore, in this study, the acute toxicity of prochloraz-manganese chloride complex to Coturnix coturnix japonica, Apis mellifera L., Eisenia foetida, Bombyx mori, Trichogramma japonicum, Brachydonio rerio, Daphnia magna Straus, and Scenedesmus obliquus was determined, in accordance with the OECD test guidelines and the Test Guidelines on Environmental Safety Assessment for Chemical Pesticides[13-20], providing a data basis for the toxicity and environmental safety assessment of the agent.
Materials and Methods
Test materials
Test pesticide: 50% prochloraz-manganese chloride complex wettable powder, provided by Hunan Research Institute of Chemical Industry.
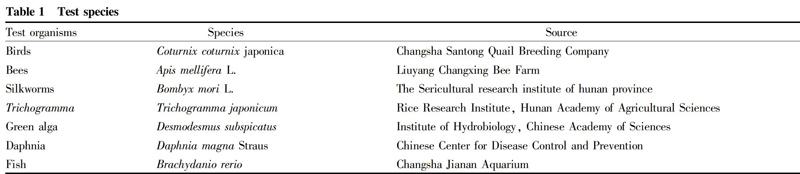
Test organisms are shown in Table 1.
Test instruments
High performance liquid chromatograph (LC-20AT, Japan, Shimadzu); digital acidity meter (STARTER 3C); dissolved oxygen meter (JPBJ-608); water hardness tester (YD200); electronic balance (AUY220); light incubator; circulating water vacuum pump.
Test methods
Acute toxicity test on bees
The test bees selected were adult worker bees of A. mellifera L., which were reared under natural conditions. The bees were taken out of the hives in the morning or evening before the test, and they were kept in the bee boxes, fed with 50% sucrose aqueous solution, and placed in the test environment for a 24 h adaptation period. Dead or abnormal individuals were excluded, and lively and healthy adult worker bees with the same size were chosen as the standard bees for the test. The test was carried out under dark conditions at the temperature at (25±2) ℃ and the relative humidity of 50%-70%.
Oral test: A certain amount of the test substance was weighed and prepared with 50% sucrose water to the test concentration of 1.00×104 mg a.i./L. Its exposure dose was 100 μg a.i./bee, and a blank control was set at the same time. Each treatment was set with three replicates, each with 10 bees. In each replicate, a feeder was used to hold 100 μl of the liquid drug for feeding. After the liquid was consumed, the feeder was taken out, and 50% sucrose water was used for normal feeding. After observing and cultivating continuously for 48 h, the symptoms of poisoning and death were recorded every day, and the 48 h-LD50 was calculated.
Contact test: A certain amount of the test substance was weighed and prepared with 50% sucrose water to the test concentration of 1.00×104 mg a.i./L. Its exposure dose was 100 μg a.i./bee, and a blank control and a solvent control were set at the same time. Each treatment was set with three replicates, each with 10 bees. After the bees were anesthetized with nitrogen, 1 μl of the medicinal liquid was dropped on the mesonotum of each bee, which was then transferred to rearing cages. The bees were fed with 50% sucrose water and observed for 48 h. The symptoms of poisoning and death were recorded every day, and the 48 h-LD50 was calculated.
Acute oral toxicity test on quails
The test quails were lively quails of C. coturnix japonica, which were 30 days old, hatched in the same batch, healthy, disease-free, weighing about 95-100 g. Before the test, they were placed in the test environment for 7 d, during which the room temperature was controlled at (25±2)℃, the humidity was 50%-75%, and the light/dark time ratio was 16 h/8 h.
A certain amount of the test substance was weighed and prepared with distilled water to 2.00×105, 1.43×105, 1.02×105, 7.29×104, and 5.21×104 mg a.i./L. The birds were exposed to the poison according to one percent of the body weight, so the doses of the poison were 2.00×103, 1.43×103, 1.02×103, 7.29×102, and 5.21×102 mg a.i./kgbody weight. Each treatment included 10 quails, half male and half, and a control group was set at the same time. The birds were fasted for 16 h before the test, and fed at 2 h after the poisoning treatment. They were observed continuously for 7 d while ensuring sufficient feed and drinking water. The poisoning symptoms and death of quails were recorded every day, and the 7 d-LD50 were calculated.
Acute toxicity test on silkworms
The selected silkworm species was Chunlei×Zhenzhu. The test was carried out on the second-instar larvae by the soaking method. The mulberry leaves were controlled at about 5 g per piece. The test conditions were as follows: test temperature (25±2) ℃, humidity 0%-85%, and light 16 h∶8 h.
A certain amount of the test substance was weighed and prepared with distilled water to 5 concentrations of 6.00×102, 3.75×102, 2.34×102, 1.46×102 and 91.6 mg a.i./L, 500 ml each. The mulberry leaves were immersed in the liquid drug completely for about 10 s, taken out, dried in the shade and added into petri dishes. Then, 20 silkworms were put per petri dish, and a control group was set at the same time. The silkworms were continuously cultured and observed for 96 h, and the poisoning symptoms and death of silkworms were recorded every day. The 96 h-LC50 was calculated.
Acute toxicity test on natural enemy T. japonicum
T. japonicum was selected and propagated in Corcyra cephalonica eggs. T. japonicum eggs were hatched under dark conditions at the temperature of (25±2) ℃ and the humidity of 50%-80%. The test was carried out on adult bees within 48 h of emergence under dark conditions with the temperature of (25±2) ℃ and the humidity of 70%-80%.
A certain amount of the test substance was weighed and prepared with acetone to 5 concentrations of 5.00×102, 3.12×102, 1.95×102, 1.22×102, and 76.3 mg a.i./L. Then, 37.5 cm2 finger tubes were selected and added with 0.50 ml of corresponding drug solution. The finger tubes were rolled to prepare a drug film. No treatment was set as a blank control, and acetone treatment was set as a solvent control. Each concentration was set with 3 replicates, and each finger tube was added with (100±10) adult bees. After the bees crawled in the drug film tubes for 1 h, they were then transferred to drug-free tubes, and fed with 10% honey water, and the mouth of each tube was sealed with black cloth. The treatments and the controls were done at the same time. The test period was 24 h, and the 24 h-LR50 was calculated.
Acute toxicity test on earthworms
For earthworms, Eisenia foetida was selected, and healthy adult earthworms of the same size that had shown breeding rings and weighed between 300 and 600 mg were tested. Before the test, the earthworms were domesticated in the artificial soil to be tested for one day. After the domestication, they were cleaned with distilled water, and the excess water was absorbed by filter paper for the test.
A certain amount of the test substance was weighed, dissolved and diluted with distilled water. The solution was then mixed evenly with artificial soil, and prepared to 100 mg a.i./kg dry soil. A blank control group was set, and each group had three replicates, each of which was added with 10 earthworms. The test lasted for 14 d, during which the poisoning symptoms and death of earthworms were observed and recorded on the 7th and 14th d, and the 14 d-LC50 was calculated.
Growth inhibition toxicity test on green alga
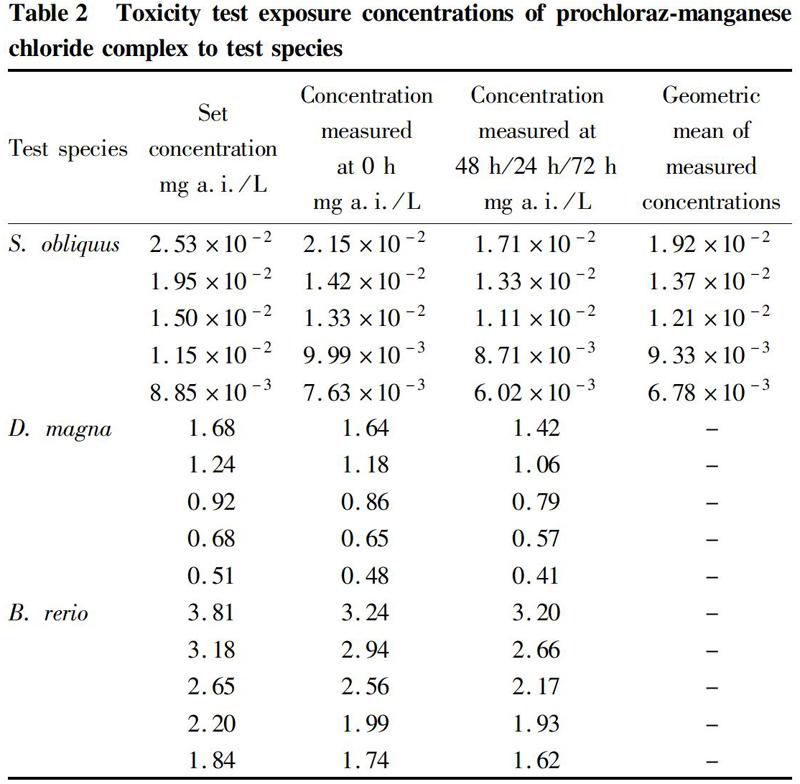
S. obliquus was introduced from the freshwater alga species bank of the Institute of Hydrobiology, Chinese Academy of Sciences, and transferred using aquatic medium No. 4 to expand the culture. After 3 times of transfer and culture, it basically achieved synchronous growth. The S. obliquus cultured for 72 h after transfer was used for the test. According to the results of the preliminary test, 1.000 5 g of the sample was accurately weighed, dissolved with 100 ml of sterile water, stirred, and filtered through a 0.45 μm filter membrane to obtain mother liquor 1, which was then diluted by 3 500, 4 550, 5 915, 7 689.5 and 9 996.35 times, respectively. The mother liquor 1 was determined by liquid chromatography to be 88.5 mg a.i./L, and the 5 concentrations set were thus 2.53×10-2, 1.95×10-2, 1.50×10-2, 1.15×10-2, and 8.85×10-3 mg a.i./L, respectively. A blank control was set at the same time, and each treatment was repeated 3 times. With a pipette, 1.00 ml of mother liquor 1 was accurately transferred to a 100 ml volumetric flask and diluted with sterilized 10% aquatic culture medium No.4 to constant weight, obtaining stock solution 2, from which 14.29, 10.99, 8.45, 6.50 and 5.00 ml were transferred, respectively, to 250 ml volumetric flasks, and prepared with sterilized 10% aquatic culture medium No. 4 to a series of 2-time concentrations of drug solutions for use. The same sterilized medium was used to inoculate the pre-cultured S. obliquus cells. The drug-containing culture media and the algal cell diluent were mixed at 1∶1 to obtain the exposure culture media. The test was carried out for 72 h, and samples were taken at 24, 48 and 72 h of culture, for counting under a microscope with a hemocytometer. The 72 h 50% inhibitory concentration (EC50) value was calculated.
Acute inhibition toxicity test on D. magna
D. magna was introduced from National Institute of Environmental Health, Chinese Center for Disease Control and Prevention. Health individuals 6-24 h after birth by female that had undergone more than 3 generations of parthenogenetic reproduction under laboratory conditions, were selected. A certain amount of the sample (1.000 0 g) was accurately weighed, dissolved with 100 ml of distilled water, stirred, and filtered through 0.45 μm filter membrane to obtain mother liquor A, which was then diluted by 40.0, 54.0, 72.9, 98.415 and 132.86 times, respectively. The mother liquor A was detected by liquid chromatography to be 67.16 mg a.i./L, and the 5 test concentrations set were thus 1.68, 1.24, 0.92, 0.68, and 0.51 mg a.i./L. A blank control was set at the same time, and each treatment was repeated 4 times.
From mother liquor A, 6.25, 4.63, 3.43, 2.54 and 1.88 ml were transferred into a 250 ml volumetric flasks, and prepared into a series of concentrations required for the test with D. magna test water. The test solution was divided and added into 5 beakers (one of which was used for water quality determination), each of which was added with 30 ml of corresponding drug solution, and a blank control was set without adding the drug. Samples were taken at 0 and 48 h during the test for concentration analysis. The 48 h 50% inhibitory concentration value (EC50) was calculated.
Acute toxicity test on B. rerio
B. rerio was purchased from Changsha City Aquarium. The fish were domesticated for more than 7 d under laboratory conditions, with 12 to 16 h of light every day, and timely removal of feces and food residues, and the mortality rate remained below 5%. During the domestication period, they were fed 1-2 times a day with commercial finished baits. Feeding was stopped 24 h before the test, and no feed was input during the test period. Healthy and lively individuals with a body length of (2 ± 1) cm were selected for the test.
The semi-static test method was adopted, and the drug solution was changed every 24 h.
A certain amount of the sample (10.000 2 g) was quantitatively weighed, dissolved with 1 000 ml of distilled water, stirred, and filtered through 0.45 μm filter membrane, obtaining mother liquor A, which was then diluted by 20.0, 24.0, 28.8, 34.56 and 41.472 times, respectively, 5 concentrations in total. The mother liquor A was determined to be 76.2 mg a.i./L by liquid chromatography, and the concentrations set were thus 3.81, 3.18, 2.65, 2.20, and 1.84 mg a.i./L. A blank control was set at the same time, and no replicates were set.
Then, 150, 125, 104.17, 86.81 and 72.33 ml of the mother liquor were transferred to fish tanks with 2.5 L of water. The fish tanks were rinsed for 3 times, and all the rinsing fluid was transferred to the fish tanks, diluted to 3 L and stirred evenly, and no addition of the drug was set as a blank control. The drug liquids were changed every 24 h.
Samples were taken before and after the chemical solution was changed, for concentration analysis. The 96 h-LC50 value was calculated.
Data processing
All experimental data was processed and analyzed with SPSS 16.0.
Results and Analysis
Acute toxicity of prochloraz-manganese chloride complex on non-target species
During the experiment, no abnormal performance was found in each experimental control group and the bee and earthworm toxicity experimental treatment groups. The behaviors of other species in the low-concentration groups were basically similar to those in the control groups, but as the exposure concentration increased, the poisoning status increased, leading to death. Specifically, the observation on the treatment groups of quails showed that most of them showed symptoms of listlessness, nap, slow movement, unstable standing, and loss of appetite, and most of the surviving quails returned to normal after 96 h. The poisoned silkworms exhibited soft and shrank bodies, and refused to feed, the feed intake of silkworms decreased with the increase of the concentration of the drug, and dead silkworms were lying sideways. With the increase of the concentration of the drug, the number of dead T. japonicum gradually increased, dead T. japonicum fell on the bottoms of the test tubes, and some T. japonicum were out of balance. S. obliquus grew slowly after chemical treatment, and with the increase of concentration, the color gradually changed from dark green to lighter, the number of algal cells decreased, and the algal cells in the 2.53×10-2 mg ai/L treatment deformed and broke. About 6 h after poisoning, D. magna in the 1.68 mg a.i./L treatment group all began to swim in rotation, and some D. magna could not swim normally. After 24 h, the activities of some D. magna were inhibited, and with the concentration increased, the number of inhibited D. magna increased. The bodies of the inhibited D. magna became whitish, no longer transparent, and sunk to the bottoms of the containers. The fish were observed to swim slowly in some concentrations about 6 h after exposure, and rolled over unbalanced, and some fish in high concentrations died. The dead B. rerio sank to the bottom, showing swollen head and congested belly. After 72 h, the B. rerio no longer died.
According to the results of concentration analysis (Table 2), the measured concentration at the beginning of the tests was lower than the theoretical concentration we set. After the prochloraz-manganese chloride complex was added with water, the hydrolysis and photolysis effects would occur under the influences of pH and temperature. Meanwhile, biodegradation would occur, leading to a decrease in the measured concentration. For D. magna and B. rerio, the measured concentration during the tests was higher than 80% of the set concentration. The set concentration could be used for calculation to reflect its toxic effects, and then the safety evaluation could be carried out. However, the concentration of S. obliquus in the 72 h test was basically lower than 80% of the set concentration. In order to maintain the test concentration, this test adopted a semi-static method, and because the calculated results of the indicated concentration could not truly reflect the toxic effect of prochloraz-manganese chloride complex, the geometric mean of the measured concentrations during the test period was selected to calculate the final result for safety evaluation[21].
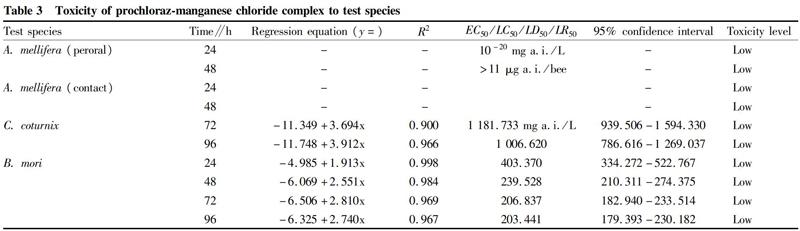
Table 3 shows the toxic effects, regression equations and confidence intervals of prochloraz-manganese chloride complex on the test species. For T. japonicum, we got their LR50, and the concentration of prochloraz-manganese chloride complex used in the field was 333.33 mg/L, which was calculated with 60 L of water per 667 m2. It was concluded that its safety factor was 8.43. Therefore, prochloraz-manganese chloride complex has a low risk to T. japonicum.
We tested the test substance as a reference substance, and the result was within the scope of Test Guidelines on Environmental Safety Assessment for Chemical Pesticides, which proved the reliability of the test.
Classification of toxicity levels
According to the Test Guidelines on Environmental Safety Assessment for Chemical Pesticides, prochloraz-manganese chloride complex had low toxicity to A. mellifera, C. coturnix, B. mori and E. foetida, medium toxicity to D. magna and B. rerio, and high toxicity and low risk to S. obliquus and T. japonicum.
Conclusions and Discussion
Prochloraz-manganese chloride complex has different toxicity to non-target organisms in the environment. It had low toxicity to terrestrial organisms such as A. mellifera, C. coturnix, B. mori and E. foetida, and low risk to T. japonicum. However, it had medium toxicity to aquatic organisms, D. magna and B. rerio, and high toxicity to S. obliquus. Therefore, in farming, it should be used away from the water body, so as to avoid damage to the aquatic ecosystem caused by pouring the liquid drug into the water body, or pouring the liquid drug into the water body when cleaning the sprayer.
In this study, we only judged and evaluated the environmental safety of prochloraz-manganese chloride complex based on its hazards to various test species. In actual work, it must be considered comprehensively according to the specific application method, field microclimate, temperature and light, etc. There are few environmental evaluations of prochloraz-manganese chloride complex in literatures, and we need to further study and improve the toxicity data of prochloraz-manganese chloride complex to aquatic and terrestrial organisms.
References
[1]LI AN, WANG AQ, CHEN JM. Studies on poly (acrylic acid)/attapulgite superabsorbent composite. I. Synthesis and characterization[J]. Journal of Applied Polymer Science, 2004, 92(3): 1596-1603.
[2]HUANG YJ, SONG HM, DING P, et al. Field efficacy evaluation of difenoconazole-prochloraz 75% WP against Melanconium oblongum Berk on Carya cathayensis Sarg.[J]. Journal of Agricultural Catastrophology, 2018, 8(2): 1-2, 36. (in Chinese)
[3]HU JJ, KANG T, ZHUANG QF, et al. Toxicity of 15 kinds of microbicides to the pathogen of peach shoot blight[J]. Shanghai Agricultural Science and Technology, 2018(4): 118-119. (in Chinese)
[4]DU GF, LIU ZJ, LI HF, et al. Pathogen identification of cherry tomato leaf spot and its fungicides screening [J]. China Plant Protection, 2017, 37(1): 13-16. (in Chinese)
[5]ZHAO J. Preliminary report on the antimicrobial tests of 8 microbicides against pear black spot[J]. Shanghai Agricultural Science and Technology, 2016(1): 144. (in Chinese)
[6]SUN ZX, DENG L, ZHANG CQ, et al. Toxicity of several fungicides and chemical mixture against Sclerotinia sclerotiorum[J]. Hubei Agricultural Sciences, 2015, 54(7): 1606-1608. (in Chinese)
[7]ZHANG XY, LAI WL, XU Y. Laboratory screening of fungicides against Colletotrichum in navel orange[J]. Journal of Gannan Normal University, 2015, 36(6): 54-57. (in Chinese)
[8]ZHENG FQ, XU CX, MA YP, et al. The Fresh-keeping effect of ‘Shatangju (Citrus reticulata) fruits dipped with prochloraz-manganese chloride complex solution[J]. Chinese Journal of Tropical Agriculture, 2016, 36(10): 50-53, 58. (in Chinese)
[9]WANG J, MAO CL, YIN XH, et al. Degradation of prochloraz-manganese chloride complex in paddy water and soil [J]. Agrochemicals, 2014, 53(5): 356-358. (in Chinese)
[10]ZHAO J, CHEN XM, YU L, et al. The efficacy of mixture of pyrametostrobin and prochloraz against Citrus anthracnose[J].Agrochemicals, 2016, 55 (4) : 290-292. (in Chinese)
[11]GU WH, FAN QS. Effect of 40% prochloraz water emulsion on controlling Citrus anthracnose[J]. XianDai NongYe KeJi, 2015, (9): 134-135. (in Chinese)
[12]WEI L, LEI HZ, SHAO WW, et al. Acute lethal toxic effects of metolachlor, kresoxim-methyl and prochloraz-manganese chloride complex to the Chinese toad (Bufo gargarizans) Tadpoles [J]. Chinese Journal of Zoology, 2016, 51(1): 45-56. (in Chinese)
[13]Standardization Administration, General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China. GB/T 31270-2014 Guidance for evaluating and calculating degradation kinetics in environmental media for pesticide registration[S]. Beijing: China Standards Press, 2014. (in Chinese)
[14]OECD. Guidelines for testing of chemicals, guideline 213: Honeybees, acute oral toxicity test [S]. Paris: OECD, 1998
[15]OECD. Guidelines for testing of chemicals, guideline 214: Honeybees, acute contact toxicity test [S]. Paris: OECD, 1998.
[16]OECD. Guidelines for testing of chemicals, guideline 223: Avian acute oral toxicity test[S]. Paris: OECD, 2010.
[17]OECD. Guidelines for testing of chemicals, guideline 207: Earthworm, acute toxicity tests[S]. Paris: OECD, 1984.
[18]OECD. Guidelines for testing of chemicals, Guideline 201: Freshwater alga and cyanobacteria, growth inhibition test [S]. Paris: OECD, 2011.
[19]OECD. Guidelines for testing of chemicals, guideline 202: Daphnia sp., acute immobilization test [S]. Paris: OECD, 2004.
[20]OECD. Guidelines for testing of chemicals, guideline 203: Fish, acute toxicity test [S]. Paris: OECD, 1992.
[21]OUYANG XQ, WU C, WANG CB, et al. Acute toxicity and safety evaluation of trifloxystrobin to environmental organisms[J]. Asian Journal of Ecotoxicology, 2017, 12(4): 327-336. (in Chinese)
杂志排行
农业生物技术(英文版)的其它文章
- Review on Effects of Sunlight on the Internal Quality of Peach Fruit
- Research Progress on Genetic Breeding of Sweet Sorghum Related to Sugar Traits
- Screening of Red-flesh Small Watermelon Varieties for Substrate Cultivation in Spring Greenhouses
- Planting Techniques of Pennisetum giganteum in Huanghuai Area
- Bibliometric Analysis of Status Quo and Trend of the Research on Duck Based on the Web of Science Database
- Preparation and Insecticidal Activity of Sea Anemone Peptide AP-GI from Aiptasia pallida
